Abstract
Objective: Coronary collateral circulation is an alternative source of blood supply to myocardium in the presence of advanced coronary artery disease. We sought to determine which clinical and angiographic variables are associated with collateral development in patients with stable angina and chronic total coronary occlusion. Methods: Demographic variables, biochemical measurements, and angiographic findings were collected from 478 patients with stable angina and chronic total coronary occlusion. The presence and extent of collaterals supplying the distal aspect of a total coronary occlusion from the contra-lateral vessel were graded from 0 to 3 according to the Rentrop scoring system. Results: Low (Rentrop score of 0 or 1) and high (Rentrop score of 2 or 3) coronary collateralizations were detected in 186 and 292 patients, respectively. Despite similar age, cigarette smoking, and medical treatment, patients with low collateralization were female in a higher proportion and less hypertensive, and had higher rates of type 2 diabetes and dyslipidemia than those with high collateralization (for all comparisons, P<0.05). In addition, patients with low collateralization exhibited more single-vessel disease, less right coronary artery occlusion, more impaired renal function, and higher serum levels of high-sensitivity C-reactive protein (hsCRP) compared with those with high collateralization. Multivariate analysis revealed that age of ≥65 years, female gender, diabetes, no history of hypertension, dyslipidemia, moderate to severe renal dysfunction, single-vessel disease, and elevated hsCRP levels were independently associated with low coronary collateralization. Conclusions: Coronary collateralization was reduced in almost 40% of stable angina patients with chronic total occlusion, which was related to clinical and angiographic factors. The impact of coronary collateralization on outcomes after revascularization needs further investigation.
Keywords: Stable angina, Coronary collateral circulation, Risk factors, Angiography, Chronic total coronary occlusion
1. Introduction
In the human heart, the development of coronary collaterals serves as a conduit, bridging significantly stenotic or occluded coronary vessels, and constitutes a natural bypass system (Seiler, 2010; Traupe et al., 2010). Currently, routine coronary angiography reveals that about 20%–30% of patients with significant coronary artery disease had chronic total occlusion. The number is expected to rise as a result of improved survival of patients with acute coronary syndrome, due to rapid advances in medical and interventional therapy for patients with coronary artery disease. Despite the complete interruption of antegrade blood flow caused by heavy atherosclerotic plaque burden or arterial thrombosis, the frequency of myocardial infarction in areas subtended by chronic total occlusion varies considerably (Fefer et al., 2012), and the degree of myocardial injury downstream of epicardial chronic total occlusion is inversely correlated with the degree of angiographic coronary collateral circulation (Choi et al., 2013). Well-developed coronary collaterals have the potential to alleviate myocardial ischemia, preserve residual contractility, reduce cardiovascular events (Regieli et al., 2009; Meier et al., 2012), and even save lives in patients with severe coronary artery occlusion (Meier, 2011). The complex mechanism mediating the development of coronary collateral vessels in the heart is still not well-understood. In fact, the extent of coronary collateralization differs greatly among patients and is affected by multiple clinical, angiographic, and biochemical factors and inflammatory cytokines (Schirmer et al., 2009; Zorkun et al., 2013). Note that the inconclusive roles of age, gender, heart rate, and risk factors for coronary artery disease in the development of coronary collateralization reported previously may be partly due to the heterogeneity of study populations and different clinical conditions. A severe coronary artery obstruction is a well-known prerequisite for spontaneous collateral recruitment (Levin, 1974) and various clinical manifestations of coronary artery disease influence collateral formation (Hsu et al., 2012; Rocic, 2012).
Because of the apparent prognostic implications of coronary collateralization, a better understanding of the factors influencing the development of coronary collaterals is essential. Therefore, in this study we sought to determine the clinical and angiographic features associated with coronary collateralization in patients with stable angina and chronic total coronary occlusion.
