Abstract
In our study, 50 patients with dilated cardiomyopathy (DCM) were selected to investigate the correlation between virus persistent infection and cardic function. We found that 44% of patients with DCM were coxsackie virus B-RNA (CVB-RNA) positive, significantly different from that (20%) of the normal control group (P<0.05). The expression levels of coxsackie adenovirus receptor (CAR) in patients with DCM were significantly higher than those in the normal control group (P<0.01). In CVB-RNA-positive patients, expression levels of CAR were significantly higher than those in CVB-RNA-negative patients (P<0.01). There was a positive correlation between CAR expression and brain natriuretic peptide (BNP) level in patients with DCM, but no significant correlations between the CAR expression level and left ventricular ejection fraction (LVEF) or left ventricular end diastolic diameter (LVEDd). These results showed that expression levels of CAR on the surface of white cells can be used as an indicator for detecting persistent virus infection. We found that expression levels of CAR and heart function in patients with DCM were highly correlated.
DCM is characterized by left ventricle or biventricular dilated and systolic dysfunction, and excludes systemic disease and primary cardiomyopathy. In recent years, many scholars have put forward the idea that viral myocarditis (VMC) and DCM are different stages of the same disease, and that viral DCM is the terminal phase of VMC (Liu and Mason, 2001). Though comprehensive modern medical therapy is adopted widely in clinical practice, prognosis of DCM is still unsatisfactory. Therefore, the detailed study of the etiology and pathogenesis of DCM has great social significance and clinical value.
Recent studies have found that virus infection (especially with coxsackie B virus) can transform VMC into DCM. The main pathogenesis of this process might be the persistent virus infection, which results in sustained damage to the myocardial tissue and induces autoimmune-mediated myocardial injuries (Nishtala et al., 2011). Fujioka et al. (2008) found coxsackie virus RNA in the myocardial tissue of patients with DCM using a polymerase chain reaction (PCR) method, accounting for 23% and 32% of patients in survivors and the dead, respectively. Feuer et al. (2002) showed that DCM patients may suffer from persistent virus infection, especially chronic intestinal virus. Virus-infected patients were usually in an immunosuppressant and weak state, so the virus could escape the host immune response and exist continuously in myocardial cells. Once viral replication was re-activated, the clinical symptoms reappeared and would eventually develop into DCM. Jane-wit et al. (2007) and Leuschner et al. (2009) claimed that an autoimmune reaction caused sustained replication of intestinal virus, resulting in the occurrence of DCM.
In our study, we detected CVB-RNA and CAR expression levels on the surface of white cells of clinically diagnosed DCM patients using the methods of reverse transcription PCR (RT-PCR) and flow cytometry (FCM), respectively. We also determined their cardiac condition based on clinical manifestations, echocardiography, and plasma BNP detection. We aimed to investigate the relationships between persistent virus infection and the heart function of DCM patients.
