Abstract
Objective: To test the influence of homocysteine on the production and activation of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of matrix metalloproteinase-2 (TIMP-2) and on cell migration of cultured rat vascular smooth muscle cells (VSMCs). Also, to explore whether rosuvastatin can alter the abnormal secretion and activation of MMP-2 and TIMP-2 and migration of VSMCs induced by homocysteine. Methods: Rat VSMCs were incubated with different concentrations of homocysteine (50–5 000 μmol/L). Western blotting and gelatin zymography were used to investigate the expressions and activities of MMP-2 and TIMP-2 in VSMCs in culture medium when induced with homocysteine for 24, 48, and 72 h. Transwell chambers were employed to test the migratory ability of VSMCs when incubated with homocysteine for 48 h. Different concentrations of rosuvastatin (10−9–10−5 mol/L) were added when VSMCs were induced with 1 000 μmol/L homocysteine. The expressions and activities of MMP-2 and TIMP-2 were examined after incubating for 24, 48, and 72 h, and the migration of VSMCs was also examined after incubating for 48 h. Results: Homocysteine (50–1 000 μmol/L) increased the production and activation of MMP-2 and expression of TIMP-2 in a dose-dependent manner. However, when incubated with 5 000 μmol/L homocysteine, the expression of MMP-2 was up-regulated, but its activity was down-regulated. Increased homocysteine-induced production and activation of MMP-2 were reduced by rosuvastatin in a dose-dependent manner whereas secretion of TIMP-2 was not significantly altered by rosuvastatin. Homocysteine (50–5 000 μmol/L) stimulated the migration of VSMCs in a dose-dependent manner, but this effect was eliminated by rosuvastatin. Conclusions: Homocysteine (50–1 000 μmol/L) significantly increased the production and activation of MMP-2, the expression of TIMP-2, and the migration of VSMCs in a dose-dependent manner. Additional extracellular rosuvastatin can decrease the excessive expression and activation of MMP-2 and abnormal migration of VSMCs induced by homocysteine.
Keywords: Matrix metalloproteinase-2 (MMP-2), Vascular smooth muscle cells (VSMCs), Migration, Rosuvastatin, Homocysteine
1. Introduction
Migration of vascular smooth muscle cells (VSMCs) into the vascular intima and remodeling of the extracellular matrix (ECM) are important events in the pathogenesis of atherosclerosis (Weber and Noels, 2011). In the early stages of atherosclerosis, the injury of endothelial leads to aggregation of platelets and the release of platelet factors which trigger excessive migration and proliferation of VSMCs in the intima (Libby et al., 2010). The VSMCs can then abnormally secret and activate matrix metalloproteinases (MMPs, especially MMP-2) and tissue inhibitors of matrix metalloproteinases (TIMPs, especially TIMP-2). This breaks the balance between MMPs and TIMPs and results eventually in the remodeling of the ECM (Löffek et al., 2011). Thus, it is important to reduce the migration of VSMCs and the subsequent expression and activation of MMP-2 and TIMP-2 induced by VSMCs.
In the human body, the development of atherosclerosis can be promoted by several kinds of cytokines, including homocysteine (Zhou and Austin, 2009). Homocysteine is an intermediate sulfur-ontaining amino acid formed during the intracellular enzymatic metabolism of methionine. The elevation of circulating homocysteine, known as homocysteinemia, can be caused by a genetic deficiency of homocysteine catabolism pathways or by deficiency of folic acid and vitamins B12 and B6 (Castro et al., 2006). Homocysteinemia has been considered as an independent risk factor for coronary heart disease (CHD) (Guo et al., 2003; Zhou and Austin, 2009). In a previous study, we observed that homocysteine was involved in the pathogenesis of coronary artery disease via the induction of excessive expression and activation of MMP-2 in cultured VSMCs (Guo et al., 2007). However, whether homocysteine influences the migration of VSMCs and how to effectively modulate homocysteine-induced excessive expression and activation of MMP-2 and TIMP-2 and abnormal migration of VSMCs remain unclear and are in need of urgent clarification.
Currently, mainly statins, hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are employed in the prevention and treatment of CHD (Ward et al., 2007). Statins can prevent the occurrence of atherosclerosis by lowing circulating cholesterol. Furthermore, recent studies have suggested that the beneficial effects of statins may extend to other mechanisms. Specific non-lipid lowering effects of statins include antioxidant properties, maintenance of atherosclerotic plaque stabilization, anti-inflammatory action, modulation of endothelial function, and anti-proliferation of VSMCs (Arnaboldi et al., 2007; Puccetti et al., 2007). Rosuvastatin, a relatively new HMG-CoA reductase inhibitor, has a lot of favorable characteristics, such as low lipophilicity, high hepatocyte selectivity, minimal metabolism, and a low propensity for cytochrome P450 drug interactions (Cheng-Lai, 2003). So, could the pleiotropic statin, rosuvastatin, reduce the homocysteine-induced excessive expression and activation of MMP-2 and abnormal cell migration of VSMCs? In the present study, we tried to further confirm the influence of homocysteine on the production and activation of MMP-2 and cell migration of cultured rat VSMCs, and explore whether rosuvastatin could modulate these effects.
