Abstract
Aims
Left ventricular (LV) circumferential strain (Ecc) is a sensitive index of regional myocardial function. Currently, no studies have assessed its prognostic value in general population. We sought to investigate whether Ecc has a prognostic value for predicting incident heart failure (HF) and other major cardiovascular events in asymptomatic individuals without a history of previous cardiovascular diseases.
Methods and results
We, prospectively, assessed incident HF and atherosclerotic events during a 5.5 ± 1.3-year period in 1768 asymptomatic individuals aged 45–84 (mean age 65 years; 47% female) who underwent tagged magnetic resonance imaging for strain determination. During the follow-up period, 39 (2.2%) participants experienced incident HF and 108 (6.1%) participants had atherosclerotic cardiovascular events. Average of peak Ecc of 12-LV segments (Ecc-global) and mid-slice (Ecc-mid) was −17.0 ± 2.4 and −17.5 ± 2.7%, respectively. Participants with average absolute Ecc-mid lower than −16.9% had a higher cumulative hazard of incident HF (log-rank test, P = 0.001). In cox regression analysis, Ecc-mid predicted incident HF independent of age, diabetes status, hypertension, interim myocardial infarction, LV mass index, and LV ejection fraction (hazard ratio 1.15 per 1%, 95% CI: 1.01–1.31, P = 0.03). This relationship remained significant after adjustment for LV-end-systolic wall stress into covariates. In addition, by adding Ecc-mid to risk factors, LV ejection fraction, and the LV mass index, both the global χ2 value (76.6 vs. 82.4, P = 0.04) and category-less net-reclassification index (P = 0.01, SE = 0.18, z = 2.53) were augmented for predicting HF. Circumferential strain was also significantly related to the composite atherosclerotic cardiovascular events, but its relationship was attenuated after introducing the LV mass index.
Conclusion
Circumferential shortening provides robust, independent, and incremental predictive value for incident HF in asymptomatic subjects without any history of previous clinical cardiovascular disease.
Clinical Trial Registration
http://www.clinicaltrials.gov. Unique identifier: NCT00005487.
Keywords: Myocardial function, Heart failure, Cardiovascular events
Introduction
Myocardial contractility is an important determinant of ventricular function.1 Traditionally, the left ventricular (LV) ejection fraction (EF) has been used as a global index of ventricular systolic function. However, LVEF is affected by the ventricular geometry and loading conditions and this may remain unchanged in affected patients until the underlying disease process is advanced. As a result, LVEF frequently overestimates myocardial systolic function in patients with concentric hypertrophy or volume overload.1 Regional circumferential shortening or strain is a sensitive index of myocardial function and may be an earlier marker of incipient myocardial dysfunction.1,2 Cardiac-tagged magnetic resonance imaging (MRI) is considered to be the reference method to measure myocardial circumferential strain (Ecc).3 An increasing number of studies have used Ecc as an accurate marker of myocardial dysfunction in various disease entities.4 However, the ability of this parameter to predict clinical outcomes in a population without previous history of cardiovascular disease has not been determined. Only the prognostic value of strain in patients with advanced heart failure (HF) has been reported in smaller clinical studies.5 Moreover, while it is well established that a higher LV mass is a strong predictor of HF,6,7 the joint effects of LV mass and regional LV dysfunction on the future development of cardiovascular events, especially HF, have not been thoroughly studied.8,9 While there is evidence from previous studies supporting a relationship between reduced LVEF and regional wall thickening on the future development of HF,9–11 the influence of the interactions of myocardial function with geometric LV remodelling and afterload measured by LV end-systolic wall stress on the development of HF and other cardiovascular events have not been fully evaluated.
In this study, we sought to examine whether regional Ecc, measured by tagged MRI, can predict the development of HF and major cardiovascular events. Our second aim was to investigate whether Ecc added incremental information to traditional risk factors as well as to the LV mass and LVEF among asymptomatic individuals without a history of cardiovascular disease.
