Abstract
Several previous studies have shown that renal failure decreases not only renal elimination but also metabolic clearance of drugs, particularly those metabolized by CYP3A. However, whether recovery of renal function results in recovery of hepatic CYP3A activity remains unknown. In this study, we evaluated the effect of renal function on CYP3A activity after kidney transplantation in patients with end-stage renal disease (ESRD) by measuring the change in CYP3A activity using plasma concentration of 4β-hydroxycholesterol as a biomarker. The study enrolled 13 patients with ESRD who underwent the first kidney allograft transplantation. Morning blood samples were collected before and 3, 7, 10, 14, 21, 30, 60, 90, 120, 150 and 180 days after kidney transplantation. Plasma concentration of 4β-hydroxycholesterol was measured using GC-MS. Compared with before kidney transplantation, creatinine clearance increased significantly from day 3 after kidney transplantation and stabilized thereafter. Plasma concentration of 4β-hydroxycholesterol was elevated significantly on days 90 and 180 after kidney transplantation. In conclusion, this study suggests the recovery of CYP3A activity with improvement in renal function after kidney transplantation in patients with ESRD.
Keywords: CYP3A activity, end-stage renal disease, renal failure, plasma
Impaired renal function alters the clearance of many drugs mainly by decreasing their renal elimination. However, several studies in rats (1–6) and in patients (7–9) have shown that renal failure also decreases the metabolic clearance of drugs, particularly those metabolized by cytochrome P450 (CYP). The underlying causes of altered CYP functional expression observed in kidney disease remain unclear, but several studies indicate that uremic toxins (10–13) and inflammation (14) may play a role via transcriptional or translational modifications of CYP enzymes.
Phenotyping of cytochrome CYP3A seems to be of special importance because enzymes belonging to this family, particularly CYP3A4, are involved in the metabolism of more than 50% of currently prescribed drugs. However, the selection of an appropriate CYP3A phenotyping substrate and metric is still a matter of discussion, although some CYP3A test substrates, including midazolam, erythromycin, alprazolam, and nifedipine have been proposed (15). However, administration of these drugs poses challenges in specific patient populations such as pediatric and elderly patients, transplant recipients, and cancer patients, in whom administration of probe drugs may have a negative impact on patient safety. Identification of an appropriate endogenous CYP3A substrate and corresponding metrics would be a promising alternative, allowing simple and possibly broader application of the phenotyping approach. Oxidation of the endogenous compound cortisol to 6β-hydroxycortisol has been shown to be a CYP3A-dependent pathway in humans. Consequently, a urinary 6β-hydroxycortisol-to-cortisol ratio has been proposed as a suitable phenotyping marker for the assessment of CYP3A activity (16, 17). However, the sensitivity of the method to detect small changes in enzyme activity is low because of pronounced background variation (17). Another compound, 4β-hydroxycholesterol, which is identified as one of the major oxysterols in humans, was reported to be formed solely by CYP3A4 (18). Markedly elevated concentrations of 4β-hydroxycholesterol were found in patients treated with CYP3A4 inducers (19–23), and decreased concentrations in patients treated with CYP3A4 inhibitors (22–24). Furthermore, a relationship between blood 4β-hydroxycholesterol concentration and the number of active CYP3A5*1 alleles has been demonstrated, suggesting that 4β-hydroxycholesterol is formed not only by CYP3A4 but also by CYP3A5 (25). Accordingly, 4β-hydroxycholesterol has been proposed to be a potential endogenous biomarker of CYP3A activity.
In general, plasma levels of some biomarkers indicating uremia or inflammation decrease in patients with end-stage renal disease (ESRD) after kidney transplantation (26, 27). However, it is not known whether CYP3A activity recovers with improvement in renal function after kidney transplantation. In this study, we evaluated the effect of renal function on CYP3A activity after patients with ESRD received kidney transplantation by measuring the change in CYP3A activity using plasma concentration of 4β-hydroxycholesterol as a biomarker.