2. Materials and methods
2.1. Materials
The chemicals used in this study were obtained from the following sources: Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from GIBCO (Grand Island, NY, USA); D-, L-homocysteine was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA); rabbit anti-MMP-2 antibody and rabbit anti-TIMP-2 antibody were obtained from Lab Vision Co. (Westinghouse Drive, Fremont, CA, USA). Rosuvastatin was a generous gift from AstraZeneca (Shanghai, China). All other chemicals were of reagent grade or the highest grade commercially available.
2.2. Preparation of VSMCs
Rat aortic VSMCs were isolated by enzymatic digestion from the thoracic aortas of 6-week-old male Wistar rats as described previously (Uzui et al., 2000). Animal studies conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health (NIH) Publication No. 85-27, revised in 1996) and were approved by the Institutional Animal Care and Use Committee of Shaoxing University, China. The cells were cultured in DMEM supplemented with 10% FBS (0.1 g/ml) at 37 °C in a humidified 5% CO2/95% air atmosphere. At confluence, cells displayed a ‘hill and valley’ growth pattern and abundant myofilaments in their cytoplasm. They were identified as VSMCs by immunocytochemistry using HHF35, a monoclonal antibody that recognizes muscle-specific actin. All VSMC cultures used in this study were between passages 4 and 7. At the subconfluent stage, the culture medium was replaced with serum-free medium and then the cells were exposed to various treatments.
2.3. Preparation of culture medium
Homocysteine was added to DMEM to a final concentration of 50–5 000 μmol/L. Rosuvastatin was added to DMEM to a final concentration of 10−9–10−5 mol/L before use. All rosuvastatin treatments were sustained in the 1 000 μmol/L homocysteine-treated medium.
2.4. Analysis of gelatinase production
After various treatments for 24, 48, and 72 h, medium samples were harvested, centrifuged at 12 000×g at 4 °C for 10 min, and normalized for cell protein content measurement using a Bradford assay. A total of 20 μg proteins of various treatments were mixed with sodium dodecyl sulfate (SDS) sample buffer without a reducing agent and loaded onto a 10% SDS-polyacrylamide gel (0.1 g/ml) containing 0.1% gelatin (1 g/L) as described previously (Guo et al., 2010). Clear zones against the blue background indicated the presence of gelatinase. The amount of gelatinase production was quantified by means of densitometric scanning of the zymograms using a digital camera, and measured using Quantity One 4.4 (Bio-Rad).
2.5. Western blotting
After various treatments for 24, 48, and 72 h, medium samples were harvested from the cells, centrifuged at 12 000×g at 4 °C for 10 min, and separated by 10% SDS-polyacrylamide gel electrophoresis, followed by transfer onto polyvinylidene difluoride membranes (Immobilon P, Millipore, 0.22 μm pore size). The membranes were blocked in TBST (100 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl, 0.05% Tween 20) containing 5% skimmed milk (0.05 g/ml) at room temperature for 2 h, and probed with anti-MMP-2 (or anti-TIMP-2) monoclonal antibody at 4 °C overnight. They were then washed twice (10 min/time) with TBST and once (10 min) with TBS (100 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl) at room temperature. The membranes were incubated with secondary antibody conjugated with horseradish peroxidase (HRP) for 1 h and detected by chemiluminescence according to the manufacturer’s recommendations (ECL Plus, Beyotime, China). The membranes were exposed to X-ray films (Kodak X-OMAT BT). Finally, the films were scanned on a densitograph (ChemiDoc XRS, Bio-Rad) and measured using Quantity One 4.4 (Bio-Rad).
2.6. Cell migration assay
The migration of VSMCs was determined using Transwell chambers (8 μm pore size; Corning Inc.) as described previously (Kuzuya et al., 2003). VSMCs (4×104 cells) were seeded onto the upper well. VSMC migration (48 h) assays were performed for various treatments. The cells that migrated onto the outer side of the membrane were fixed and stained. Migrated cells were counted in four randomly chosen fields of duplicate chambers for each sample using microscopy.
2.7. Statistical analysis
Results are presented as percentages of the control and represent the mean±standard error (SE) for experiments performed in duplicate. Differences among all data were analyzed for statistical significance by one-way analysis of variance (ANOVA) followed by unpaired Student’s t-test. Differences at P<0.05 were considered statistically significant.