2. Materials and methods
2.1. Study population
Between January 2008 and March 2013, a total of 612 consecutive patients with stable angina and chronic total occlusion (>3 months) of at least one major epicardial coronary artery were screened, from the database of Shanghai Ruijin Hospital Percutaneous Coronary Intervention (PCI) Outcomes Program. This program utilizes clinical and angiographic information to estimate risk-adjusted outcomes. Stable angina was diagnosed according to the criteria recommended by the American College of Cardiology/American Heart Association (Fraker et al., 2007). The duration of coronary artery occlusion was estimated from the date of occurrence of myocardial infarction in the area of myocardium supplied by the occluded vessel, from an abrupt worsening of existing angina pectoris, or from information obtained from a previous angiogram (Wang et al., 2013). For the purpose of this study and to avoid confounding data, patients who had a history of coronary artery bypass grafting (n=49) or who had received PCI within the prior three months (n=54) were excluded. Patients with type 1 diabetes, identified by measurement of C-peptide levels, were excluded (n=8). We also excluded those with heart failure, pulmonary heart disease, malignant tumor, or immune system disorders (n=23). The remaining 478 eligible patients with stable angina and chronic total occlusions (363 men and 115 women, mean age (65±10) years) were enrolled in this study.
2.2. Clinical parameters and biochemical measurement
Demographic variables, risk factors for coronary artery disease, lipid profile, renal function, and medical treatment were recorded. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg. The diagnosis of type 2 diabetes mellitus was made according to the criteria of the American Diabetes Association, and included symptoms of diabetes plus a casual plasma glucose concentration beyond 200 mg/dl (11.1 mmol/L), or an increased fasting glucose level (126 mg/dl (7.0 mmol/L)) or 2-h postprandial glucose level (200 mg/dl (11.1 mmol/L)) during an oral glucose tolerance test. Hyperlipidemia was diagnosed according to the guidelines of the National Cholesterol Education Program (ATP III). The abbreviated modification of diet in renal disease (MDRD) equation was used to estimate the glomerular filtration rate (eGFR): eGFR=186(c/88.4)−1.154A−0.203b, where c is the concentration of creatinine, A is age, and b is the constant, which equals to 0.742 for female. At least moderate renal dysfunction was defined as eGFR <60 ml/(min∙1.73 m2). Serum levels of high-sensitivity C-reactive protein (hsCRP) were measured using an enzyme-linked immunosorbent assay (ELISA) kit.
2.3. Coronary angiography and collateral scoring
Coronary angiography was performed through the femoral or radial approach (Pu et al., 2012; Shen et al., 2012). Intracoronary administration of nitroglycerin was encouraged prior to angiography. Coronary angiograms were reviewed by two experienced cardiologists who were blind to the study protocol and biochemical measurements. Any difference in interpretation was resolved by a third reviewer who was blind to the readings of the first two reviewers.
Significant coronary artery disease was diagnosed if there was ≥70% diameter stenosis in at least one major epicardial coronary artery. Left main coronary artery narrowing of ≥50% was considered as 2-vessel disease. The severity of coronary artery disease was determined by the number of significantly diseased coronary arteries. Multi-vessel coronary disease was defined as the presence of ≥70% luminal diameter stenosis involving at least two major epicardial coronary arteries. The recorded data also included the vessel to which the collaterals were connected and the grade of coronary collateral circulation.
The presence and extent of collaterals supplying the distal aspect of a total coronary occlusion from the contra-lateral vessel were graded on a 4-point scale from 0 to 3 according to the Rentrop scoring system (Rentrop et al., 1985): 0, no collateral vessels; 1, thread-like, poorly opacified collaterals with faint visualization of the distal vessel; 2, moderately opacified collateral channels; 3, large, brightly filled collateral channels with immediate visualization of the entire distal vessel >10 mm. Patients were then classified as having low (Rentrop score of 0 and 1) or high (Rentrop score of 2 and 3) coronary collateralization. In patients with more than one total coronary occlusion, the vessel with the highest collateral grade was chosen for analysis.
2.4. Statistical analysis
Continuous variables are presented as mean±standard deviation (SD) and categorical data are summarized as frequencies or percentages. The differences between groups were analyzed by Chi-square test for categorical clinical variables and by Student’s unpaired t-test for continuous variables. Subsequently, significantly correlated variables in the univariate analysis or relevant variables were further analyzed by logistic or multiple regression analysis. All P-values were 2-sided with a significance level of P<0.05. The Statistical Package for the Social Sciences (SPSS) 11.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
3. Results
3.1. Clinical and angiographic characteristics
Of the total 478 patients, 75.9% were men, 38.1% currently smoked cigarettes, 33.1% had type 2 diabetes, 67.8% suffered hypertension, and 45.8% had hyperlipidemia. Moderate to severe renal dysfunction occurred in 17.6% of the population, and 77.2% of patients had multi-vessel coronary disease.
