Abstract
Adiponectin plays an important role in the development of hypertension, atherosclerosis, and cardiomyocyte hypertrophy, but very little was known about the influence of serum adiponectin or the adiponectin gene polymorphism on myocardial fibrosis. Our study investigates the influence of the SNP +45 polymorphism of the adiponectin gene and serum levels of adiponectin on myocardial fibrosis in patients with essential hypertension. A case-control study was conducted on 165 hypertensive patients and 126 normotensive healthy controls. The genotypes of adiponectin gene polymorphisms were detected by the polymerase chain reaction (PCR) method. Serum concentrations of procollagen were measured by a double antibody sandwich enzyme-linked immunosorbent assay (ELISA) in all subjects. The integrated backscatter score (IBS) was measured in the left ventricular myocardium using echocardiography. The serum levels of adiponectin in hypertensive patients were significantly lower than those in the normal control group ((2.69±1.0) μg/ml vs. (4.21±2.89) μg/ml, respectively, P<0.001). The serum levels of type-I procollagen carboxyl end peptide (PICP) and type-III procollagen ammonia cardinal extremity peptide (PIIINP) in the hypertension group were significantly higher than those in the control group. In the hypertension group, serum levels of adiponectin were significantly and negatively related to the average acoustic intensity and corrected acoustic intensity of the myocardium (r=0.46 and 0.61, respectively, P<0.05 for both). The serum levels of PICP and PIIINP were significantly different among the three genotypes of SNP +45 (P<0.01). Logistic regression analyses showed that sex and genotype (GG+GT) were the major risk factors of myocardial fibrosis in hypertensive patients (OR=5.343 and 3.278, respectively, P<0.05). These data suggest that lower levels of adiponectin and SNP +45 polymorphism of the adiponectin gene are likely to play an important role in myocardial fibrosis in hypertensive patients.
Keywords: Hypertension, Myocardial fibrosis, Adiponectin gene, Type-I procollagen carboxyl end peptide (PICP), Type-III procollagen ammonia cardinal extremity peptide (PIIINP)
1. Introduction
Adiponectin is a protein produced and secreted by the adipocytes that affects the metabolism of the human body (Kadowaki et al., 2006; Matsuzawa, 2006; Robinson et al., 2011). Several studies have demonstrated that adiponectin plays an important role in the development of hypertension, atherosclerosis, and cardiomyocyte hypertrophy (Fujita et al., 2008; Pischon et al., 2011; Ikonomidis et al., 2012). Adiponectin is encoded by the adiponectin gene on chromosome 3q27 (Guo et al., 2006). Several single nucleotide polymorphisms (SNPs) in the adiponectin gene have been shown to be associated with diabetes mellitus or insulin resistance syndrome (the metabolic syndrome) (Hara et al., 2002; Vasseur et al., 2002; Melistas et al., 2009; Namvaran et al., 2012).
Myocardial fibrosis is one of the important mechanisms of ventricular remodeling and chronic heart failure caused by hypertension (Díez, 2008). However, little is known about the influence of plasma levels of adiponectin or SNPs in the adiponectin gene on myocardial fibrosis in humans. We attempt here to elucidate the relationship between the adiponectin levels and the SNP +45T/G polymorphism of the adiponectin gene with myocardial fibrosis in Chinese hypertensive patients.
2. Materials and methods
2.1. Study population
From September 2008 to December 2011, 165 patients with primary hypertension and 126 normotensive control subjects were recruited into this study. All these subjects were selected from healthy physical examination volunteers and no medication therapies were used in these subjects. All the hypertensive patients met the criteria set by the World Health Organization/International Society on Hypertension (WHO/ISH) in 1999: systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg (Kjeldsen et al., 2002); the below diseases were excluded: history of cardiovascular disease or cancer, diabetes mellitus, abnormal liver or renal function, and thyroid or pituitary disease. The nomotensive control group comprised healthy volunteers who had no history of heart disease, hypertension, diabetes mellitus, chronic liver disease, or kidney disease, and no abnormal indices detected by electrocardiography (ECG), echocardiography, chest radiography, or analyses of their renal function and liver function.