METHODS
Patients
The study enrolled patients with ESRD (creatinine clearance less than 15 ml/min) who underwent the first kidney allograft transplantation between January 2011 and November 2012 in the Department of Urology, Faculty of Medicine at Oita University. After kidney transplantation, all patients were followed for 180 days. Morning blood samples were collected in tubes containing EDTA anticoagulant before and 3, 7, 10, 14, 21, 30, 60, 90, 120, 150, and 180 days after kidney transplantation. All blood samples were centrifuged and plasma samples frozen at −40°C within 2 h of peripheral venipuncture. The following clinical data were collected: gender; age; body weight; prescribed drugs; and laboratory data including white blood cell count, hemoglobin, C-reactive protein, alanine transaminase (ALT), γ-glutamyl transpeptidase (γ-GTP), total bilirubin, serum creatinine, and blood urea nitrogen. Creatinine clearance was calculated according to the Cockcroft-Gault equation (28). Before kidney transplantation, all patients were hemodialyzed three times a week and received medications for hypertension (amlodipine, benidipine, olmesartan, telmisartan, carvedilol, or bisoprolol) and hyperphosphatemia (lanthanum carbonate or precipitated calcium carbonate). No patient received medications known to inhibit or induce CYP3A. All patients were treated with a triple-therapy immunosuppression protocol, consisting of tacrolimus, mycophenolate mophetil, and methylprednisolone. Furthermore, all patients received induction therapy with basiliximab before and 4 days after kidney transplantation. Patients were excluded if they had an episode of rejection or received medications known to inhibit or induce CYP3A during the study. This study was approved by the ethics committee of Oita University. Each subject received information about the scientific purpose of the study, and gave written informed consent.
Materials
4β-Hydroxycholesterol and 4β-hydroxycholesterol-d7 (internal standard) were purchased from Avanti Polar Lipids (Alabaster, AL). Tert-butyldimethylsilylimidazole-dimethylformamide was purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were analytical reagent grade from commercial sources.
Measurement of plasma concentration of 4β-hydroxycholesterol
Plasma concentration of 4β-hydroxycholesterol was determined using a modification of the method previously reported (18, 29). An aliquot of 100 μl of plasma sample was mixed with 20 μl of internal standard (1,000 nM 4β-hydroxycholesterol-d7 in 2-propanol) and 200 μl of 2 M sodium methoxide solution in ethanol, and the mixture was left at room temperature for 20 min to convert esterified to free 4β-hydroxycholesterol. Then, 500 μl of water and 2 ml of n-hexane were added, and extraction was performed by vortexing for 1 min. After centrifugation at 3,000 g for 5 min at 20°C, the organic phase was transferred to a glass tube and evaporated to dryness by a stream of nitrogen at 40°C. The residue was reconstituted with 100 μl of tert-butyldimethylsilylimidazole-dimethylformamide and incubated at room temperature for 12 h to convert 4β-hydroxycholesterol into a tert-butyldimethylsilyl ether. Subsequently, 1 ml of water and 2 ml of ethyl acetate were added, and the mixture was vortexed for 1 min. After centrifugation at 3,000 g for 5 min at 20°C, the organic phase was transferred to a glass tube and evaporated to dryness by a stream of nitrogen at 40°C. The residue was reconstituted with 100 μl of n-hexane. The sample was immediately transferred to auto sampler vials, and 1 μl of sample was splitless injected into the GC/MS system. An Agilent 7890GC gas chromatograph equipped with an HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm phase thickness) was used and was connected to an HP 5975 mass selective detector and an HP 7693 automatic sample injector (Agilent Technologies). The oven temperature program was as follows: 180°C for 1 min; increasing at 35°C/min to 270°C, and then 20°C/min to 310°C; followed by 310°C for 15 min. Helium was used as a carrier gas at a flow rate of 1 ml/min. The detector temperature was 270°C, and the detector transfer line temperature was set at 280°C. The mass spectrometer was used in the selected ion-monitoring mode, and the following ions (m/z) were monitored: 573.5 for 4β-hydroxycholesterol and 580.6 for 4β-hydroxycholesterol-d7 (internal standard). The electron ionization energy was 70 eV. Inter-assay coefficients of variation for 20 and 200 ng/ml samples were 3.8% and 4.2%, respectively (n = 6). Intra-assay coefficients of variation for 20 and 200 ng/ml samples were 3.4% and 4.7%, respectively (n = 6). The lower limit of quantification was 1 ng/ml, and the accuracy of the analysis ranged from 94.6% to 105.8%.