3. Results
3.1. Effects of homocysteine on expression and activation of MMP-2 in VSMC culture medium
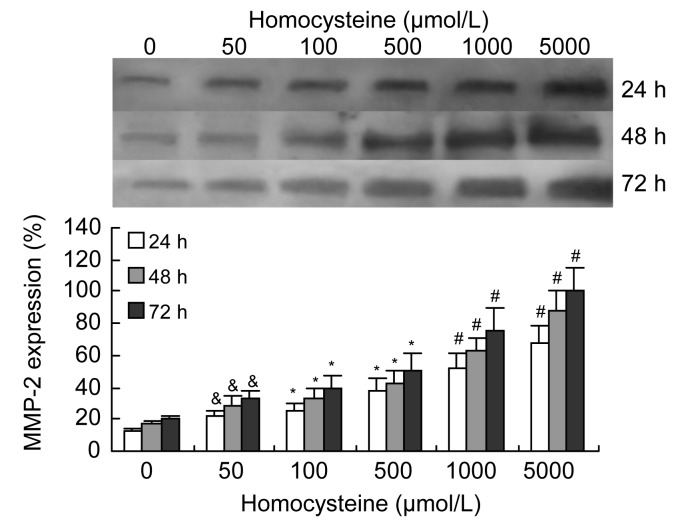
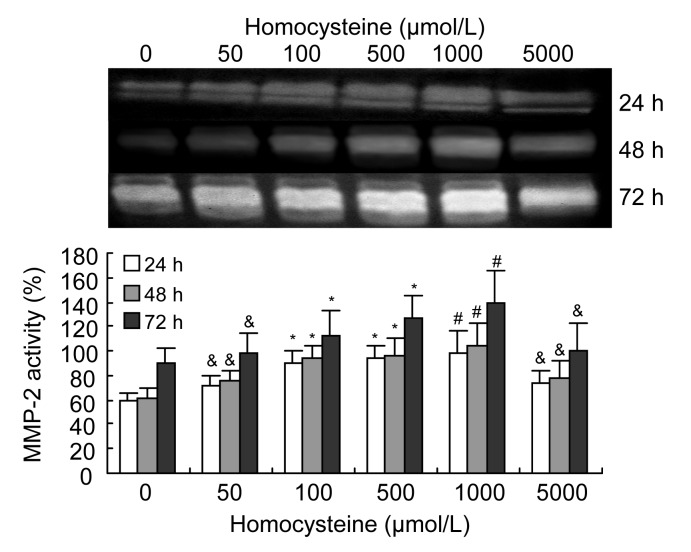
Western blotting of the culture medium showed that the expression of MMP-2 by VSMCs increased significantly in a dose-dependent manner when induced by 50–5 000 μmol/L homocysteine, and tended to increase with a longer incubation time (Fig. 1). Gelatin zymograms of condition media showed that the activity of MMP-2 was also significantly increased by 50–1 000 μmol/L homocysteine in a dose-dependent manner, but decreased at the highest level of homocysteine (5 000 μmol/L) (Fig. 2).
Fig. 1.
Effects of homocysteine on expression of MMP-2 in VSMC culture medium
Homocysteine (50–5 000 μmol/L) significantly increased the expression of MMP-2, as determined by Western blotting, in a dose-dependent manner. Columns indicate data of protein expression of MMP-2 as percentages of the 72 h intervention group (5 000 μmol/L homocysteine), and represent the mean±SE of three separate experiments performed in duplicate. The group treated with 0 μmol/L homocysteine was regarded as the control. & P<0.05, * P<0.01, # P<0.001, vs. control group
Fig. 2.
Effects of homocysteine on activity of MMP-2 in the culture medium containing VSMCs
Homocysteine (50–1 000 μmol/L) significantly increased the activity of MMP-2, as determined by zymography, in a dose-dependent manner, and reduced the MMP-2 activity at 5 000 μmol/L homocysteine. Columns indicate the gelatinolytic activity of MMP-2 as a percentage of the 72 h intervention group (5 000 μmol/L homocysteine), and represent the mean±SE for three separate experiments performed in duplicate. The group treated with 0 μmol/L homocysteine was regarded as the control. & P<0.05, * P<0.01, # P<0.001, vs. control group
3.2. Effects of rosuvastatin on expression and activation of MMP-2 induced by homocysteine in VSMC culture medium
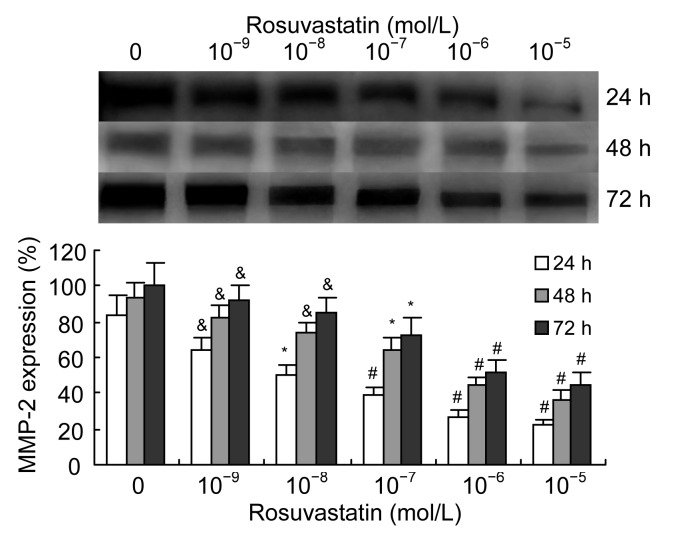
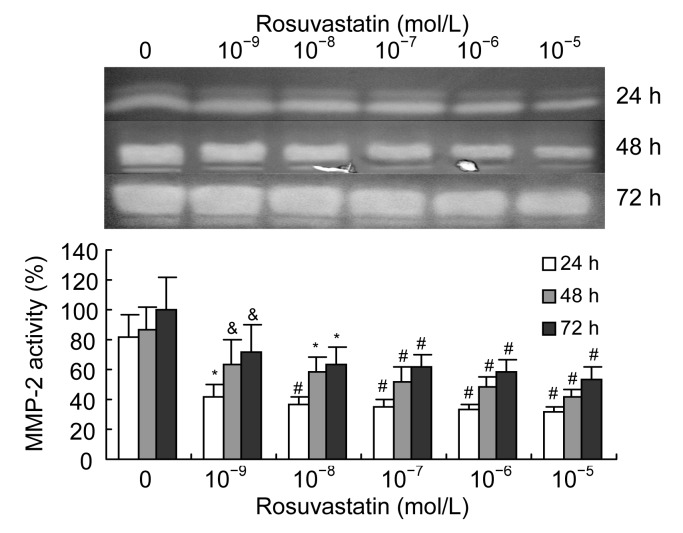