2.2. Evaluation of clinical and biochemical parameters
The body weight and height of all the subjects were measured to calculate the body mass index (BMI). Blood pressure (BP) was measured using a mercury sphygmomanometer during three separate intervals. Blood was taken in the fasting state. Blood and levels of the total cholesterol (TC), triglycerides (TGs), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), and plasma glucose were assessed using an automatic biochemical analyzer. The serum adiponectin level was measured by radioimmunoassay.
2.3. Measurement of the myocardial integrated backscatter score
The standard four-chamber view was used to acquire data. The parameters were set as: depth was 16 cm; probe frequency was 2.5 MHz; total gain was 50 dB; lateral gain compensation (LGC) was set at the lowest level; mechanic index was 1.6; and the focus was at the middle of the heart. All the parameters described above were kept constant throughout the study. With respect to the acoustic density-integrated backscatter score (AD-IBS) status, six sites (basal septum, middle septum, apical septum, basal lateral wall, middle lateral wall, and apical lateral wall) were sampled to measure the average image intensity (AII). The mean AII of the six sites was defined as the average acoustic intensity (AAI). The AII in the left ventricular cavity (AIILVC) was measured to calculate the corrected acoustic intensity (CAI) using the following equation: CAI=AAI/AIILVC.
2.4. DNA extraction and genotyping of the SNP +45T/G polymorphism of the adiponectin gene
Genomic DNA was extracted from frozen whole blood using a DNA extraction kit (BioTeke Corporation, Beijing, China). Fragments comprising the 45T/G of the adiponectin gene sequence were amplified by the polymerase chain reaction (PCR) using the forward primer 5′-CTGTTGCTGGGAGCTGTTCTACT-3′ and the reverse primer 5′-GATGAAAGAGGCCAGAAACATTCT-3′ under the conditions described previously (Sabouri et al., 2011). Real-time fluorescence quantitative PCR was used to detect the SNPs+45T/G of the adiponectin gene. Primer synthesis, design of the detection probe, and detection of SNPs+45T/G of the adiponectin gene were completed by the Shanghai GeneCore BioTechnologies, China.
2.5. Assessment of serum levels of PICP and PIIINP
The 3-ml blood was taken from patients in the fasting state. Serum was separated by centrifugation at 3 000 r/min for 10 min at 4 °C. Serum levels of type-I procollagen carboxyl end peptide (PICP) and type-III; procollagen ammonia cardinal extremity peptide (PIIINP) were measured using enzyme-linked immunosorbent assay (ELISA). The human PICP and PIIINP kits were supplied by RapidBio (Beijing, China).
2.6. Statistical analyses
Continuous data can be defined as the mean±standard deviation (SD). Differences in continuous parameters such as levels of PICP and PIIINP between the two groups were analyzed using the non-paired t-test. The frequencies of allelic and genotypic associations with PICP and PIIINP and estimation of the odds ratio (OR) were assessed using the χ 2 test with the TT genotype as the reference genotype. Multivariate logistic regression was used to estimate the influence of genotype on myocardial fibrosis to adjust for other known risk factors for myocardial fibrosis, including age, sex, BMI, BP, fasting plasma glucose, current smoking as well as levels of TC and TGs. P<0.05 was considered statistically significant. All statistical analyses were completed using SPSS ver13.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Clinical characteristics of the study population
The clinical characteristics of the study participants are shown in Table 1. Levels of glucose and TG in the hypertension group were significantly higher than those in the control group. Also, BMI was significantly different between the two groups. Serum adiponectin levels in hypertensive patients were significantly lower than those in the normal control group ((2.69±1.00) μg/ml vs. (4.21±2.89) μg/ml, P<0.001). There were no significant differences between the two groups with respect to sex, age, and other indices.
Table 1.