Determination of CYP3A5 genotype
A venous blood sample (5 ml) was obtained from each patient, and DNA was prepared using the Maxwell® 16 DNA Purification Kit (Promega; Tokyo, Japan). All samples were analyzed for the single nucleotide polymorphism A6986G (CYP3A5*3). Allelic discrimination reaction was performed using TaqMan genotyping assays (C_26201809_30) in a LightCycler® Nano System (Roche Applied Science; Penzberg, Germany). When the CYP3A5*3 allele was not detected, the allele was designated CYP3A5*1.
Statistical analysis
Data are expressed as mean ± standard deviation. Variables before and after kidney transplantation were compared by Dunnet test. A P value less than 0.05 was considered statistically significant. Statistical analyses were performed using Predictive Analysis Software Statistics version 18 (SPSS Inc.; Chicago, IL).
RESULTS
Fourteen patients signed the informed consent form for this study. Among 14 patients, a patient who had rejection during the study was excluded from analysis. Finally, the data of 13 patients were analyzed. Table 1 shows the clinical data of the 13 patients with ESRD before and 30 and 180 days after kidney transplantation. Six patients were heterozygous (CYP3A5*1/*3) and 5 patients were homozygous (CYP3A5*3/*3) for the CYP3A5*3 allele. As expected, blood urea nitrogen decreased significantly after kidney transplantation. On the other hand, no significant differences in ALT, γ-GTP, and total bilirubin were observed between before and after kidney transplantation, suggesting that hepatic function was stable during the study.
TABLE 1.
Characteristics of patients in the study
| Parameter | Before Transplantation | 30 Days after Transplantation | 180 Days after Transplantation |
| No. of patients | 13 | ||
| Males/females | 8/5 | ||
| Cause of kidney disease | |||
| Glomerulonephritis | 6 | ||
| Immunoglobulin A nephropathy | 2 | ||
| Thin basement membrane disease | 1 | ||
| Unknown nephritis | 4 | ||
| Living/cadaveric kidney transplantation | 10/3 | ||
| Age (years) | 46.1 ± 11.2 [24–66] | ||
| CYP3A5 polymorphism | |||
| CYP3A5*1/*1 | 2 | ||
| CYP3A5*1/*3 | 6 | ||
| CYP3A5*3/*3 | 5 | ||
| Body weight (kg) | 57.4 ± 11.8 [44.7–83.1] | 54.7 ± 10.2 [40.7–71.9] | 54.0 ± 10.0 [40.7–71.5] |
| White blood cell count (/μl) | 7,022 ± 2,187 [3,290–9,860] | 5,688 ± 1,791 [2,810–8,630] | 4,980 ± 1,846 [2,920–9,270] |
| Hemoglobin (g/dl) | 11.7 ± 1.8 [9.5–16.1] | 10.5 ± 1.5 [8.5–13.9] | 11.7 ± 1.2 [9.1–13.4] |
| C-reactive protein (mg/dl) | 0.08 ± 0.08 [0.01–0.27] | 0.10 ± 0.11 [0.01–0.37] | 0.06 ± 0.09 [0.01–0.35] |
| ALT (IU/l) | 15.9 ± 12.5 [4.6–44.6] | 14.3 ± 10.8 [4.0–32.6] | 13.4 ± 8.6 [5.8–32.5] |
| γ-GTP (IU/l) | 20.8 ± 9.4 [10.3–35.4] | 23.6 ± 10.1 [10.3–43.5] | 26.6 ± 12.0 [10.4–52.9] |
| Total bilirubin (mg/dl) | 0.44 ± 0.14 [0.28–0.70] | 0.39 ± 0.10 [0.26–0.60] | 0.46 ± 0.16 [0.27–0.74] |
| Blood urea nitrogen (mg/dl) | 52.9 ± 17.2 [28.5–100.7] | 24.3 ± 6.6†† [12.7–38.5] | 25.9 ± 5.2†† [18.8–35.0] |
CYP, cytochrome P450; ALT, alanine aminotransaminase; γ-GTP, γ-glutamyl transpeptidase. Data are expressed as numbers or mean ± SD [range]. †P < 0.01 versus before kidney transplantation.