Low (Rentrop score of 0 or 1) and high (Rentrop score of 2 or 3) coronary collateralization was detected in 186 and 292 patients, respectively. Despite similar age, cigarette smoking, and medical treatment, patients with low collateralization were female in a higher proportion and less hypertensive, and had higher rates of type 2 diabetes and dyslipidemia before statin medications than those with high collateralization (for all comparisons, P<0.05). In addition, patients with low collateralization exhibited more single-vessel coronary artery disease, less right coronary artery occlusion, more impaired renal function, and higher serum levels of hsCRP compared with those with high collateralization (P<0.05) (Table 1).
Table 1.
Baseline characteristics of patients with low or high coronary collateralization
| Parameter | Low collateralization group (n=186) | High collateralization group (n=292) | P value |
| Age (year) | 66±10 | 64±11 | 0.07 |
| Age ≥65 years | 102 (55.7%) | 136 (46.1%) | 0.04 |
| Male | 130 (71%) | 233 (79%) | 0.048 |
| Cardiovascular risk factors | |||
| Smoking | 69 (37.1%) | 113 (38.3%) | 0.72 |
| Hypertension | 115 (61.8%) | 209 (71.6%) | 0.026 |
| Hyperlipidemia | 107 (57.5%) | 112 (38.4%) | <0.001 |
| Diabetes mellitus | 83 (45.4%) | 75 (25.4%) | <0.001 |
| Systolic blood pressure (mmHg) | 136±17 | 140±16 | 0.008 |
| Diastolic blood pressure (mmHg) | 82±10 | 85±8 | 0.001 |
| Triglyceride (mmol/L) | 1.91±1.19 | 1.79±0.99 | 0.06 |
| Total cholesterol (mmol/L) | 4.37±1.43 | 4.14±1.12 | 0.05 |
| HDL-C (mmol/L) | 0.98±0.25 | 1.05±0.26 | 0.02 |
| LDL-C (mmol/L) | 2.69±1.40 | 2.57±0.89 | 0.20 |
| Apoprotein-A (g/L) | 1.22±0.25 | 1.19±0.23 | 0.45 |
| Apoprotein-B (g/L) | 1.00±0.33 | 0.88±0.27 | 0.009 |
| Lipoprotein (a) (g/L) | 0.24±0.19 | 0.24±0.21 | 0.57 |
| Fasting glucose (mmol/L) | 6.02±1.97 | 5.39±1.39 | <0.001 |
| HbA1c (%) | 6.44±1.10 | 6.19±1.11 | <0.001 |
| BUN (mmol/L) | 5.50±1.95 | 5.07±1.65 | 0.001 |
| Creatinin (μmol/L) | 82.8±19.8 | 80.8±27.3 | 0.003 |
| Uric acid (μmol/L) | 339±80 | 328±79 | 0.15 |
| eGFR<60 ml/(min∙1.73 m2) | 48 (26.2%) | 36 (12.2%) | <0.001 |
| High-sensitivity CRP (mg/L) | 5.85±3.01 | 5.28±2.71 | 0.03 |
| Medications | |||
| Oral antiplatelet | 168 (100%) | 292 (100%) | 1.00 |
| ACEI | 79 (42.5%) | 138 (47.3%) | 0.30 |
| β-blockers | 86 (46.2%) | 121 (41.4%) | 0.31 |
| Nitrates | 48 (25.8%) | 72 (25.7%) | 0.77 |
| Calcium antagonists | 23 (28.4%) | 15 (25.0%) | 0.653 |
| Statins | 175 (94.1%) | 285 (97.6%) | 0.89 |
| Number of significant CAD | 0.004 | ||
| 1-vessel disease | 51 (27.9%) | 58 (19.7%) | |
| 2-vessel disease | 53 (29.0%) | 129 (43.7%) | |
| 3-vessel disease | 79 (43.2%) | 108 (36.6%) |
Values are expressed as mean±SD or number of patients (percentage). Chi-square for categorical variables. HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; HbA1c: glycosylated hemoglobin; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate; CRP: C-reactive protein; ACEI: angiotensin-converting enzyme inhibitor; CAD: coronary artery disease
3.2. Binary logistic regression analysis
Variables that presented a level of statistical significance of <0.20 in the univariate analyses (Table 1) were chosen for multivariate logistic regression analysis to determine independent risk factors for low coronary collateralization. We found that age of ≥65 years, female gender, diabetes, no history of hypertension, hyperlipidemia, moderate to severe renal dysfunction, and single-vessel coronary disease were independently associated with low coronary collateralization (Table 2).