We examined the effect of rosuvastatin on the expression and activation of MMP-2 in VSMCs when stimulated with 1 000 μmol/L homocysteine. We found that rosuvastatin (10−9–10−5 mol/L) decreased the excessive expression of MMP-2 induced by homocysteine in a dose-dependent manner (Fig. 3). The activation of MMP-2 induced by homocysteine was also decreased by rosuvastatin in a dose-dependent manner (Fig. 4).
Fig. 3.
Effects of rosuvastatin on homocysteine-induced expression of MMP-2 in VSMC culture medium
As determined by Western blotting, rosuvastatin (10−9–10−5 mol/L) significantly and dose-dependently decreased the production of MMP-2 in cultured VSMCs induced by 1 000 μmol/L homocysteine. Columns indicate protein expression as a percentage of the 72 h intervention group (0 mol/L rosuvastatin), and represent the mean±SE for three separate experiments performed in duplicate. The group treated with 0 mol/L rosuvastatin and 1 000 μmol/L homocysteine was regarded as the control. & P<0.05, * P<0.01, # P<0.001, vs. control group
Fig. 4.
Effects of rosuvastatin on homocysteine-induced activation of MMP-2 in VSMC culture medium
As determined by zymography, rosuvastatin (10−9–10−5 mol/L) significantly and dose-dependently decreased the activation of MMP-2 in cultured VSMCs induced by 1 000 μmol/L homocysteine. Columns indicate gelatinolytic activity as a percentage of the 72 h intervention group (0 mol/L rosuvastatin), and represent the mean±SE for three separate experiments performed in duplicate. The group treated with 0 mol/L rosuvastatin and 1 000 μmol/L homocysteine was regarded as the control. & P<0.05, * P<0.01, # P<0.001, vs. control group
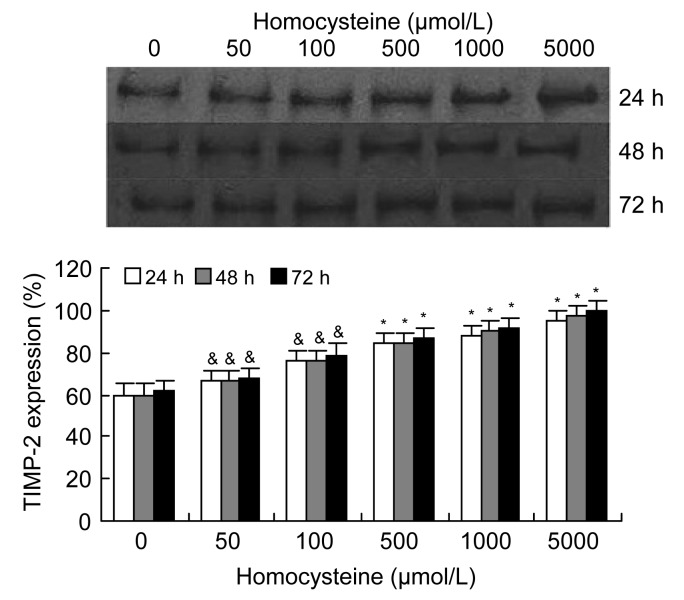
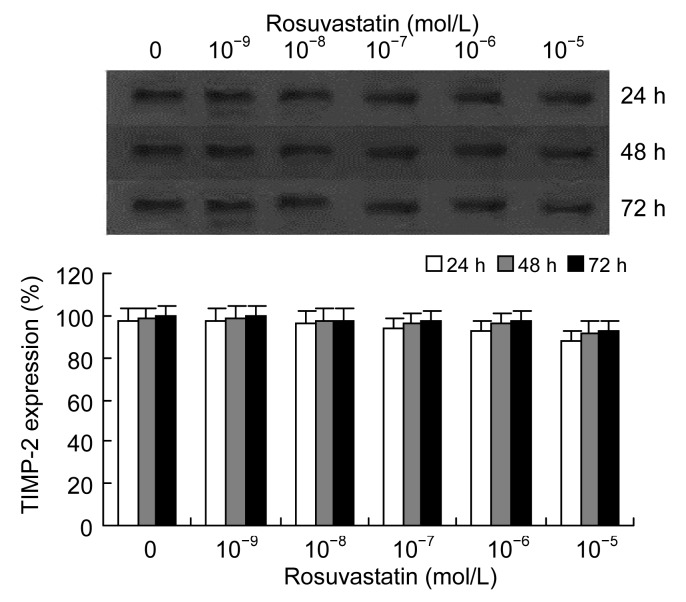
3.3. Effects of homocysteine and rosuvastatin on expression of TIMP-2 in VSMC culture medium
Similar to the effect of homocysteine on expression of MMP-2, Western blotting of the VSMC culture medium showed that the expression of TIMP-2 increased significantly in a dose- and time-dependent manner following induction by homocysteine (50–5 000 μmol/L) (Fig. 5). Following incubation of cells with 1 000 μmol/L homocysteine and different concentrations of rosuvastatin (10−9–10−5 mol/L), the secretion of TIMP-2 by VSMCs decreased in a dose-dependent manner, but unlike the effect on MMP-2, the effect of rosuvastatin on TIMP-2 was not remarkable (Fig. 6).