Clinical characteristics of the study population
| Item | Control group (n=126) | Hypertension group (n=165) |
| Age (year) | 52.00±10.33 | 53.78±9.29 |
| BMI (kg/m2) | 24.65±2.47 | 25.82±2.41* |
| Sex, male/female | 59/67 | 92/73 |
| GLU (mmol/L) | 4.96±0.68 | 5.50±1.21* |
| TG (mmol/L) | 1.46±0.53 | 1.57±0.75 |
| TC (mmol/L) | 5.13±1.11 | 5.24±1.32 |
| HDL-C (mmol/L) | 1.61±0.40 | 1.59±0.51 |
| LDL-C (mmol/L) | 3.14±0.97 | 3.27±1.21 |
| Serum adiponectin (μg/ml) | 4.21±2.89 | 2.69±1.00* |
| PICP (ng/ml) | 4.12±1.19 | 13.10±6.51* |
| PIIINP (ng/ml) | 66.70±11.72 | 128.94±56.37* |
P<0.001, vs. the control group
The genotype frequencies of the adiponectin gene+45T/G polymorphism were in accordance with the Hardy-Weinberg equilibrium law in the hypertension group and normal control group using the genetic equilibrium test (normal control group, χ 2=0.029, P>0.05; hypertension group, χ 2=0.503, P>0.05).
3.2. Influence of blood pressure on myocardial fibrosis
In the hypertension group, the Pearson correlation analyses showed that the serum adiponectin levels were negatively and significantly correlated with both their SBP and DBP (correlation coefficient (r) −0.274 and −0.272, respectively, P<0.05 for both).
Levels of PICP and PIIINP are considered to be biochemical markers of myocardial fibrosis (Jellis et al., 2011). Serum levels of PICP in the hypertension group were significantly higher than those of the control group ((13.10±6.51) ng/ml vs. (4.12±1.19) ng/ml, P<0.001) according to the non-paired t-test. The serum PIIINP level of the hypertension group was significantly higher than that of the control group ((128.94±56.37) ng/ml vs. (66.70±11.72) ng/ml, P<0.001) (Table 1).
3.3. Relationship between the myocardial integrated backscatter score, biochemical markers of myocardial fibrosis, and serum levels of adiponectin
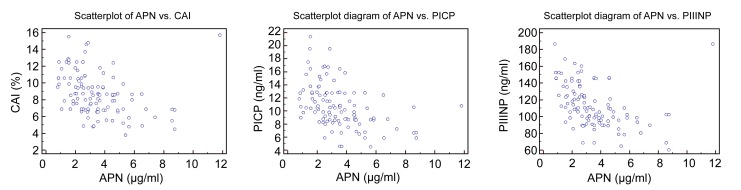
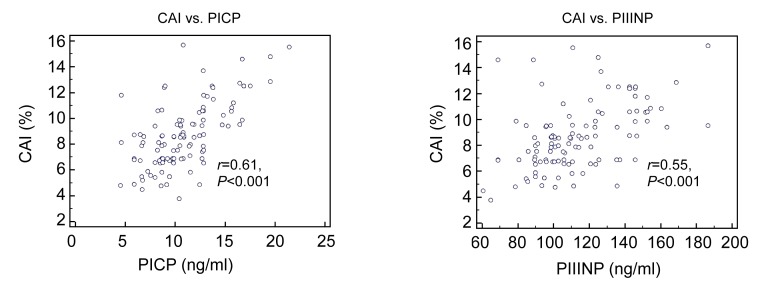
In the hypertension group, serum adiponectin levels were significantly and negatively related to myocardial AAI and CAI (r=0.46 and 0.61, respectively, P<0.05 for both; Fig. 1). Similarly, serum adiponectin levels significantly and negatively correlated with PCIP and PIIINP (r=0.39 and 0.35, respectively, P<0.05 for both; Fig. 1). Stepwise multiple regression analyses showed that serum adiponectin levels were significantly and negatively correlated with CAI after adjustment for BP, BMI, age, left ventricular myocardial mass, and blood levels of lipids and glucose (Table 2). Also, the Pearson analysis showed that CAI was significantly and positively correlated to PICP and PIIINP levels (r=0.612 and 0.552, respectively, P<0.001 for both; Fig. 2).
Fig. 1.
Scatterplot diagrams of adiponectin (APN) levels with CAI, PICP, and PIIINP
Table 2.
Stepwise regression analysis of factors influencing CAI
| Item | β | R 2 | F | P |
| SBP | 0.476 | 0.351 | 73.41 | <0.0001 |
| Adiponectin | 0.249 | 0.21 | 41.74 | 0.009 |
| DBP | 0.160 | 0.19 | 32.31 | 0.045 |
| Blood glucose | 0.142 | 0.13 | 22.13 | 0.065 |
β: partial regression coefficient
Fig. 2.