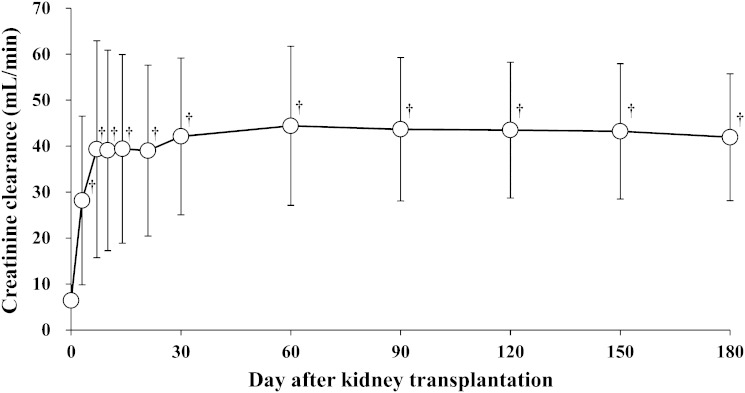
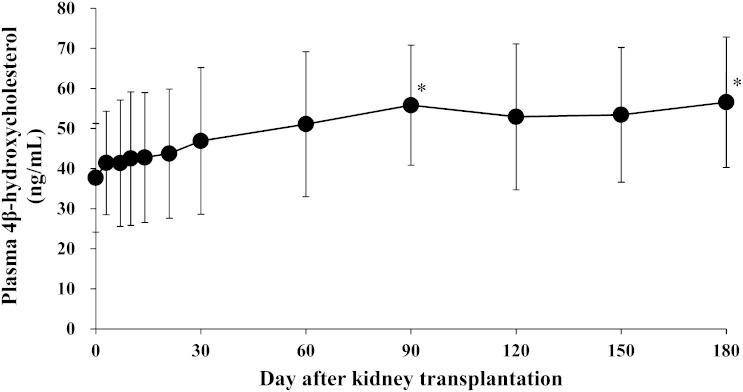
Figure 1 shows the change in creatinine clearance over time in patients with ESRD after kidney transplantation. Creatinine clearance increased significantly on day 3 after kidney transplantation, and thereafter remained almost stable until day 180 after kidney transplantation. Figure 2 shows the change in plasma concentration of 4β-hydroxycholesterol over time in patients with ESRD after kidney transplantation. Compared with before kidney transplantation (37.7 ± 13.6 ng/ml), plasma 4β-hydroxycholesterol concentration was significantly elevated on days 90 and 180 after kidney transplantation (55.8 ± 15.0 ng/ml, P = 0.021 and 56.6 ± 16.3 ng/ml, P = 0.015, respectively).
Fig. 1.
Change in creatinine clearance over time in patients with end-stage renal disease after kidney transplantation. Data are presented as mean ± SD, n = 13. †P < 0.01 versus before transplantation.
Fig. 2.
Change in plasma concentration of 4β-hydroxycholesterol over time in patients with end-stage renal disease after kidney transplantation. Data are presented as mean ± SD, n = 13. *P < 0.05 versus before transplantation.