Fig. 5.
Effects of homocysteine on expression of TIMP-2 in VSMC cultures
Homocysteine (50–5 000 μmol/L) significantly increased the expression of TIMP-2, as determined by Western blotting, in a dose-dependent manner. Columns indicate protein expression of TIMP-2 as a percentage of the 72 h intervention group (5 000 μmol/L homocysteine), and represent the mean±SE for three separate experiments performed in duplicate. The group treated with 0 μmol/L homocysteine was regarded as the control. & P<0.05, * P<0.01, vs. control group
Fig. 6.
Effects of rosuvastatin on homocysteine-induced expression of TIMP-2 in VSMC cultures
As determined by Western blotting, it seemed that rosuvastatin (10−9–10−5 mol/L) dose-dependently decreased the production of TIMP-2 in cultured VSMCs induced by homocysteine (1 000 μmol/L), but this effect was not significant. Columns indicate protein expression of TIMP-2 as a percentage of the 72 h intervention group (0 mol/L rosuvastatin), and represent the mean±SE for three separate experiments performed in duplicate. The group treated with 0 mol/L rosuvastatin and 1 000 μmol/L homocysteine was regarded as the control group
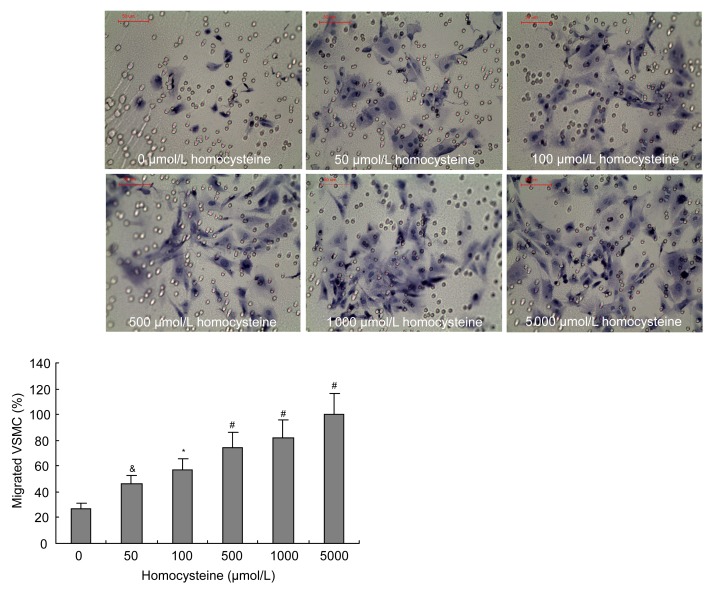
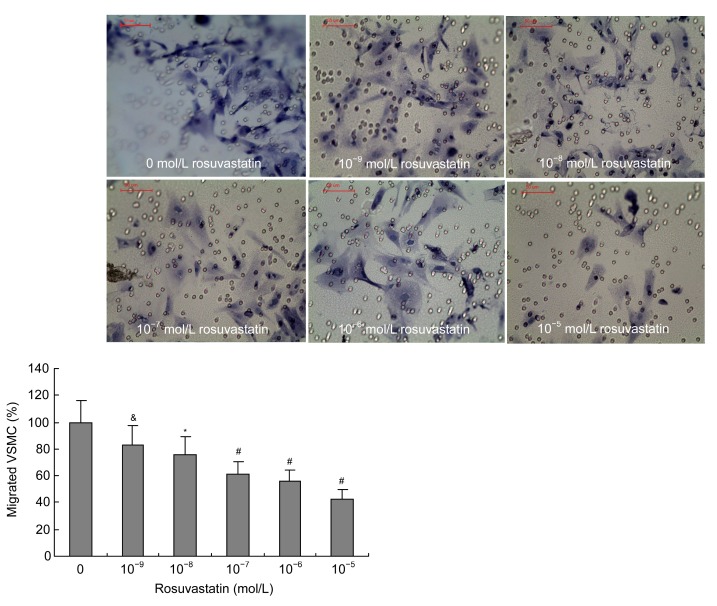
3.4. Effects of homocysteine and rosuvastatin on migration of VSMCs
Transwell chambers were used to examine the effect of rosuvastatin on homocysteine-induced migration of VSMCs. Migration of VSMCs increased following induction by homocysteine (50–5 000 μmol/L) in a dose-dependent manner (Fig. 7). The treatment of VSMCs with different concentrations of rosuvastatin (10−9–10−5 mol/L) when induced by 1 000 μmol/L homocysteine showed that rosuvastatin could significantly inhibit the homocysteine-induced migration of VSMCs in a dose-dependent manner (Fig. 8).