Relationship between CAI and PICP or PIIINP
3.4. SNP +45T/G polymorphism of the adiponectin gene and biochemical markers of myocardial fibrosis
Because of the smaller number of cases of the 45GG genotype, the 45TG and 45GG genotypes were combined as the 45TG+GG group. In the hypertension group, the serum level of PICP in the 45TG+GG subgroup was significantly higher than that in the 45TT subgroup ((16.56±7.73) ng/ml vs. (10.83±4.14) ng/ml, P<0.001) according to the non-paired t-test. The serum level of PIIINP in the 45TG+GG subgroup was significantly higher than that of the 45TT subgroup ((148.15±78.63) ng/ml vs. (116.32±26.04) ng/ml, P<0.001). However, in the control group, there were no significant differences in serum levels of PICP and PIIINP between the 45TG+GG and 45TT subgroups (P>0.05 for both, Table 3). Similarly, in the hypertensive group, the CAI in the 45TG+GG subgroup was significantly higher than that in the 45TT subgroup ((9.24±3.01)% vs. (8.12±2.56)%, P=0.02).
Table 3.
Comparison of PICP and PIIINP levels between different genotypes in hypertension and control groups
| Group | Genotype | PICP (ng/ml) | PIIINP (ng/ml) | Adiponectin level (μg/ml) |
| Hypertension | TT (n=123) | 10.83±4.14 | 116.32±26.04 | 2.92±0.94 |
| TG+GG (n=42) | 16.56±7.73* | 148.15±78.63* | 2.03±0.86* | |
| Control | TT (n=102) | 3.79±1.12 | 66.27±13.22 | 4.26±2.83 |
| TG+GG (n=24) | 4.21±1.10 | 67.61±11.09 | 4.04±3.05 |
P<0.001, vs. TT genotype
The adiponectin level of the 45TT subgroup in the hypertension patients was significantly higher than that of the 45TG+GG subgroup (Table 3), but there was no difference between the 45TT and 45TG+GG subgroups in normotensive subjects (Table 3).
Additionally, our results showed that there was no significant difference in adiponectin levels between genders (female (3.63±2.19) μg/ml vs. male (3.49±1.87) μg/ml, P=0.66). Similarly, no significant differences were detected in the TT and TG+GG genotypes between genders (TT allele frequency was 73.97% (female) and 74.73% (male), respectively, P=0.94).
3.5. Association of SNP +45T/G of the adiponectin gene with myocardial fibrosis
The means of the PICP and PIIINP levels were set as the cut-off point to differentiate the severe and mild fibrosis groups to calculate the OR for certain risk factors. Hypertensive patients were divided into two subgroups according to the serum levels of PICP and PIIINP: severe fibrosis group (PICP≥13 ng/ml and/or PIIINP≥128 ng/ml) and the mild fibrosis group (PICP<13 ng/ml and PIIINP<128 ng/ml). The fibrosis level was set as a dependent variable, and age, sex, BP, genotype (GG+GT or TT) as well as blood levels of TGs, LDL-C and HDL-C were set as independent variables. After adjustment of other risk factors by logistic regression analyses, sex and genotype could be entered into the regression equation (OR=5.343 and 3.278, respectively, P<0.04). These data suggest that sex and genotype could be novel independent risk factors of myocardial fibrosis in hypertensive patients (Table 4).
Table 4.
Logistic regression analysis of risk factors for myocardial fibrosis in hypertensive patients
| RF | B | WV | SE | OR (95% CI) | P |
| Sex (male) | 1.676 | 8.416 | 0.578 | 5.343 (1.722, 16.577) | 0.004 |
| GG+TG | 1.187 | 4.207 | 0.579 | 3.278 (1.054, 10.191) | 0.04 |
RF: risk factor; B: regression coefficient; WV: Wald value; SE: standard error
4. Discussion
The occurrence and development of myocardial fibrosis in hypertensive patients have been previously reported to be related to stress, humoral factors and inflammatory factors (Kaya et al., 2011). Studies have shown that angiotensin II (the main active substance in the renin-angiotensin system) can stimulate the hypertrophy of the myocardial cells and hyperplasia of the cardiac fibroblasts (Castoldi et al., 2012). However, these results cannot fully explain the mechanism of myocardial fibrosis caused by high BP (Kai et al., 2009). Hence, finding other risk factors which can promote or inhibit myocardial fibrosis is very important.