DISCUSSION
In this study, we investigated the change in CYP3A activity over time after kidney transplantation in patients with ESRD. To evaluate hepatic CYP3A activity in humans, some CYP3A test substrates have been proposed, including midazolam, erythromycin, alprazolam, and nifedipine (15). However, these substrates have the problem of protein binding. For example, midazolam is normally 96% protein bound, and the midazolam unbound fraction may be increased in patients with ESRD, resulting in increased hepatic clearance. Using midazolam administration, Nolin et al. (30) reported that hepatic and intestinal CYP3A activities were not affected in patients with ESRD compared with healthy control subjects. Furthermore, administration of these drugs poses challenges in kidney transplant recipients, and may have a negative impact on patient safety. As a traditional and safer method to evaluate hepatic CYP3A activity in these specific patients, the urinary 6β-hydroxycortisol-to-cortisol ratio has been used as an endogenous CYP3A substrate (16, 17). However, this method depends on the renal clearance of both compounds as well as on the formation clearance of 6β-hydroxycortisol. Therefore, the urinary 6β-hydroxycortisol-to-cortisol ratio is a valid index of CYP3A activity only in the absence of significant intra- and inter-individual variations in cortisol renal clearance (31). Then, this method is unsuitable for kidney transplant recipients. As a more-accurate endogenous probe of CYP3A4 activity, the formation clearance of 6β-hydroxycortisol has been proposed (17, 31, 32). However, this method is not suitable for the estimation of changes over time because of the need for urine collection. In the present study, we used plasma concentration of 4β-hydroxycholesterol to evaluate hepatic CYP3A activity in patients with ESRD before and after kidney transplantation. Because 4β-hydroxycholesterol is formed by CYP3A4 and CYP3A5 (18, 25), it has been proposed as a potential endogenous biomarker of CYP3A activity. A previous study showed that 4β-hydroxycholesterol is slowly eliminated from the circulation, probably due to slow 7α-hydroxylation (33), suggesting that the kinetics of 4β-hydroxycholesterol is not affected by renal function. Hence, 4β-hydroxycholesterol is suitable for the evaluation of hepatic CYP3A activity in kidney transplant recipients. In this study, mean plasma concentrations of 4β-hydroxycholesterol in patients with ESRD changed from 37.7 ng/ml before transplantation to 56.6 ng/ml after transplantation. To the best of our knowledge, plasma concentration of 4β-hydroxycholesterol in patients with renal failure has not been reported previously, which makes comparison impossible. However, our levels are within the range of 18.8–77.0 ng/ml reported by other groups in healthy volunteers (18, 21, 23, 25, 34, 35).
Several previous studies have shown that renal failure decreases not only renal elimination but also metabolic clearance of drugs, particularly those metabolized by CYP3A. Dowling et al. (7) reported that CYP3A activity in patients with ESRD was significantly lower than that in controls using erythromycin breath test. Kirwan et al. (8, 9) showed that increasing severity and duration of acute kidney injury were associated with decreased midazolam elimination. However, there is no report of whether recovery of renal function results in recovery of hepatic CYP3A activity. In our study, we investigated the change in CYP3A activity over time after kidney transplantation in patients with ESRD, using plasma concentration of 4β-hydroxycholesterol as a biomarker, aiming to evaluate the effect of renal function on CYP3A activity. Creatinine clearance increased significantly from day 3 after kidney transplantation and remained stable up to the end of study, indicating successful kidney transplantations in the patients who participated in this study. Then, plasma concentration of 4β-hydroxycholesterol was significantly elevated on days 90 and 180 after kidney transplantation, compared with before kidney transplantation. Posttransplantation increase in plasma 4β-hydroxycholesterol, a product of the hepatic CYP3A system, suggests that successful kidney transplantation increases hepatic CYP3A activity in patients with ESRD. This is the first report suggesting recovery of CYP3A activity with improvement in renal function in ESRD patients after kidney transplantation. Further studies, including measuring activities of the CYP3A enzyme system, may validate and elucidate the mechanism of recovery of CYP3A activity.
Our study has several limitations. First, 4β-hydroxycholesterol has a long half-life of approximately 62 h (33). Therefore, the actual recovery of CYP3A activity after kidney transplantation may be earlier than the results in this study. Second, it has been reported that basiliximab may inhibit CYP3A, although tacrolimus, mycophenolic acid, and methylprednisolone have no potency to inhibit or induce CYP3A (36–40). Then, recovery of CYP3A activity may be slightly masked after induction therapy with basiliximab. Third, we were not able to evaluate the clearance of plasma 4β-hydroxycholesterol. Demonstration of no variation in the clearance of plasma 4β-hydroxycholesterol in each patient would have provided additional evidence for the recovery of CYP3A activity in these patients.