Fig. 7.
Effects of homocysteine on the migration of VSMCs
Homocysteine (50–5 000 μmol/L) promoted the migration of VSMCs in a dose-dependent manner. Cell migration assay was performed using Transwell chambers following various treatments of homocysteine as indicated for 48 h. The cells that migrated to the outer side of the membrane were fixed and stained. Migrated cells (mean±SE) were counted in four randomly chosen fields of duplicate chambers for each sample using microscopy. The group treated with 0 μmol/L homocysteine was regarded as the control group. & P<0.05, * P<0.01, # P<0.001, vs. control group
Fig. 8.
Effects of rosuvastatin on the homocysteine-induced migration of VSMCs
Rosuvastatin (10−9–10−5 mol/L) inhibited homocysteine-induced (1 000 μmol/L) migration of VSMCs in a dose-dependent manner. A cell migration assay was performed using Transwell chambers in various treatments for 48 h. The cells that migrated to the outer side of the membrane were fixed and stained. Migrated cells (mean±SE) were counted in four randomly chosen fields of duplicate chambers for each sample using microscopy. The group treated with 0 mol/L rosuvastatin and 1 000 μmol/L homocysteine was regarded as the control group. & P<0.05, * P<0.01, # P<0.001, vs. control group
4. Discussion
Atherosclerosis is a multistep disease. A chronic inflammatory process is involved in its occurrence and development, including interactions between various soluble mediators, monocytes, endothelial cells, and VSMCs. The growth of atherosclerotic plaque requires the migration and proliferation of VSMCs in the arterial wall, and this process is largely dependent on the balance of MMPs and TIMPs (Raffetto and Khalil, 2008). Among the MMPs, MMP-2 is widely distributed in vessel walls and plays an important role in the turnover of basement membrane type IV collagen and in controlling cell migration (Löffek et al., 2011). TIMP-2 is an important member of TIMPs and can effectively inhibit the activity of MMP-2. In normal arteries, MMP-2 and TIMP-2 keep in balance and maintain the homeostasis of the vessel wall. However, in atherosclerotic plaques, the expression and activation of MMP-2 and TIMP-2 are increased, which promotes the degradation of the ECM, thereby supplying space for the migration and proliferation of VSMCs. The VSMCs can then secrete and activate more MMP-2 and aggravate the imbalance of MMPs/TIMPs, resulting eventually in the development and rupture of atherosclerotic plaques (Newby, 2005). Thus, it is important to find a way to inhibit the development of atherosclerosis by reducing the abnormal expression and activation of MMP-2 and TIMP-2, and the migration of VSMCs.
Homocysteine is considered as an independent risk factor of atherosclerotic cardiovascular disease. However, the mechanisms by which homocysteine affects the pathogenesis of atherosclerosis are not completely known, especially those relating to its effects on the migration of VSMCs and the balance between MMP-2 and TIMP-2. In this study, we explored these questions using cultured VSMCs and found that the expressions of MMP-2 and TIMP-2 by VSMCs and the migration of VSMCs were increased by homocysteine (50–5 000 μmol/L) in a dose-dependent manner. Previous studies have demonstrated that the synergy between VSMC migration and an imbalance of MMPs/TIMPs can promote the development of atherosclerosis. It seems that the results observed in this study are in accordance with this mechanism. However, in the study we found that the expressions of both MMP-2 and TIMP-2 were increased, suggesting that the increased TIMP-2 may inhibit the proteolytic effect of MMP-2. This raises the question of whether the activity of MMP-2 is activated by homocysteine or inhibited by the up-regulated TIMP-2. The promotional effect of MMP-2 on cell migration is attributable mainly to the activated MMP-2, via degradation of the ECM around the cells in the vessel wall and by supplying space for cell migration (Busti et al., 2010). So, we considered that the detection of MMP-2 activity should reveal the result of this new balance between MMP-2 and TIMP-2. To explore this possibility, we detected the activity of MMP-2 in VSMC cultures by gelatin zymography. We found that the activity of MMP-2 in cultured VSMCs was up-regulated when stimulated with homocysteine at low concentrations (50–1 000 μmol/L), but the tendency for up-regulation was attenuated following treatment with homocysteine at a concentration of 5 000 μmol/L. These results revealed that although the secretion of TIMP-2 was increased, the activity of MMP-2 was still increased. This may be in accordance with the increased migratory ability of VSMCs. Several other questions remained. It seemed incompatible that the activation of MMP-2 was decreased while the migration of VSMCs was increased when VSMCs were incubated with 5 000 μmol/L homocysteine. How can this inconsistency be explained and why was the activity of MMP-2 weakened in this case?