Methods
Study sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective study designed to evaluate mechanisms that underlie the development and progression of subclinical cardiovascular diseases among asymptomatic individuals across populations.12 A sample of 6814 men and women from the USA aged 45–84 years of age at enrolment and free of known cardiovascular disease representing four different ethnic backgrounds were enrolled by six participating centres in the USA. Upon entry, all participants underwent an extensive evaluation that consisted of clinical questionnaires, physical examination, and laboratory tests. Individuals with symptoms or documentation of previous cardiovascular disease were excluded. Cardiac MRI was performed in 5098 participants. Of those, 1773 individuals were randomly selected to undergo tagged MRI for Ecc measurement as an ancillary study protocol at the time of conventional MRI (n = 1481) or at a separate examination (n = 292). Clinical characteristics of this subcohort were similar to the entire MESA cohort except for having a lower body mass index. Among the subcohort, four cases were excluded due to cardiac events reported to have happened before the tagged MRI, and one case was excluded due to the loss of follow-up data. Finally, 1768 cases were analysed in this study. The institutional review boards in each of the participating centres approved the study protocol and informed consent was obtained from each participant.
Conventional and tagged magnetic resonance imaging
MRI data were acquired using 1.5T scanners. Images were obtained using segmented k-space and electrocardiographic-triggered fast spoiled gradient-echo pulse sequence. After acquisition of standard scout images, two- and four-chamber cine MRI series were acquired. Short-axis cine images were then obtained with retrospective gating at 20 frames per cardiac cycle from above the mitral valve plane to the LV apex. Blood pressure was measured immediately before and after the MRI aortic measurements with the patient in the supine position on the MRI scanner gantry. Three-tagged short-axis slices (base to apex) were obtained using an electrocardiographic-triggered segmented k-space fast spoiled gradient-echo pulse sequence during breath holds. Parallel striped tags were prescribed in two orthogonal orientations using spatial modulation of magnetization encoding gradients. Parameters for tagged images were as follows: field of view 40 cm; slice thickness 7–8 mm; gap of 10 mm; repetition time 6 ms; echo time 3.0 ms; flip angle 10°–12°; phase encoding views 128 with 6 phase encoding views per segment; temporal resolution 21–40 ms; tag spacing 7 mm. The detailed protocol used for tagged MRI studies has been previously described.13
Left ventricular geometry and chamber performance assessment
The endocardial and epicardial myocardial borders were contoured using a semi-automated method (MASS 4.2, Medis, Leiden, the Netherlands). The difference between the epicardial and endocardial areas for all slices was multiplied by the slice thickness and section gap, and then multiplied by the specific gravity of the myocardium to determine the ventricular mass. All of these measurements were performed at end diastole. The papillary muscle mass was included in the LV cavity and excluded from the LV mass measurements.6 End-systolic pressure (ESP) was calculated as (2 × systolic blood pressure + diastolic pressure)/314 The stroke volume (SV) was calculated as LV end-diastolic volume (LVEDV) minus end-systolic volume (LVESV). The LV mass index was calculated by the LV mass/body surface area. The body size and the gender-adjusted LV mass (LV massadj) was calculated as LV mass/(a × height0.54 × weight0.61), where a = 6.82 for women and 8.25 for men with mass grams, height in meters, weight in kilograms.6 As an index of afterload LV-end-systolic wall stress (LVESWS, unit KPa) was calculated as ESP/{[(LVESV + LV mass volume)/LVESV]2/3– 1} from LaPlace's law.15
Myocardial strain analysis
Short-axis-tagged slices were analysed using the HARP method (embedded in MATLAB software or HARP1.15, Diagnosoft, Palo Alto, CA, USA), which enables fast determination of myocardial strain during the cardiac cycle.16 Circumferential strain was determined in four mid-wall segments (anterior, lateral, posterior, and septal) from three LV short-axis slices16 using in-house developed software (Figure 1). By convention, systolic Ecc, which denotes circumferential shortening, is normally negative, and less negative values of Ecc reflect decreased regional function. The intraclass correlation coefficients for inter-observer and intra-observer agreement for peak systolic mid-wall Ecc were 0.80 and 0.84 in studies with good tag persistence, and 074 and 0.82 in those with fair tag persistence.13 Segments without well-defined peak Ecc due to significant noise were excluded (2051 among 21 252 segments; 9%). We defined global Ecc is defined as the average of any existing 12-segments and Ecc-mid as average of any existing mid-LV segments.
Figure 1.