Table 2.
Multivariable analysis of risks of low collateralization in patients with chronic total occlusion
| Variable | OR | 95% CI | P value |
| Female | 1.770 | 1.022–3.329 | 0.049 |
| Age ≥65 years | 1.556 | 1.002–2.369 | 0.039 |
| Hypertension | 0.524 | 0.336–0.818 | 0.004 |
| Hyperlipidemia | 2.168 | 1.426–3.296 | <0.001 |
| Diabetes mellitus | 2.567 | 1.650–3.995 | <0.001 |
| eGFR<60 ml/(min∙1.73 m2) | 3.069 | 1.761–5.350 | <0.001 |
| Single-vessel disease | 1.902 | 1.155–3.302 | 0.011 |
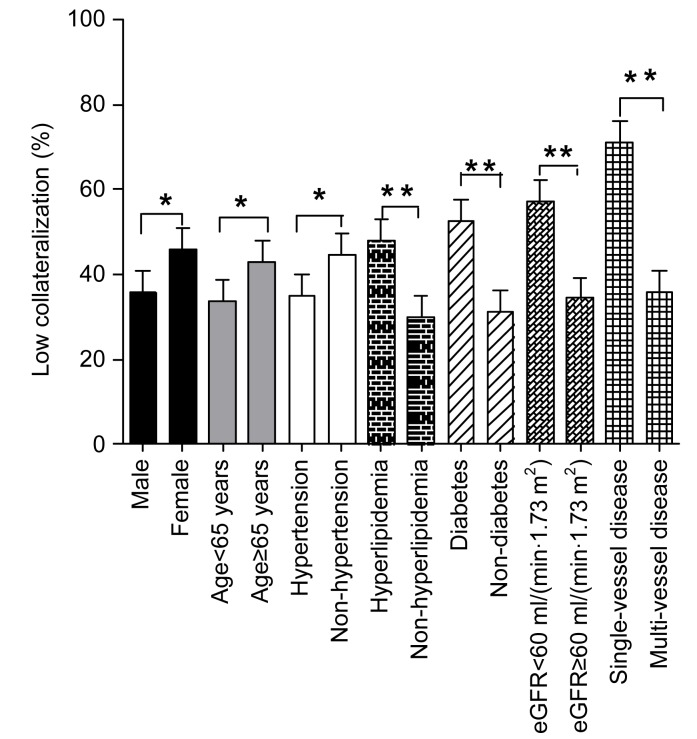
The data were then dichotomized according to the age, gender, presence or absence of hypertension, hyperlipidemia, diabetes, renal dysfunction, and the number of significant diseased coronary arteries. We found that the rate of low collateralization was significantly higher in patients older than 65 years or female with no prior hypertension, but who had hyperlipidemia, diabetes, at least moderate renal insufficiency, and single-vessel disease (all P<0.05) (Fig. 1).
Fig. 1.
Occurrence of low coronary collateralization in patients with various clinical and angiographic features
The significance of differences is indicated by * P<0.05 or ** P<0.01
4. Discussion
It is well known that the development of coronary collateral circulation is influenced by multiple clinical, biochemical, and angiographic factors. In this unique cohort of stable angina patients with chronic total coronary occlusion, we have shown that old age, female gender, no prior hypertension, presence of diabetes, hyperlipidemia, at least moderate renal dysfunction, and single-vessel coronary disease were independently associated with low coronary collateralization.