Firstly, it has been reported that homocysteine exerts a dual effect, activating proMMP-2 at a low molar ratio (MR) of 10:1 and inhibiting the activation of MMP-2 at high MRs (>1 000:1) (Bescond et al., 1999). This may explain why the activity of MMP-2 was weakened following incubation with homocysteine at a concentration of 5 000 μmol/L. Secondly, we recognized that the migration of VSMCs was caused by a combination of the migratory ability of VSMCs themselves and the hydrolytic effect of MMPs on the ECM. High concentrations of homocysteine might directly elevate the migratory ability of VSMCs. This might also activate other MMPs, including MMP-1 and MMP-9 (Moshal et al., 2006; Steed and Tyagi, 2011), which can also degrade the ECM. Finally, although the up-regulation tendency of MMP-2 activity was attenuated, the actual activity of MMP-2 was still stronger compared with the control (incubated without homocysteine). So the activated MMP-2 also may contribute to the migration of VSMCs. However, more research is required to confirm this.
Statins are widely used in the treatment of atherogenic dyslipidemia. In addition to their potent action on cholesterol-rich lipoproteins, statins have pleiotropic properties that may be beneficial for CHD. In this study, we found that rosuvastatin could inhibit the expression and activation of MMP-2 and the migration of VSMCs induced by homocysteine in vitro. The secretion of TIMP-2 was also decreased by rosuvastatin in a dose-dependent manner, but no significant difference was observed. As an HMG-CoA reductase inhibitor, treatment with rosuvastatin may cause mevalonate starvation in VSMCs. Mevalonate metabolism yields squalene, the precursor of cholesterol and geranylgeranyl pyrophosphate (GGPP), which is important in prenylation of proteins. Luan et al. (2003) showed that the secretion of MMPs could be regulated by GGPP or mevalonate and depressed by lovastatin in rabbit smooth muscle cells (SMCs) or foam cells. The inhibition of prenylation was considered to be the mechanism responsible for the decrease in MMPs caused by lovastatin. This mechanism may also apply to the interpretation of the results of the present study. Thus, the results of this study suggest that rosuvastatin may reduce the risk of CHD in patients with hyperhomocysteinemia, by reducing the abnormal migration of VSMCs and the expression and activation of MMP-2 in VSMCs induced by hyperhomocysteinemia. Holven et al. (2002) demonstrated that homocysteine exerts atherogenic effects in part by enhancing chemokine responses in cells involved in atherogenesis, and Li et al. (2002) showed that the intervention of rosuvastatin could down-regulate these inflammatory reactions. Furthermore, homocysteinemia can aggravate myocardial infarction via oxidative stress mechanisms (Hagar, 2002), and the antioxidative effect of rosuvastatin may attenuate the oxidative stress caused by homocysteinemia (Verreth et al., 2007). Overall, we consider that rosuvastatin is suitable for preventing or reducing the occurrence and development of atherosclerosis, especially in populations with dyslipidemia and homocysteinemia.
5. Conclusions
In cultured rat VSMCs, homocysteine (50–1 000 μmol/L) significantly increased the production and activation of MMP-2, the expression of TIMP-2, and the migration of VSMCs in a dose-dependent manner. Additional extracellular rosuvastatin may decrease the excessive expression and activation of MMP-2 and the abnormal migration of VSMCs induced by homocysteine, but not the secretion of TIMP-2. These data suggest that the beneficial effect of rosuvastatin on homocysteine-related vascular disease processes may be related, in part at least, to their inhibitory effect on homocysteine-induced MMP-2 expression and the migration of VSMCs.