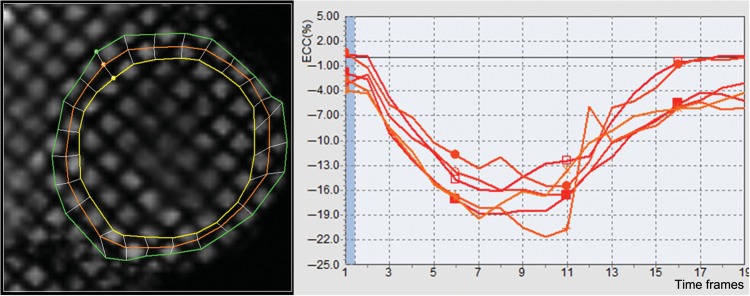
Tagged cardiovascular magnetic resonance imaging study with a sample circumferential strain curve.
Clinical follow-up
A telephone interviewer contacted each participant (or representative) every 6–9 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. Medical records were successfully obtained from an estimated 96% of hospitalized cardiovascular events and 95% of outpatient cardiovascular diagnostic encounters. Two physicians reviewed all records for independent endpoint classification and assignment of event dates. Reviewers classified HF as definite, probable, or absent. Definite or probable HF required HF symptoms, such as shortness of breath or oedema. In addition to symptoms, probable HF required HF diagnosed by a physician and patient receiving medical treatment for HF. Definite HF required one or more other criteria, such as pulmonary oedema/congestion by chest X-ray; dilated ventricle or poor LV systolic function by echocardiography or ventriculography; or evidence of LV diastolic dysfunction suggesting increased LV filling pressure. The documented LVEF at the diagnosis of HF was obtained from medical records. We considered participants not meeting any criteria, including only a physician's diagnosis of HF without any other evidence, as having no HF. Moreover, we studied four secondary outcome measures previously defined in the MESA based on pre-specified clinical event definitions: (i) myocardial infarction (MI), resuscitated cardiac arrest, and death from coronary disease were classified as hard coronary events; (ii) composite of all coronary events additionally included definite angina and probable angina followed by revascularization; (iii) hard cardiovascular events encompassed hard coronary events plus fatal and nonfatal stroke; and (iv) the composite endpoint of all cardiovascular events was defined by deaths related to atherosclerotic diseases and any of the coronary and hard cardiovascular events.
Statistical analysis
Baseline characteristics are presented as mean ± standard deviation or proportions. For the correlation analysis, the Pearson correlation coefficient was used. We used Ecc-global and Ecc-mid as independent variables. Receiver-operating characteristic (ROC) curve analysis was used to determine optimal cut-off values for incident HF with continuous Ecc. The best cut-off value was defined as the point with the highest sum of the sensitivity and specificity. The overall event-free survival rates were calculated using the Kaplan–Meier analysis and the event rates were compared using the log-rank test. The overall adequacy of the final parsimonious risk prediction model was evaluated by the c-statistics for the censored survival data using the Cox regression model of Uno et al.17 Cox proportional hazards regressions for the probability distribution of tagged MRI scan time to HF or other cardiovascular events were performed with conventional risk factors and MRI-derived indices as covariates. Variables significant in the univariate analysis (P < 0.05) were included in the multiple Cox regression analysis (stepwise forward and enter method). For the analysis with interim MI, a time-dependent covariate was used. The incremental prognostic value of covariates was assessed by stepwise changes in model χ2 values of the Cox proportional hazards model. We also evaluated the added predictive ability of Ecc for the distributions of time to HF using ‘Net Reclassification Improvement’ (NRI) and ‘Integrated Discrimination Index’ (IDI).18 We calculated the predicted probabilities of having HF within 6 years using the Cox Proportional Hazard models with and without Ecc for each MESA participant who had the observed covariates. All statistical analyses were performed using SPSS (version 15.0, SPSS, Inc., Chicago, IL, USA), Splus 8 (TIBCO, Palo Alto, CA, USA), and the ‘survC1’ statistical package in R. Two-sided P-values <0.05 were considered to be significant.
Results
Baseline clinical characteristics and the global left ventricular functional index
The mean age of the study sample was 65 ± 10 years. Forty-seven per cent of the participants were female, 47% of the participants had a history of hypertension and 14% had diabetes. The mean LVEF was 69 ± 8% and the LV mass index was 78.7 ± 17.1 g/m2 (men: 85.8 ± 17.5 g/m2, women: 70.5 ± 12.8 g/m2, P < 0.001) (Table 1).
Table 1.