Adiponectin is a peptide hormone secreted by adipocytes (Yamauchi et al., 2001; Robinson et al., 2011). Several studies have shown that adiponectin has an important role in the development of hypertension, atherosclerosis, and cardiomyocyte hypertrophy (Fujita et al., 2008; Pischon et al., 2011). Nevertheless, the relationship between adiponectin levels and myocardial fibrosis has not been clarified. We used serum levels of PICP and PIIINP and IBS as indicators of myocardial fibrosis in vivo to clarify the influence of serum levels of adiponectin and its gene polymorphism on myocardial fibrosis (Lin et al., 2004).
4.1. Relations between hypertension, adiponectin levels, and myocardial fibrosis
Serum levels of PICP and PIIINP in hypertensive patients were significantly higher than those in the normal control group. This finding suggested that collagen synthesis has increased in patients with essential hypertension. High BP could promote myocardial fibrosis, which is consistent with previous findings (Devi et al., 2006).
It has been reported that IBS is directly related to the myocardial collagen volume fraction when assessed by morphometric analyses (Kosmala et al., 2012). In the present study, myocardial IBS was used to represent the degree of myocardial fibrosis. The results demonstrated that serum levels of adiponectin were significantly and negatively correlated with myocardial IBS. Adiponectin plays an important part in inhibiting collagen synthesis in the heart probably through adjustment of the metabolism in essential hypertension.
Our results showed that serum levels of adiponectin were significantly and negatively correlated with the markers of myocardial fibrosis (PICP and PIIINP). This finding cohered with the previous study results (Tsai et al., 2008). Therefore, we speculated that low serum levels of adiponectin could lead to myocardial fibrosis through an unknown mechanism. The pathway through which adiponectin regulates myocardial fibrosis requires further study. It has been reported that adiponectin can protect against angiotensin II-induced cardiac fibrosis through activation of peroxisome proliferator-activated receptor α (PPAR-α) in cultured cells (Fujita et al., 2008). Also, studies have shown that pressure overload can lead to concentric cardiac hypertrophy in adiponectin gene knockout mice (Liao et al., 2005). Those results and the present study suggest that adiponectin could affect cardiac remodeling in pathological conditions.
4.2. Relation between polymorphism of the adiponectin gene and myocardial fibrosis
The present study showed that serum levels of PICP and PIIINP in patients with the TT genotype were significantly lower than those in individuals with the GT and GG genotype. Logistic regression analyses demonstrated that the G allele was significantly and positively associated with myocardial fibrosis independent of known risk factors such as age, sex, BMI, BP, and blood levels of lipids. The OR of the G allele at the +45T/G polymorphism for myocardial fibrosis was 3.278 (95% confidence interval (CI), 1.054–10.191, P=0.04). Furthermore, the “fibrosing” effect of the G allele at the +45T/G polymorphism may be related to lower serum levels of adiponectin.
Cartegni et al. (2002) proposed that the SNP +45T/G of exon 2 belongs to the synonymous mutation G15G. The location of this mutation is close to the junctions of exons and introns, so silent mutation of this coding district may influence expression of the adiponectin gene through an mRNA splicing mechanism. This reduces expression of adiponectin by down-regulation of RNA levels. In theory, the mutation of the adiponectin gene could influence the expression of adiponectin. Therefore, the T allele may inhibit myocardial fibrosis by increasing adiponectin levels. Our study showed that there were significant differences between TT and TG+GG allele in hypertension, but Mohammadzadeh and Zarghami (2009) reported that there was no statistical difference between TT and TG+GG allele in non-diabetic and type 2 diabetes mellitus patients. Probably the smaller sample size impacted their study result or there was the presence of racial differences.
It was reported that female patients had higher levels of adiponectin (Khabour et al., 2013). However, our results showed that there were no significant differences between genders. Similarly, no significant differences were detected in TT and TG+GG genotypes between genders. Our results were in accordance with Kuo and Halpern (2011). Indeed, there were many inconsistent results concerning the impact of genders on adiponectin levels. We inferred that the probable causes included different race, the sum of the sample, and complicated disease, etc.