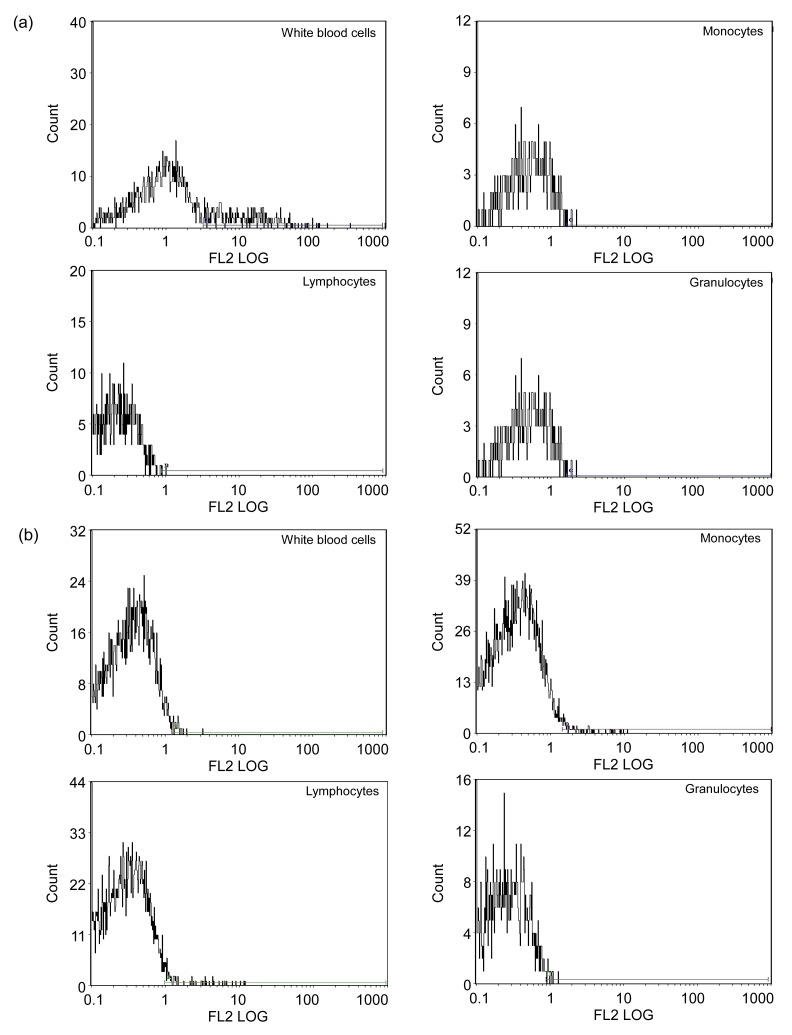
Among 50 patients with DCM, 22 were found to be CVB-RNA-positive by RT-PCR, a positive rate of 44%; 6 were found to be CVB-RNA-positive in the normal control group (n=30), a positive rate of 20%, but the difference between the two groups was statistically significant (P<0.05). The results of FCM of the patients with DCM and the healthy subjects are shown in Fig. 1. Compared with the control group, CAR expression levels on the cell surfaces including white blood cells, lymphocytes, monocytes, and granulocytes were significantly higher in patients in the DCM group. The differences were statistically significant (t=6.74, 9.77, 11.07, and 12.23, respectively, P<0.01) (Table 1). FCM analysis showed that the CAR expression levels of 22 patients who were CVB-RNA-positive increased more than those of the 28 CVB-RNA-negative patients. The differences were also statistically significant (t=8.04, 3.59, 0.13, and −2.15, respectively, P<0.01) (Table 2).
Fig. 1.
Results of flow cytometry (FCM) of white blood cells, monocytes, lymphocytes, and granulocytes of control group (a) and patients with DCM (b)
Table 1.
A comparison of CAR expression levels in different cells of patients with DCM and individuals in the control group
| Group | n | CAR expression level |
|||
| White blood cells | Lymphocytes | Monocytes | Granulocytes | ||
| Control | 30 | 1.97±0.96 | 1.42±0.93 | 1.05±0.48 | 0.90±0.70 |
| DCM | 50 | 3.26±0.93 | 3.22±0.90 | 3.01±0.90 | 2.84±0.86* |
Data are expressed as mean±SD
P<0.01, compared with the control group
Table 2.