Baseline clinical characteristics and global left ventricular functional indices
| Variable | |
|---|---|
| Age, years | 64.9 ± 9.8 |
| Females, n (%) | 835 (47) |
| Ethnicity, n (%) | |
| Caucasian | 520 (29) |
| African American | 509 (29) |
| Hispanic | 475 (27) |
| Chinese American | 264 (15) |
| Risk factors | |
| Current smoking, n (%) | 201 (11) |
| Hypertension, n (%) | 836 (47) |
| Normal/IFG/untreated/treated diabetesa | 1214 (69)/311 (18)/53 (3)/188 (11) |
| Body mass index, kg/m2 | 27.7 ± 4.7 |
| Estimated GFR, mL/min | 80.7 ± 18.1 |
| Haemodynamic parameters | |
| Systolic blood pressure, mmHg | 127.6 ± 20.8 |
| Diastolic blood pressure, mmHg | 71.8 ± 10.2 |
| Pulse pressure, mmHg | 55.8 ± 16.8 |
| Heart rate, b.p.m. | 62.6 ± 9.6 |
| Ventricular function or geometry | |
| LV ejection fraction, % | 69.0 ± 7.8 |
| LV end-diastolic volume, mL | 123.4 ± 31.9 |
| LV end-systolic volume, mL | 39.2 ± 17.7 |
| LV mass index, g/m2 | 78.7 ± 17.1 |
| LV massadj, g/m0.54. kg0.61 | 103.8 ± 19.6 |
| LV mass/volume, g/mL | 1.2 ± 0.3 |
| LVESWS, kPa | 8.9 ± 2.5 |
| Current use of cardiovascular medication | |
| Any ACE inhibitors/ARBs, n (%) | 352 (20) |
| Any β-blockers, n (%) | 182 (10) |
| Any calcium channel blockers, n (%) | 266 (15) |
| Any diuretics, n (%) | 228 (13) |
| Aspirin, n (%) | 446 (25) |
| Any statins, n (%) | 300 (17) |
IFG, impaired fasting glucose; GFR, glomerular filtration rate; LV, left ventricular; Ea, effective arterial elastance; Ees, end-systolic elastance; LVESWS, LV end-systolic wall stress; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
aTwo cases are missing.
Patterns of regional circumferential strain and their haemodynamic determinants
Average Ecc-global was −17.0 ± 2.4%. The average values for the basal, mid, and apical slice were −15.6 ± 3.3, −17.5 ± 2.7, and −18.0 ± 2.7%, respectively, reflecting an increase in myocardial shortening from the base to the apex in a stepwise fashion. Anterior and lateral segments had more negative strain values than posterior and septal segments (Supplemental material online, Appendix). Women also had more negative Ecc than men (−17.8 ± 2.7 vs. −17.2 ± 2.7%, P < 0.001 with Ecc-mid) reflecting greater myocardial function. Diabetes was related to more positive Ecc than non-diabetic individuals (−16.8 ± 2.6 vs. −17.6 ± 2.7%, P < 0.001 with Ecc-mid) reflecting reduced function. Higher heart rate was also related to more positive Ecc-mid (r = 0.164, P < 0.001) reflecting reduced function. Similarly, greater LV mass (r = 0.224, P < 0.001), LVESV (r = 0.219, P < 0.001), LVEDV (r = 0.066, P = 0.008), lower LVEF (r = −0.305, P < 0.001), and SV (r = −0.091, P < 0.001) were all related with a more positive Ecc-mid reflecting reduced myocardial function. Circumferential strain was significantly correlated with LVESWS (r = 0.145, P < 0.001), as an index of LV afterload.
Clinical follow-up results
The mean duration of follow-up after tagged MR scanning was 5.5 ± 1.3 years. Thirty nine (2.2%) participants experienced incident HF. Among the HF patients, 22 (56%) experienced HF with LV EF <50%, 11 (28%) had HF with preserved EF (LVEF ≥50%), and 6 participants did not have documented LVEF at the time that HF was diagnosed. Eleven (28%) participants with HF had interim MI during the follow-up. Moreover, 79 (4.5%) and 108 (6.1%) participants in the study cohort who sustained at least one of the outcomes used in the composite endpoints of ‘all coronary’ and ‘all cardiovascular’ events, respectively.