Logistic regression analyses showed that male patients in the hypertension group had a higher risk of myocardial fibrosis than female patients. Sex was another risk factor for myocardial fibrosis in hypertensive patients (OR=5.343, 95% CI, 1.722–16.577, P=0.004).
In summary, carriers of the polymorphism of the adiponectin gene +45TT had lower levels of PICP and PIIINP in hypertensive patients. The +45T/G polymorphism of the adiponectin gene was related to myocardial fibrosis in hypertension patients.
Acknowledgments
We sincerely thank Dr. Hai-feng HOU (Department of Epidemiology, Taishan Medical University, Tai’an, China) for his assistance in the statistical analysis.
Footnotes
Project supported by the Natural Science Foundation of Shandong Province, China (No. ZR2012HL19), and the Science and Technology Development Plan of Tai’an City, China (No. 20113096)
Compliance with ethics guidelines: Cheng-jun YAN, Su-mei LI, Qiang XIAO, Yan LIU, Jian HOU, Ai-fang CHEN, Li-ping XIA, and Xiu-chang LI declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the Ethics Committee of Taishan Medical College and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
References
- 1.Cartegni L, Chew SL, Krainer AR. Listening to silent and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet. 2002;3(4):285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- 2.Castoldi G, di Gioia CR, Bombardi C, Catalucci D, Corradi B, Gualazzi MG, Leopizzi M, Mancini M, Zerbini G, Condorelli G, et al. miR-133a regulates collagen 1A1: potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. J Cell Physiol. 2012;227(2):850–856. doi: 10.1002/jcp.22939. [DOI] [PubMed] [Google Scholar]
- 3.Devi S, Kennedy RH, Joseph L, Shekhawat NS, Melchert RB, Joseph J. Effect of long-term hyperhomocysteinemia on myocardial structure and function in hypertensive rats. Cardiovasc Pathol. 2006;15(2):75–82. doi: 10.1016/j.carpath.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Díez J. Diagnosis and treatment of myocardial fibrosis in hypertensive heart disease. Circ J. 2008;72(Suppl. A):A8–A12. doi: 10.1253/circj.CJ-07-1067. [DOI] [PubMed] [Google Scholar]
- 5.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, Nishida M, Hiuge A, Kurata A, Kihara S, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-α. Arterioscler Thromb Vasc Biol. 2008;28(5):863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 6.Guo X, Saad MF, Langefeld CD, Williams AH, Cui J, Taylor KD, Norris JM, Jinagouda S, Darwin CH, Mitchell BD, et al. Genome-wide linkage of plasma adiponectin reveals a major locus on chromosome 3q distinct from the adiponectin structural gene: the IRAS family study. Diabetes. 2006;55(6):1723–1730. doi: 10.2337/db05-0428. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K, et al. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51(2):536–540. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- 8.Ikonomidis I, Kadoglou N, Tsiotra PC, Kollias A, Palios I, Fountoulaki K, Halvatsiotis I, Maratou E, Dimitriadis G, Kremastinos DT, et al. Arterial stiffness is associated with increased monocyte expression of adiponectin receptor mRNA and protein in patients with coronary artery disease. Am J Hypertens. 2012;25(7):746–755. doi: 10.1038/ajh.2012.42. [DOI] [PubMed] [Google Scholar]
- 9.Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011;4(6):693–702. doi: 10.1161/CIRCIMAGING.111.963587. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kai H, Kudo H, Takayama N, Yasuoka S, Kajimoto H, Imaizumi T. Large blood pressure variability and hypertensive cardiac remodeling—role of cardiac inflammation. Circ J. 2009;73(12):2198–2203. doi: 10.1253/circj.CJ-09-0741. [DOI] [PubMed] [Google Scholar]
- 12.Kaya Z, Leib C, Werfel S, Göser S, Öttl R, Leuchs B, Pfitzer G, Katus HA, Müller OJ. Comparison of IL-10 and MCP-1-7ND gene transfer with AAV9 vectors for protection from murine autoimmune myocarditis. Cardiovasc Res. 2011;91(1):116–123. doi: 10.1093/cvr/cvr063. [DOI] [PubMed] [Google Scholar]