4.1. Traditional risk factors
Consistent with previous angiographic studies (Boodhwani et al., 2006; Wang et al., 2013; van der Hoeven et al., 2013), our results indicated that coronary collateral development was lower in females and declined with increasing age of the patients. In contrast, well-developed collaterals were more frequently detected in hypertensive patients than in their non-hypertensive counterparts. Clearly, high blood pressure (especially elevated diastolic blood pressure) provides an increased gradient between central aortic pressure and left ventricular end-diastolic pressure, which is one of the main prerequisites for coronary collateral formation. Recently, Wang et al. (2013) found that elevated diastolic blood pressure is related to high coronary collateralization by altering blood flow velocity in diastole and tangential fluid shear stress on the endothelial surface. Differences in coronary collateral growth may be one of the pathophysiological mechanisms responsible for the J-curve phenomenon in the relationship between diastolic blood pressure and cardiovascular risk. In experimental animal studies, hyperlipidemia, particularly hypercholesterolemia, was shown to be an inhibitor of arteriogenesis (Lin et al., 2010). However, data on the relationship between coronary collateral formation and hyperlipidemia remain controversial, partly because of the use of different techniques for grading the collateral flow (van der Hoeven et al., 2013).
In this study, we observed that diabetes correlated negatively with the development of collaterals and was also an independent predictor of poorly developed collateral circulation. Kilian et al. (2002) found that even in patients with pre-diabetes, the fasting glucose level was an independent predictor of coronary collateral formation. The mechanism by which diabetes induces poor collateral growth is complex and remains unclear. In vitro and animal experiments have shown that high glucose levels can cause endothelial dysfunction (Williamson et al., 1990), and endothelial cell function is vital for coronary collateral formation. Hyperglycemia reduced coronary collateral blood flow through a nitric oxide-mediated mechanism in a dog model (Kersten et al., 2001). In diabetic patients, increased levels of endostatin and angiostatin are negatively correlated with coronary collateral formation (Kadi et al., 2011).
4.2. Renal dysfunction
So far, data regarding the prediction of low collateralization in chronic renal dysfunction patients with stable coronary artery disease remain scant (Hsu et al., 2012). In this study, a high percentage of patients with at least moderate renal dysfunction had poor coronary collateralization. Xie et al. (2011) reported that coronary collateral formation is significantly reduced in stable coronary artery disease patients with mild to moderate renal insufficiency (eGFR<80 ml/(min∙1.73 m2)). Recently, Duran et al. (2013) hypothesized that an association between mild to moderate renal impairment and the presence of coronary collateral vessels may be explained by increased ischemia and the extent and severity of coronary artery disease. In patients with chronic renal dysfunction, hypoxia in the kidney induces the expression of proteins, such as the hypoxia-inducible factor and vascular endothelial growth factor, which are involved in angiogenesis and are associated with coronary collateral development.
We postulated that there was a synergistic effect of hypertension and diabetes on low coronary collateralization. Blood pressure is usually elevated in patients with renal dysfunction as a result of fluid retention and over-production of vasoactive hormones via the rennin-angiotensin system. These changes may aggravate hypertension. Diabetes is also an important cause of chronic renal insufficiency. Thus, chronic renal dysfunction may further potentiate hypertension and diabetes and cause low coronary collateralization.
4.3. Coronary disease severity and occluded vessels
Numerous studies have demonstrated that coronary collateral formation is dependent mainly on the severity of coronary artery disease, with high collateralization in patients with extensive coronary lesions. In line with previous findings, our present study showed that the number of significantly diseased coronary arteries was associated with high collateralization not only in univariate analysis but also in multivariate analysis. Hence, the extent of coronary atherosclerosis plays an important role in coronary collateral formation for stable angina patients with chronic total occlusion.
Coronary collaterals were significantly better developed in patients with right coronary artery occlusion, which is consistent with previous findings (Elsman et al., 2004). This may imply better collateralization from the left coronary artery (especially the left anterior descending artery) than from other vessels, possibly due to the larger size of the artery.
4.4. Inflammatory cytokines
Inflammation has emerged as being central to the initiation and progression of atherosclerosis, and a complex interaction exists between new blood vessel formation and inflammation (Imhof and Aurrand-Lions, 2006). In the present study, an increased hsCRP level in serum was detected in patients with low collateralization. This is consistent with previous reports and supports a notion that a graded inverse independent association exists between CRP levels and collateral development in patients with stable angina pectoris (Kerner et al., 2007). Seiler (2010) demonstrated that tumor necrosis factor-α was detectable more often in patients with insufficient coronary collateral. Guray et al. (2004) reported an association between poor collateral circulation and an elevated concentration of soluble adhesion molecules, a marker of cytokine-induced endothelial activation.