Prediction of heart failure and atherosclerotic events by circumferential strain
The area under the ROC curve (AUC) was 0.646 for Ecc-global (P = 0.002) and 0.664 for Ecc-mid (P = 0.001), respectively. The optimal cut-off values of Ecc-global and Ecc-mid for the prediction of HF were −16.3% (sensitivity of 59% and specificity of 66%) and −16.9% (sensitivity of 67% and specificity of 61%), respectively. The cumulative hazard of HF was significantly higher in participants with Ecc-global worse than −16.3% (P = 0.001) and Ecc-mid LV worse than −16.9% (P = 0.002) by the log-rank test (Figure 2). For comparison, the AUCs of the LV mass index, the best predictor for incident HF and all cardiovascular events was 0.719 (P < 0.001; 0.714 and P < 0.001 with LV massadj) and 0.626 (P < 0.001), respectively. Throughout the multivariable model proportionality assumption held. In the univariate analysis, using the Cox proportional hazard model, age, diabetes status (normal, impaired fasting glucose, untreated diabetes, treated diabetes), hypertension and interim MI were significantly associated with incident HF. For MRI-based parameters, LV mass index, LVEF, LVEDV, LVESWS, SV, and Ecc (both global and mid) were significantly associated with incident HF. The relationship between Ecc and incident HF was attenuated after controlling for LVESWS, LVEDV, or SV, but it remained significant. However, LVEDV demonstrated significant colinearity with the LV mass index (r = 0.614, P < 0.001), and it was not significant (P = 0.219) after controlling for the LV mass index. In the multivariable analysis, age, LV mass index, interim MI, and Ecc-mid were significantly related to incident HF [hazard ratio (HR): 1.15 per 1%, 95% CI: 1.01–1.31, P = 0.03]. When SV (1.16; 1.02–1.32, P = 0.02) or LVESWS was added into the model, Ecc-mid was still remained significant (Table 2). The relationship remained significant when the LV mass index was replaced by the LV massadj. To avoid overfitting problems in limited events number, we performed stepwise multivariable analysis with age, LV mass index, Ecc-mid, and interim MI, which are the most significant variable in the univariate analysis. Circumferential strain -mid remain significant. In addition for the Cox model with age, LV mass index, Ecc-mid, and interim MI as predictors, the overall c-statistics (for τ = 6) is 0.850 with 95% confidence interval (0.766, 0.933).
Figure 2.
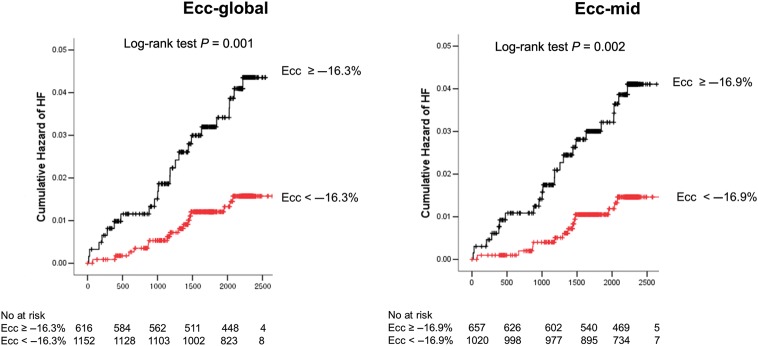
The Kaplan–Meier curve of cumulative hazard of incident heart failure according to circumferential strain. Circumferential strain-global and Ecc-mid.
Table 2.