In conclusion, we measured plasma concentration of 4β-hydroxycholesterol, a product of the hepatic CYP3A system, in patients with ESRD, and found that the concentration increased after kidney transplantation, suggesting that activity of the hepatic CYP3A system may recover with improvement in renal function. Further studies are required to validate and elucidate the mechanism of recovery of CYP3A activity.
Footnotes
Abbreviations:
- ALT
- alanine transaminase
- CYP
- cytochrome P450
- ESRD
- end-stage renal disease
- γ-GTP
- γ-glutamyl transpeptidase
REFERENCES
- 1.Uchida N., Kurata N., Shimada K., Nishimura Y., Yasuda K., Hashimoto M., Uchida E., Yasuhara H. 1995. Changes of hepatic microsomal oxidative drug metabolizing enzymes in chronic renal failure (CRF) rats by partial nephrectomy. Jpn. J. Pharmacol. 68: 431–439. [DOI] [PubMed] [Google Scholar]
- 2.Leblond F. A., Giroux L., Villeneuve J. P., Pichette V. 2000. Decreased in vivo metabolism of drugs in chronic renal failure. Drug Metab. Dispos. 28: 1317–1320. [PubMed] [Google Scholar]
- 3.Leblond F., Guévin C., Demers C., Pellerin I., Gascon-Barré M., Pichette V. 2001. Downregulation of hepatic cytochrome P450 in chronic renal failure. J. Am. Soc. Nephrol. 12: 326–332. [DOI] [PubMed] [Google Scholar]
- 4.Rege B., Krieg R., Gao N., Sarkar M. A. 2003. Down-regulation of hepatic CYP3A in chronic renal insufficiency. Pharm. Res. 20: 1600–1606. [DOI] [PubMed] [Google Scholar]
- 5.Naud J., Michaud J., Beauchemin S., Hébert M. J., Roger M., Lefrancois S., Leblond F. A., Pichette V. 2011. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab. Dispos. 39: 1363–1369. [DOI] [PubMed] [Google Scholar]
- 6.Kusaba J., Kajikawa N., Kawasaki H., Kurosaki Y., Aiba T. 2012. Comparative study on altered hepatic metabolism of CYP3A substrates in rats with glycerol-induced acute renal failure. Biopharm. Drug Dispos. 33: 22–29. [DOI] [PubMed] [Google Scholar]
- 7.Dowling T. C., Briglia A. E., Fink J. C., Hanes D. S., Light P. D., Stackiewicz L., Karyekar C. S., Eddington N. D., Weir M. R., Henrich W. L. 2003. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin. Pharmacol. Ther. 73: 427–434. [DOI] [PubMed] [Google Scholar]
- 8.Kirwan C. J., Lee T., Holt D. W., Grounds R. M., MacPhee I. A., Philips B. J. 2009. Using midazolam to monitor changes in hepatic drug metabolism in critically ill patients. Intensive Care Med. 35: 1271–1275. [DOI] [PubMed] [Google Scholar]
- 9.Kirwan C. J., MacPhee I. A., Lee T., Holt D. W., Philips B. J. 2012. Acute kidney injury reduces the hepatic metabolism of midazolam in critically ill patients. Intensive Care Med. 38: 76–84. [DOI] [PubMed] [Google Scholar]
- 10.Guévin C., Michaud J., Naud J., Leblond F. A., Pichette V. 2002. Down-regulation of hepatic cytochrome p450 in chronic renal failure: role of uremic mediators. Br. J. Pharmacol. 137: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanada K., Ogawa R., Son K., Sasaki Y., Kikkawa A., Ichihara S., Ogata H. 2006. Effects of indoxylsulfate on the in vitro hepatic metabolism of various compounds using human liver microsomes and hepatocytes. Nephron. Physiol. 103: 179–186. [DOI] [PubMed] [Google Scholar]
- 12.Nolin T. D., Appiah K., Kendrick S. A., Le P., McMonagle E., Himmelfarb J. 2006. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J. Am. Soc. Nephrol. 17: 2363–2367. [DOI] [PubMed] [Google Scholar]