A comparison of CAR expression levels in different cells of CVB-RNA-positive and CVB-RNA-negative patients
| Group | n | CAR expression level |
|||
| White blood cells | Lymphocytes | Monocytes | Granulocytes | ||
| CVB-RNA-positive | 22 | 3.80±0.70 | 3.54±0.76 | 3.02±0.91 | 2.97±0.88 |
| CVB-RNA-negative | 28 | 2.31±0.46* | 2.67±0.91* | 2.98±0.94∆ | 2.62±0.84* |
Data are expressed as mean±SD
P<0.01, compared with the CVB-RNA-positive group
P>0.05, compared with the CVB-RNA-positive group
There was a positive and significant correlation between the CAR expression level and the BNP level in patients with DCM (r=0.34, P<0.05). The CAR expression level showed some association with the LVEF and LVEDd of patients, but it did not reach statistical significance (r=−0.32 and 0.30, respectively, P>0.05) (Table 3), possibly because of the small sample size. However, the result still showed that persistent virus infection may cause deterioration of heart function in patients with DCM. Among 50 patients with DCM, there was a significant correlation between grades of heart function and BNP levels (r=0.84; P<0.01) (Table 4). This suggests that BNP detection may be helpful for the diagnosis, prognosis, and risk stratification of DCM in clinics.
Table 3.
BNP, LVEF, and LVEDd data of patients (n=50) and their correlations with the CAR level
| Parameter | Value | r | P |
| BNP | (1 107.11±846.41) pg/ml | 0.34 | <0.05 |
| LVEF | (39.97±10.55)% | −0.32 | >0.05 |
| LVEDd | (6.11±0.81) cm | 0.30 | >0.05 |
Table 4.
A comparison between plasma BNP levels and cardiac function classification in patients with dilated cardiomyopathy
| Cardiac function classification | n | BNP (pg/ml) |
| I | 5 | 266.67±122.56 |
| II | 16 | 588.47±432.57 |
| III | 20 | 1 145.91±429.65 |
| IV | 9 | 2 414.16±787.27 |
r=0.84, P<0.01
Though the pathogenesis of DCM has not yet been clearly elucidated, recent studies have confirmed that virus infection (especially of coxsackie B virus) can transform VMC into DCM. In our study, we used RT-PCR and FCM methods to detect virus infection. The results confirmed that persistent virus infection is one of the most important factors in the pathogenesis of DCM. Patients who were CVB-RNA-positive showed high expression of CAR while those who were CVB-RNA-negative showed low expression of CAR. So we conclude that expressions of CVB-RNA and CAR could become useful indicators of virus infection due to their high sensitivity and specificity. Moreover, because detection of CAR was by quantitative analysis, the experimental results were relatively accurate compared with clinical RT-PCR. FCM detection also has the advantages of a large sample size and simple operability. Therefore, the CAR expression level on the surface of white blood cells can be used as one of the quantitative indicators of clinical patients with DCM.
The main clinical manifestations of advanced DCM are heart failure and arrhythmia. Echocardiography has been clinically proven to play an important role in the diagnosis of severe heart dysfunction, but in recent years many studies have shown that the detection of plasma BNP levels has diagnostic significance for different degrees of heart failure. BNP is a heart neurohormone, secreted by the ventricles, which promotes natriuresis by inhibiting the renin-angiotensin-aldosterone system. Thus, it has important pathological and physiological significance. Recently, plasma BNP levels have become an objective diagnosis indicator of heart failure (McCullough et al., 2003). Our study confirmed that there was a significant positive correlation between BNP and the heart function grading of DCM patients. Due to the restrictions of time, low disease incidence, the number and compliance of patients, and the small number of cases involved, the results of this study may have certain limitations. This research, therefore, represents a preliminary study of the correlation between virus infection and heart function in patients with DCM, and follow-up studies of therapeutic interventions are in progress.
Materials and methods
Research subjects, reagents, and apparatus
Fifty patients with DCM were selected from outpatients and wards in our hospital from October 2009 to December 2011. The group comprised 29 males and 21 females, aged 41–75 years old, with an average age of (58.5±10.0) years. Thirty healthy people, including 16 males and 14 females aged from 26 to 67 years, with an average age of (49.4±10.4) years, were selected from our hospital medical center during the same period, as a normal control group for the PCR, enzyme-linked immunosorbent assay (ELISA) and FCM assessments.