Univariate and multivariable Cox proportional hazards analysis for incident HF
| Variable | Univariate analysis |
Multivariable analysis (enter) |
Multivariable analysis (stepwise forward) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age, per year | 1.06 | 1.02–1.10 | 0.001 | 1.07 | 1.03–1.11 | 0.002 | 1.06 | 1.02–1.11 | 0.002 |
| Male | 1.47 | 0.77–2.80 | 0.24 | ||||||
| Ethnicity | |||||||||
| Caucasian | 0.50 | 0.22–1.13 | 0.10 | ||||||
| African American | 1.40 | 0.73–2.68 | 0.32 | ||||||
| Hispanic | 1.38 | 0.71–2.68 | 0.35 | ||||||
| Chinese | 0.90 | 0.35–2.28 | 0.81 | ||||||
| Presence of diabetes | 1.71 | 0.79–3.72 | 0.18 | ||||||
| Diabetes status | 1.36 | 1.05–1.77 | 0.02 | 1.17 | 0.87–1.57 | 0.31 | |||
| Hypertension | 2.01 | 1.04–3.86 | 0.037 | 0.67 | 0.31–1.46 | 0.29 | |||
| Heart rate, per b.p.m. | 1.02 | 0.98–1.05 | 0.34 | ||||||
| Annual family income, dollar | 0.92 | 0.84–1.01 | 0.07 | ||||||
| Current smoking | 1.22 | 0.48–3.12 | 0.68 | ||||||
| Hypertensive medication | 1.72 | 0.92–3.23 | 0.09 | ||||||
| Statin use | 1.23 | 0.57–2.70 | 0.60 | ||||||
| Aspirin use | 0.75 | 0.35–1.64 | 0.47 | ||||||
| Estimated GFR, per mL/min | 0.99 | 0.97–1.00 | 0.14 | ||||||
| LV mass index, per g/m2 | 1.05 | 1.03–1.06 | <0.001 | 1.04 | 1.02–1.06 | <0.001 | 1.04 | 1.02–1.05 | <0.001 |
| LV massadja, per g | 1.04 | 1.03–1.04 | <0.001 | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–10.4 | <0.001 |
| LV end-diastolic volume, per mL | 1.02 | 1.01–1.03 | <0.001 | ||||||
| Mass/volume ratio | 1.06 | 0.95–8.85 | 0.06 | ||||||
| LV ejection fraction, per % | 0.92 | 0.88–0.95 | <0.001 | 1.01 | 0.95–1.07 | 0.84 | |||
| Stroke volume, per mL | 1.02 | 1.00–1.03 | 0.04 | ||||||
| LVESWS, per KPa | 1.22 | 1.10–1.35 | <0.001 | 1.13 | 0.98–1.31 | 0.10 | |||
| Interim myocardial infarction (time dependent) | 19.28 | 9.59–38.74 | <0.001 | 10.27 | 4.60–22.92 | <0.001 | 15.84 | 7.45–33.70 | <0.001 |
| Ecc-global, per % | 1.24 | 1.12–1.37 | <0.001 | 1.15 | 0.002–1.33 | 0.047 | 1.14 | 1.00–1.30 | 0.045 |
| Ecc-midb, per % | 1.25 | 1.13–1.37 | <0.001 | 1.18 | 1.03–1.35 | 0.015 | 1.18 | 1.05–1.33 | 0.007 |
See abbreviations in Table 1.
aIn replacement of LV mass index.
bAverage Ecc-global in multivariate analysis.
In a subgroup analysis including only individuals with baseline LV EF ≥50% (n = 1645, 93%), Ecc was related to incident HF independent of age, diabetes status, and hypertension (1.18, 1.04–1.33, P = 0.011 with Ecc-global; 1.18, 1.05–1.32, P = 0.006 with Ecc-mid), but was attenuated after the introduction of the LV mass index into the model (P > 0.05). Relationships of Ecc-global and Ecc-mid with composite coronary events were borderline while they were statistically significant with composite atherosclerotic cardiovascular disease in univariate analysis. Their relationships were also attenuated after introduction of the LV mass index into the model (Table 3).
Table 3.
Unadjusted hazard ratios and hazard ratios after adjustment of the left ventricular mass index for heart failure and atherosclerotic adverse outcomes
| Unadjusted |
After adjustment for LV mass index |
|||
|---|---|---|---|---|
| Ecc-global/Ecc-mid |
Ecc-global/Ecc-mid |
|||
| HRa (95% CI) | P-value | HR (95% CI) | P-value | |
| Hard CHD (n = 51) | 1.11 (0.998–1.23) | 0.055 | 1.09 (0.98–1.22) | 0.109 |
| 1.11 (1.01–1.22) | 0.038 | 1.09 (0.99–1.21) | 0.089 | |
| All CHD (n = 79) | 1.09 (1.00–1.19) | 0.040 | 1.07 (0.98–1.17) | 0.120 |
| 1.10 (1.02–1.19) | 0.017 | 1.08 (0.99–1.17) | 0.068 | |
| Hard CVD (n = 77) | 1.09 (1.00–1.19) | 0.041 | 1.07 (0.98–1.18) | 0.127 |
| 1.11 (1.03–1.20) | 0.010 | 1.09 (1.00–1.18) | 0.048 | |
| All CVD (n = 108) | 1.10 (1.03–1.18) | 0.009 | 1.08 (0.99–1.16) | 0.059 |
| 1.11 (1.04–1.19) | 0.001 | 1.09 (1.02–1.17) | 0.016 | |
| Heart failure (n = 39) | 1.24 (1.12–1.37) | <0.001 | 1.21 (1.07–1.36) | 0.002 |
| 1.25 (1.13–1.37) | <0.001 | 1.22 (1.09–1.36) | 0.001 | |
CHD, coronary heart disease; CVD, cardiovascular disease.
aPer 1% increase in Ecc.