4.5. Study limitations
We recognize several limitations in our study. First, this study was cross-sectional, thereby allowing us to detect associations, but not to formulate predictions. Likewise, the potential mechanisms were not fully elucidated. Second, coronary collateralization was assessed by angiographic images. Measuring the collateral flow index using the intravascular Doppler technique may provide a more objective physiological assessment of collateral grade.
5. Conclusions
Our data revealed that almost 40% of stable angina patients with chronic total occlusion had low coronary collateralization. Old age, female gender, no prior hypertension, hyperlipidemia, diabetes, at least moderate renal dysfunction, and less severe coronary artery disease were associated with low collateralization and may have a negative synergistic effect on collateral development. These findings provide new insights for risk stratification and medical and revascularization treatments in patients with coronary artery disease.
Footnotes
Compliance with ethics guidelines: Zhen SUN, Ying SHEN, Lin LU, Rui-yan ZHANG, Li-jin PU, Qi ZHANG, Zheng-kun YANG, Jian HU, Qiu-jing CHEN, and Wei-feng SHEN declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000(5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81(2):634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 2.Choi JH, Chang SA, Choi JO, Song YB, Hahn JY, Choi SH, Lee SC, Lee SH, Oh JK, Choe Y, et al. Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation. 2013;127(6):703–709. doi: 10.1161/CIRCULATIONAHA.112.092353. [DOI] [PubMed] [Google Scholar]
- 3.Duran M, Uysal OK, Gunebakmaz O, Yilmaz Y, Vatankulu MA, Turfan M, Duran AO, Ornek E, Cetim M, Kaya MG. Renal impairment and coronary collaterals in patients with acute coronary syndrome. Herz. 2013 doi: 10.1007/s00059-013-3823-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Elsman P, van′t Hof AW, de Boer MJ, Hoorntje JC, Suryapranata H, Dambrink JH, Zijlstra F. Zwolle Myocardial Infarction Study Group. Role of collateral circulation in the acute phase of ST-segment elevation myocardial infarction treated with primary coronary intervention. Eur Heart J. 2004;25(10):854–858. doi: 10.1016/j.ehj.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, Gannot S, Samuel M, Weisbrod M, Bierstone D, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59(11):991–997. doi: 10.1016/j.jacc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Fraker TD, Jr, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Gardin JM, et al. Chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50(23):2264–2274. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Guray U, Erbay AR, Guray Y, Yilmaz MB, Boyaci AA, Sasmaz H, Korkmaz S, Kutuk E. Poor coronary collateral circulation is associated with higher concentrations of soluble adhesion molecules in patients with single-vessel disease. Coron Artery Dis. 2004;15(7):413–417. doi: 10.1097/00019501-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hsu PC, Juo SH, Su HM, Chen SC, Tsai WC, Lai WT, Sheu SH, Lin TH. Predictor of poor coronary collaterals in chronic kidney disease population with significant coronary artery disease. BMC Nephrol. 2012;13(1):98–104. doi: 10.1186/1471-2369-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12(2):171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- 10.Kadi H, Ceyhan K, Karayakali M, Celik A, Ozturk A, Koc F, Onalan O. Effects of prediabetes on coronary collateral circulation in patients with coronary artery disease. Coron Artery Dis. 2011;22(4):233–237. doi: 10.1097/MCA.0b013e328345241b. [DOI] [PubMed] [Google Scholar]
- 11.Kerner A, Gruberg L, Goldberg A, Roguin A, Lavie P, Lavie L, Markiewicz W, Beyar R, Aronson D. Relation of C-reactive protein to coronary collaterals in patients with stable angina pectoris and coronary artery disease. Am J Cardiol. 2007;99(4):509–512. doi: 10.1016/j.amjcard.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 12.Kersten JR, Toller WG, Tessmer JP, Pagel PS, Warltier DC. Hyperglycemia reduces coronary collateral blood flow through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol. 2001;281(5):H2097–H2104. doi: 10.1152/ajpheart.2001.281.5.H2097. [DOI] [PubMed] [Google Scholar]