Rabbit anti-human CAR polyclonal antibody and rabbits with IgG1 reagents were supplied by Hangzhou Burke Medical and Biological Products Co., Ltd.; human lymphocyte separation medium by Tianjin Haoyang Biological Products Technology Co., Ltd.; ELISA kits by US 48T/96T and Trizol by the Invitrogen Company; ethidium bromide (EB) solution at a concentration of 10 mg/ml and PCR kit by the Hangzhou Haofeng Biotechnology Co., Ltd.; primers by Hangzhou Haofeng Biotechnology Co., Ltd. (synthesis primers 5′-GAAGACTAAGGACCTAACAAA-3′, 5′-TACCCAGAGTAATCAAATGC-3′; amplified fragments as 312 bp).
FCM: EPICS XL; centrifuge: SH-012; nucleic acid protein analyzer: DU 800; BIO-RAD gel imager: Gel Doc XR; fluorescence quantitative analyzer: ABI PRISM 7000; PCR synthesizer: MJ Research; triage meter plus diagnostic apparatus: Biosite; ultrasonic diagnostic apparatus: HP Sonos 5500; electrophoresis: 041BR 12412.
Criteria and cardiac functional classes
Inclusion criteria: We adopted diagnostic standards of DCM proposed in the ‘Cardiomyopathy Diagnosis and Treatment Recommendations’ of the Working Group of the Medical Society of Cardiology (Liao et al., 2007): (1) LVEDd >5.0 cm for female and >5.5 cm for male; (2) LVEF <45% and (or) left ventricular fractional shortening rate (FS) <25%; (3) infection/autoimmune DCM diagnostics: consistent with diagnostic standards of DCM, and myocarditis history or myocardial biopsy confirmed the presence of inflammatory infiltration, sustained expression of the detected viral RNA or of serum immune markers, such as anti-myocardial antibodies.
Exclusion criteria: (1) hypertension (>160/100 mmHg); (2) coronary heart disease (coronary artery main branch stenosis >50%); (3) long-term excessive drinking history (women >40 g/d, men >80 g/d, for more than five years); (4) persistent rapid supraventricular arrhythmia; (5) systemic disease; (6) pericardial disease; (7) congenital heart disease; (8) pulmonary heart disease and neuromuscular diseases; (9) pregnancy, severe infections, diabetes and autoimmune diseases; (10) severely impaired liver and kidney function.
Cardiac functional classes according to the New York Heart Association (NYHA) standards: Class I, no limit to physical activity and daily activities without symptoms; Class II, physical activity and daily activities slightly limited by the onset of symptoms, but asymptomatic at rest; Class III, physical activity obviously limited, symptoms can still occur during lighter than normal daily activities, but can be asymptomatic at rest; Class IV, not engaged in any physical activity, symptoms occur at rest also. According to established standards, details of patients’ clinical symptoms and manifestations were collected and grades of heart function were determined by collaboration of three attending physicians.
Detection
Selected patients were routinely given heart medical care. All detections were completed within 24 h of admission, including collection of fasting blood, echocardiography, BNP, and other related tests. Venous blood was taken early in the morning for detecting CVB-RNA and CAR expression levels.
Echocardiography: Selected patients were checked by ultrasound diagnostic apparatus (HP Sonos 5500, USA) in silence. Parasternal and apical conventional section examinations were used to measure the LVDEd and LVEF.
BNP test: A triage meter and diagnostic apparatus were used with a boundary of 100 pg/ml. Salts of ethylenediaminetetraacetic acid (EDTA) anticoagulant were added to 2 ml of fasting blood in a test tube and shaken. Then 250 μl of anticoagulated whole blood was added to the test board, and tested using heart failure diagnostic apparatus.
RT-PCR test: RT-PCR was carried out according to the methods described previously (Ling et al., 2001; Chen et al., 2003; Ye and Chen, 2009). The PCR products were put in 2% agarose electrophoresis gels for 60 min, then stained for 30 min in a solution of EB (10 mg/ml). Results were observed under UV light.