Footnotes
Project supported by the Health Ministry Scientific Research Fund of China (No. WKJ2011-2-018), the Zhejiang Provincial Natural Science Foundation of China (No. Y2100535), the Key Social Development Project of Zhejiang Province (No. 2010A23010), the Science and Technology Projects of Shaoxing (No. 2011A23011), the Science and Technology Plan Project of Zhejiang Province (No. 2012C33040), and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, China
Compliance with ethics guidelines: Ya-fei SHI, Ju-fang CHI, Wei-liang TANG, Fu-kang XU, Long-bin LIU, Zheng JI, Hai-tao LV, and Hang-yuan GUO declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Arnaboldi L, Guzzetta M, Pazzucconi F, Radaelli G, Paoletti R, Sirtori CR, Corsini A. Serum from hypercholesterolemic patients treated with atorvastatin or simvastatin inhibits cultured human smooth muscle cell proliferation. Pharmacol Res. 2007;56(6):503–508. doi: 10.1016/j.phrs.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Bescond A, Augier T, Chareyre C, Garçon D, Hornebeck W, Charpiot P. Influence of homocysteine on matrix metalloproteinase-2: activation and activity. Biochem Biophys Res Commun. 1999;263(2):498–503. doi: 10.1006/bbrc.1999.1391. [DOI] [PubMed] [Google Scholar]
- 3.Busti C, Falcinelli E, Momi S, Gresele P. Matrix metalloproteinases and peripheral arterial disease. Intern Emerg Med. 2010;5(1):13–25. doi: 10.1007/s11739-009-0283-y. [DOI] [PubMed] [Google Scholar]
- 4.Castro R, Rivera I, Blom HJ, Jakobs C, Tavares , de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006;29(1):3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- 5.Cheng-Lai A. Rosuvastatin: a new HMG-CoA reductase inhibitor for the treatment of hypercholesterolemia. Heart Dis. 2003;5(1):72–78. doi: 10.1097/01.HDX.0000050417.89309.F8. [DOI] [PubMed] [Google Scholar]
- 6.Guo H, Lee JD, Ueda T, Shan J, Wang J. Plasma homocysteine levels in patients with early coronary artery stenosis and high risk factors. Jpn Heart J. 2003;44(6):865–871. doi: 10.1536/jhj.44.865. [DOI] [PubMed] [Google Scholar]
- 7.Guo H, Lee JD, Uzui H, Yue H, Wang P, Toyoda K, Geshi T, Ueda T. Effects of heparin on the production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells. Can J Cardiol. 2007;23(4):275–280. doi: 10.1016/S0828-282X(07)70754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo H, Liu L, Shi Y, Sun A, Xu F, Chi J, Huang D. Chinese yellow wine and red wine inhibit matrix metalloproteinase-2 and improve atherosclerotic plaque in LDL receptor knockout mice. Cardiovasc Ther. 2010;28(3):161–168. doi: 10.1111/j.1755-5922.2009.00132.x. [DOI] [PubMed] [Google Scholar]
- 9.Hagar HH. Folic acid and vitamin B12 supplementation attenuates isoprenaline-induced myocardial infarction in experimental hyperhomocysteinemic rats. Pharmacol Res. 2002;46(3):213–219. doi: 10.1016/S1043-6618(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 10.Holven KB, Aukrust P, Holm T, Ose L, Nenseter MS. Folic acid treatment reduces chemokine release from peripheral blood mononuclear cells in hyperhomocysteinemic subjects. Arterioscler Thromb Vasc Biol. 2002;22(4):699–703. doi: 10.1161/01.ATV.0000013288.35930.90. [DOI] [PubMed] [Google Scholar]
- 11.Kuzuya M, Kanda S, Sasaki T, Tamaya-Mori N, Cheng XW, Itoh T, Itohara S, Iguchi A. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003;108(11):1375–1381. doi: 10.1161/01.CIR.0000086463.15540.3C. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Chen H, Romeo F, Sawamura T, Saldeen T, Mehta JL. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther. 2002;302(2):601–605. doi: 10.1124/jpet.102.034959. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74(2):213–220. doi: 10.1253/circj.CJ-09-0706. [DOI] [PubMed] [Google Scholar]
- 14.Löffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38(1):191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 15.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 16.Moshal KS, Sen U, Tyagi N, Henderson B, Steed M, Ovechkin AV, Tyagi SC. Regulation of homocysteine-induced MMP-9 by ERK1/2 pathway. Am J Physiol Cell Physiol. 2006;290(3):C883–C891. doi: 10.1152/ajpcell.00359.2005. [DOI] [PubMed] [Google Scholar]
- 17.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 18.Puccetti L, Acampa M, Auteri A. Pharmacogenetics of statins therapy. Recent Pat Cardiovasc Drug Discov. 2007;2(3):228–236. doi: 10.2174/157489007782418982. [DOI] [PubMed] [Google Scholar]
- 19.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15(7):1927–1943. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uzui H, Lee JD, Shimizu H, Tsutani H, Ueda T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis. 2000;149(1):51–59. doi: 10.1016/S0021-9150(99)00295-6. [DOI] [PubMed] [Google Scholar]
- 22.Verreth W, de Keyzer D, Davey PC, Geeraert B, Mertens A, Herregods MC, Smith G, Desjardins F, Balligand JL, Holvoet P. Rosuvastatin restores superoxide dismutase expression and inhibits accumulation of oxidized LDL in the aortic arch of obese dyslipidemic mice. Br J Pharmacol. 2007;151(3):347–355. doi: 10.1038/sj.bjp.0707231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, Yeo W, Payne N. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(14):1–160. iii–iv. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 24.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: causal relationship and potential mechanisms. Biofactors. 2009;35(2):120–129. doi: 10.1002/biof.17. [DOI] [PubMed] [Google Scholar]