- 13.Kilian JG, Keech A, Adams MR, Celermajer DS. Coronary collateralization: determinants of adequate distal vessel filling after arterial occlusion. Coron Artery Dis. 2002;13(3):155–159. doi: 10.1097/00019501-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Levin DC. Pathways and functional significance of the coronary collateral circulation. Circulation. 1974;50(4):831–837. doi: 10.1161/01.CIR.50.4.831. [DOI] [PubMed] [Google Scholar]
- 15.Lin TH, Wang CL, Su HM, Hsu PC, Juo SH, Voon WC, Shin SJ, Lai WT, Sheu SH. Functional vascular endothelial growth factor gene polymorphisms and diabetes: effect on coronary collaterals in patients with significant coronary artery disease. Clin Chim Acta. 2010;411(21-22):1688–1693. doi: 10.1016/j.cca.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Meier P. The sword of Damocles: an illustrative example of the life-saving effect of the collateral circulation. J Invasive Cardiol. 2011;23(3):E47–E48. [PubMed] [Google Scholar]
- 17.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33(5):614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 18.Pu LJ, Shen Y, Zhang RY, Zhang Q, Ding FH, Hu J, Yang ZK, Shen WF. Screening for significant atherosclerotic renal artery stenosis with a regression model in patients undergoing transradial coronary angiography/intervention. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2012;13(8):631–637. doi: 10.1631/jzus.B1201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regieli JJ, Jukema JW, Nathore HM, Zwinderman AH, Ng S, Grobbee DE, van der Graaf Y, Doevendans PA. Coronary collaterals improve prognosis in patients with ischemic heart disease. Int J Cardiol. 2009;132(2):257–262. doi: 10.1016/j.ijcard.2007.11.100. [DOI] [PubMed] [Google Scholar]
- 20.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5(3):587–592. doi: 10.1016/S0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 21.Rocic P. Why is coronary collateral growth impaired in type II diabetes and the metabolic syndrome? Vascul Pharmacol. 2012;57(5-6):179–186. doi: 10.1016/j.vph.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schirmer SH, van Royen N, Moerlan PD, Fledderus JO, Henriques JP, van der Schaaf RJ, Vis MM, Baan J, Jr, Koch KT, Horrevoets AJ, et al. Local cytokine concentration and oxygen pressure are related to maturation of collateral circulation in humans. J Am Coll Cardiol. 2009;53(23):2141–2147. doi: 10.1016/j.jacc.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Seiler C. The human coronary collateral circulation. Eur J Clin Invest. 2010;40(5):465–476. doi: 10.1111/j.1365-2362.2010.02282.x. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Pu LJ, Lu L, Zhang Q, Zhang RY, Shen WF. Serum advanced glycation end-products and receptors as prognostic biomarkers in diabetics undergoing coronary artery stent implantation. Can J Cardiol. 2012;28(6):737–743. doi: 10.1016/j.cjca.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation. 2010;122(12):1210–1220. doi: 10.1161/CIRCULATIONAHA.109.930651. [DOI] [PubMed] [Google Scholar]
- 26.van der Hoeven NW, Teunissen PF, Werner GS, Delewi R, Schirmer SH, Traupe T, van der Laan AM, Tijssen JG, Piek JJ, Seiler C, et al. Clinical parameters associated with collateral development in patients with chronic total coronary occlusion. Heart. 2013;99(15):1100–1105. doi: 10.1136/heartjnl-2013-304006. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Jing J, Liu CF, Jiang TM, Yang XB, Zhou Y, Chen YD. The relationship between diastolic pressure and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Am J Hypertens. 2013;26(5):630–635. doi: 10.1093/ajh/hps096. [DOI] [PubMed] [Google Scholar]
- 28.Williamson JR, Ostrow E, Eades D, Chang K, Allison W, Kilo C, Sherman WR. Glucose-induced microvascular functional changes in non-diabetic rats are stereospecific and are prevented by an aldose reductase inhibitor. J Clin Invest. 1990;85(4):1167–1172. doi: 10.1172/JCI114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie SL, Li HY, Deng BQ, Luo NS, Geng DF, Wang JF, Nie RQ. Poor coronary collateral vessel development in patients with mild to moderate renal insufficiency. Clin Res Cardiol. 2011;100(3):227–233. doi: 10.1007/s00392-010-0233-8. [DOI] [PubMed] [Google Scholar]
- 30.Zorkun C, Akkaya E, Zoulu A, Tandogan I. Determinants of coronary collateral circulation in patients with coronary artery disease. Anadolu Kardiyol Derg. 2013;13:146–151. doi: 10.5152/akd.2012.250. [DOI] [PubMed] [Google Scholar]