Flow cytometry test: The CAR expression on the cell surface in each cell sample in turn (leukocytes, neutrophils, lymphocytes and monocytes) was detected by the method described by Yu et al. (2005) and the percentage of positive cells (PPC) was recorded.
Statistical analysis
All data were analyzed using SPSS 17.0 software and expressed as mean±standard deviation (SD). Pearson correlation analysis was used for measurement data, Spearman correlation analysis for rating data, and Chi-square test for countable data, with P<0.05 regarded as being statistically significant.
Footnotes
Project (No. 2007CB144) supported by the Traditional Chinese Medicine (TCM) Project of Zhejiang Province, China
Compliance with ethics guidelines: Qiang LIU, Xiao-jia SU, Yan YU, and Yong-lin LIU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000(5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Chen SX, Xie LS, Mei SW, Qian FR, Chen MF. The significance of coxsackie virus B RNA and antibody detection in the diagnosis of viral myocarditis. Chin J Lab Med. 2003;26(3):166–168. (in Chinese) [Google Scholar]
- 2.Feuer R, Mena I, Pagarigan R, Slifka MK, Whitton JL. Cell cycle status affects coxsackievirus replication, persistence and reaction in vitro. J Vurol. 2002;76(9):4430–4440. doi: 10.1128/JVI.76.9.4430-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujioka S, Kitaura Y, Terasaki F. Etiology and quantitative evaluation of viral infection in the myocardium of patients with end-stage idiopathic dilated cardiomyopathy. J Mol Cell Cardiol. 2008;45(4):S33. doi: 10.1016/j.yjmcc.2008.09.700. [DOI] [Google Scholar]
- 4.Jane-wit D, Altuntas CZ, Johnson JM, Yong S, Wickley PJ, Clark P, Wang Q, Popović ZB, Penn MS, Damron DS, et al. β1-adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation. 2007;116(4):399–410. doi: 10.1161/CIRCULATIONAHA.106.683193. [DOI] [PubMed] [Google Scholar]
- 5.Leuschner F, Katus HA, Kaya Z. Autoimmune myocarditis: past, present and future. J Autoimm. 2009;33(3-4):282–289. doi: 10.1016/j.jaut.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Liao YH, Yang YZ, Wang CH. Dilated cardiomyopathy. Chin J Cardiovasc Dis. 2007;35(1):5–9. (in Chinese) [Google Scholar]
- 7.Ling W, Shen Q, Wang J, Xu YL, Xu Y. Experimental study of the immuno-PCR assay coxsackie virus group B antigen. J Sec Milit Med Univ. 2001;22(3):264–266. (in Chinese) [Google Scholar]
- 8.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104(9):1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 9.McCullough PA, Omland T, Maisel AS. B-type natriuretic peptides: a diagnostic breakthrough for clinicians. Rev Cardiovasc Med. 2003;4(2):72–80. [PubMed] [Google Scholar]
- 10.Nishtala K, Phong TQ, Steil L, Sauter M, Salazar MG, Kandolf R, Kroemer HK, Felix SB, Völker U, Klingel K, et al. Virus-induced dilated cardiomyopathy is characterized by increased levels of fibrotic extracellular matrix proteins and reduced amounts of energy-producing enzymes. Proteomics. 2011;11(22):4310–4320. doi: 10.1002/pmic.201100229. [DOI] [PubMed] [Google Scholar]
- 11.Ye CH, Chen ZQ. Experimental study on granzyme B expression in mouse with viral myocarditis. Chin J Microbiol Immunol. 2009;29(11):1019–1024. (in Chinese) [Google Scholar]
- 12.Yu XH, Li SJ, Tang GH. Expression and significance of coxsackie adenovirus receptor in peripheral white blood cell of children with viral myocarditis. J Clin Pediatr. 2005;23(7):470–472. (in Chinese) [Google Scholar]