- 13.Michaud J., Nolin T. D., Naud J., Dani M., Lafrance J. P., Leblond F. A., Himmelfarb J., Pichette V. 2008. Effect of hemodialysis on hepatic cytochrome P450 functional expression. J. Pharmacol. Sci. 108: 157–163. [DOI] [PubMed] [Google Scholar]
- 14.Molanaei H., Stenvinkel P., Qureshi A. R., Carrero J. J., Heimbürger O., Lindholm B., Diczfalusy U., Odar-Cederlöf I., Bertilsson L. 2012. Metabolism of alprazolam (a marker of CYP3A4) in hemodialysis patients with persistent inflammation. Eur. J. Clin. Pharmacol. 68: 571–577. [DOI] [PubMed] [Google Scholar]
- 15.Streetman D. S., Jr. Bertino J. S., Nafziger A. N. 2000. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 10: 187–216. [DOI] [PubMed] [Google Scholar]
- 16.Karayalçin U., Takeda Y., Miyamori I., Morise T., Takeda R. 1991. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor pravastatin on urinary 6 beta-hydroxycortisol excretion: a preliminary study. Steroids. 56: 598–600. [DOI] [PubMed] [Google Scholar]
- 17.Galteau M. M., Shamsa F. 2003. Urinary 6beta-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur. J. Clin. Pharmacol. 59: 713–733. [DOI] [PubMed] [Google Scholar]
- 18.Bodin K., Bretillon L., Aden Y., Bertilsson L., Broomé U., Einarsson C., Diczfalusy U. 2001. Antiepileptic drugs increase plasma levels of 4beta-hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J. Biol. Chem. 276: 38685–38689. [DOI] [PubMed] [Google Scholar]
- 19.Wide K., Larsson H., Bertilsson L., Diczfalusy U. 2008. Time course of the increase in 4beta-hydroxycholesterol concentration during carbamazepine treatment of paediatric patients with epilepsy. Br. J. Clin. Pharmacol. 65: 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemi M., Kivistö K. T., Diczfalusy U., Bodin K., Bertilsson L., Fromm M. F., Eichelbaum M. 2006. Effect of SLCO1B1 polymorphism on induction of CYP3A4 by rifampicin. Pharmacogenet. Genomics. 16: 565–568. [DOI] [PubMed] [Google Scholar]
- 21.Diczfalusy U., Kanebratt K. P., Bredberg E., Andersson T. B., Böttiger Y., Bertilsson L. 2009. 4beta-hydroxycholesterol as an endogenous marker for CYP3A4/5 activity. Stability and half-life of elimination after induction with rifampicin. Br. J. Clin. Pharmacol. 67: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephson F., Bertilsson L., Böttiger Y., Flamholc L., Gisslén M., Ormaasen V., Sönnerborg A., Diczfalusy U. 2008. CYP3A induction and inhibition by different antiretroviral regimens reflected by changes in plasma 4beta-hydroxycholesterol levels. Eur. J. Clin. Pharmacol. 64: 775–781. [DOI] [PubMed] [Google Scholar]
- 23.Goodenough A. K., Onorato J. M., Ouyang Z., Chang S., Rodrigues A. D., Kasichayanula S., Huang S. P., Turley W., Burrell R., Bifano M., et al. 2011. Quantification of 4-beta-hydroxycholesterol in human plasma using automated sample preparation and LC-ESI-MS/MS analysis. Chem. Res. Toxicol. 24: 1575–1585. [DOI] [PubMed] [Google Scholar]
- 24.Lütjohann D., Marinova M., Schneider B., Oldenburg J., von Bergmann K., Bieber T., Björkhem I., Diczfalusy U. 2009. 4beta-hydroxycholesterol as a marker of CYP3A4 inhibition in vivo - effects of itraconazole in man. Int. J. Clin. Pharmacol. Ther. 47: 709–715. [DOI] [PubMed] [Google Scholar]
- 25.Diczfalusy U., Miura J., Roh H. K., Mirghani R. A., Sayi J., Larsson H., Bodin K. G., Allqvist A., Jande M., Kim J. W., et al. 2008. 4Beta-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet. Genomics. 18: 201–208. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz M. I., Saglam M., Caglar K., Cakir E., Ozgurtas T., Sonmez A., Eyileten T., Yenicesu M., Acikel C., Oguz Y., et al. 2005. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation. 80: 1660–1666. [DOI] [PubMed] [Google Scholar]