- 13.Khabour OF, Wehaibi SH, Al-Azzam SI, Alzoubi KH, El-Akawi ZJ. Association of adiponectin with hypertension in type 2 diabetic patients: the gender effect. Clin Exp Hypertens. 2013;35(5):361–366. doi: 10.3109/10641963.2012.732645. [DOI] [PubMed] [Google Scholar]
- 14.Kjeldsen SE, Erdine S, Farsang C, Sleight P, Mancia G. 1999 WHO/ISH Hypertension Guidelines Subcommittee. 1999 WHO/ISH Hypertension Guidelines—highlights & ESH update. J Hypertens. 2002;20(1):153–155. doi: 10.1097/00004872-200201000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Kosmala W, Przewlocka-Kosmala M, Wojnalowicz A, Mysiak A, Marwick TH. Integrated backscatter as a fibrosis marker in the metabolic syndrome: association with biochemical evidence of fibrosis and left ventricular dysfunction. Eur Heart J Cardiovasc Imaging. 2012;13(6):459–467. doi: 10.1093/ejechocard/jer291. [DOI] [PubMed] [Google Scholar]
- 16.Kuo SM, Halpern MM. Lack of association between body mass index and plasma adiponectin levels in healthy adults. Int J Obes (Lond) 2011;35(12):1487–1494. doi: 10.1038/ijo.2011.20. [DOI] [PubMed] [Google Scholar]
- 17.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovas Res. 2005;67(4):705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Lin YH, Shiau YC, Yen RF, Lin LC, Wu CC, Ho YL, Huang PJ. The relation between myocardial cyclic variation of integrated backscatter and serum concentrations of procollagen propeptides in hypertensive patients. Ultrasound Med Biol. 2004;30(7):885–891. doi: 10.1016/j.ultrasmedbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3(1):35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 20.Melistas L, Mantzoros CS, Kontogianni M, Antonopoulou S, Ordovas JM, Yiannakouris N. Association of the +45T>G and +276G>T polymorphisms in the adiponectin gene with insulin resistance in nondiabetic Greek women. Eur J Endocrinol. 2009;161(6):845–852. doi: 10.1530/EJE-09-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadzadeh G, Zarghami N. Associations between single-nucleotide polymorphisms of the adiponectin gene, serum adiponectin levels and increased risk of type 2 diabetes mellitus in Iranian obese individuals. Scand J Clin Lab Invest. 2009;69(7):764–771. doi: 10.3109/00365510903137237. [DOI] [PubMed] [Google Scholar]
- 22.Namvaran F, Rahimi-Moghaddam P, Azarpira N, Dabbaghmanesh MH. Polymorphism of adiponectin (45T/G) and adiponectin receptor-2 (795G/A) in an Iranian population: relation with insulin resistance and response to treatment with pioglitazone in patients with type 2 diabetes mellitus. Mol Biol Rep. 2012;39(5):5511–5518. doi: 10.1007/s11033-011-1354-5. [DOI] [PubMed] [Google Scholar]
- 23.Pischon T, Hu FB, Girman CJ, Rifai N, Manson JE, Rexrode KM, Rimm EB. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis. 2011;219(1):322–329. doi: 10.1016/j.atherosclerosis.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15(2):221. doi: 10.1186/cc10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabouri S, Ghayour-Mobarhan M, Moohebati M, Hassani M, Kassaeian J, Tatari F, Mahmoodi-kordi F, Esmaeili HA, Tavallaie S, Paydar R, et al. Association between 45T/G polymorphism of adiponectin gene and coronary artery disease in an Iranian population. Sci World J. 2011;11:93–101. doi: 10.1100/tsw.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai WC, Lin CC, Chen JY, Huang YY, Lee CH, Li WT, Weng CM, Chen JH. Association of adiponectin with procollagen type I carboxyterminal propeptide in non-diabetic essential hypertension. Blood Press. 2008;17(4):233–238. doi: 10.1080/08037050802308895. [DOI] [PubMed] [Google Scholar]
- 27.Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, Boutin P, Vaxillaire M, Leprêtre F, Dupont S, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11(21):2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]