- 27.Simmons E. M., Langone A., Sezer M. T., Vella J. P., Recupero P., Morrow J. D., Ikizler T. A., Himmelfarb J. 2005. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation. 79: 914–919. [DOI] [PubMed] [Google Scholar]
- 28.Cockcroft D.W., Gault M. H. 1976. Prediction of creatinine clearance from serum creatinine. Nephron. 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 29.van de Merbel N. C., Bronsema K. J., van Hout M. W., Nilsson R., Sillén H. 2011. A validated liquid chromatography-tandem mass spectrometry method for the quantitative determination of 4β-hydroxycholesterol in human plasma. J. Pharm. Biomed. Anal. 55: 1089–1095. [DOI] [PubMed] [Google Scholar]
- 30.Nolin T. D., Frye R. F., Le P., Sadr H., Naud J., Leblond F. A., Pichette V., Himmelfarb J. 2009. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J. Am. Soc. Nephrol. 20: 2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta T., Suzuki A., Mori C., Shibasaki H., Yokokawa A., Kasuya Y. 2003. Evidence for the validity of cortisol 6 beta-hydroxylation clearance as a new index for in vivo cytochrome P450 3A phenotyping in humans. Drug Metab. Dispos. 31: 1283–1287. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs S. J., Martin D. E., Everitt D. E., Patterson S. D., Jorkasky D. K. 1998. Urinary excretion of 6 beta-hydroxycortisol as an in vivo marker for CYP3A induction: applications and recommendations. Clin. Pharmacol. Ther. 63: 617–622. [DOI] [PubMed] [Google Scholar]
- 33.Bodin K., Andersson U., Rystedt E., Ellis E., Norlin M., Pikuleva I., Eggertsen G., Björkhem I., Diczfalusy U. 2002. Metabolism of 4 beta-hydroxycholesterol in humans. J. Biol. Chem. 277: 31534–31540. [DOI] [PubMed] [Google Scholar]
- 34.Tomalik-Scharte D., Lütjohann D., Doroshyenko O., Frank D., Jetter A., Fuhr U. 2009. Plasma 4beta-hydroxycholesterol: an endogenous CYP3A metric? Clin. Pharmacol. Ther. 86: 147–153. [DOI] [PubMed] [Google Scholar]
- 35.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. 2009. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 50: 350–357. [DOI] [PubMed] [Google Scholar]
- 36.Strehlau J., Pape L., Offner G., Nashan B., Ehrich J. H. 2000. Interleukin-2 receptor antibody-induced alterations of ciclosporin dose requirements in paediatric transplant recipients. Lancet. 356: 1327–1328. [DOI] [PubMed] [Google Scholar]
- 37.Sifontis N. M., Benedetti E., Vasquez E. M. 2002. Clinically significant drug interaction between basiliximab and tacrolimus in renal transplant recipients. Transplant. Proc. 34: 1730–1732. [DOI] [PubMed] [Google Scholar]
- 38.Lecointre K., Furlan V., Taburet A. M. 2002. In vitro effects of tacrolimus on human cytochrome P450. Fundam. Clin. Pharmacol. 16: 455–460. [DOI] [PubMed] [Google Scholar]
- 39.Kagaya H., Miura M., Satoh S., Inoue K., Saito M., Inoue T., Habuchi T., Suzuki T. 2008. No pharmacokinetic interactions between mycophenolic acid and tacrolimus in renal transplant recipients. J. Clin. Pharm. Ther. 33: 193–201. [DOI] [PubMed] [Google Scholar]
- 40.Konishi H., Sumi M., Shibata N., Takada K., Minouchi T., Yamaji A. 2004. Decrease in oral bioavailability of ciclosporin by intravenous pulse of methylprednisolone succinate in rats. J. Pharm. Pharmacol. 56: 1259–1266. [DOI] [PubMed] [Google Scholar]