Incremental prognostic value of circumferential strain for incident heart failure
Circumferential strain -mid had an incremental predictive value not only to traditional risk factors (age, diabetes status, and hypertension), but also to the model containing traditional risk factors, LVEF and the LV mass index (model χ276.6 vs. 82.4, P = 0.04). Category less NRI after adding Ecc-mid to the model (age, diabetes status, hypertension, LVEF, and LV mass index) was 0.45 (P = 0.01, SE = 0.18, z = 2.53), and IDI was 0.03 (P = 0.002, SE = 0.01, z = 3.08). However, neither LVESWS nor SV provides an incremental value on the LV mass index. Circumferential strain -mid still provided an additive value, when LVESWS or SV was introduced (Figure 3).
Figure 3.
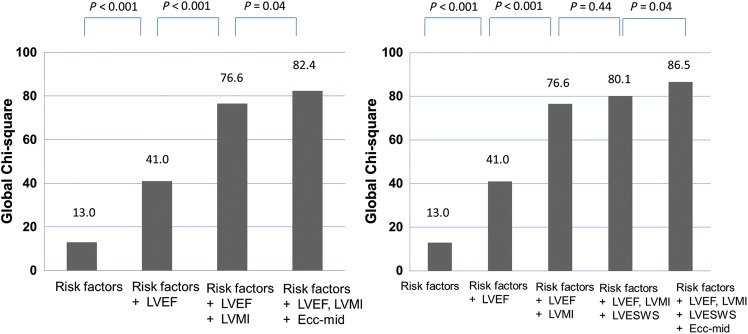
An incremental predictive value of variables for incident heart failure. Model χ2 values are presented for a series of Cox models. Risk factors include age, diabetes status, hypertension; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; LVESWS, and left ventricular end-systolic wall stress.
Discussion
In this study, we documented that regional myocardial Ecc provides a significant-independent prognostic value for the future development of symptomatic HF. Previous population-based studies focused on the association between asymptomatic LV dysfunction (assessed by reduced LVEF or impaired regional wall thickening) and future cardiovascular events.9,11,19 Moreover, the relative prognostic contributions of strain vs. EF or LV mass have not been explored previously. Therefore, our study is the first to demonstrate the importance of mid-wall regional Ecc, which has been shown to be less-load dependent and more closely related to wall stress than other indices of LV myocardial performance, as a predictor of cardiovascular events in a general multi-ethnic population. Finally, the study supports the use of myocardial strain as a more reproducible method to assess LV function in population-based studies.
Asymptomatic regional myocardial dysfunction and incident cardiovascular events
Although there is still debate regarding the relationship between asymptomatic LV dysfunction and future cardiovascular events, several studies support the hypothesis that asymptomatic reduced LVEF is related to the future development of HF and coronary events.8,9 The commonly used cut-off value for LVEF is 50%,9,10 and in our study most participants (93%) fell into the ‘preserved EF’ group when using this specific cut-off value. In this group, therefore, risk stratification is largely based on clinical risk factors as well as the presence of adverse, ventricular remodelling, and/or diastolic function. Within this subgroup, we documented a wide spectrum of regional myocardial function. Within asymptomatic subjects, risk factors, such as hypertension and diabetes (also supported by our results), have been related to circumferential or longitudinal fibre dysfunction through mechanisms that include microvascular dysfunction, fibrosis, or steatosis.20 Intrinsic myocardial function indexed as Ecc predicted HF independent of age, hypertension, and diabetes status within this subgroup, but was significantly attenuated after adding the LV mass index to the model. In this regard, our work suggests that reduced Ecc contributes to incident HF to some extent at least through adverse LV remodelling. Importantly, reduced circumferential shortening also had a significant contribution over traditional risk factors including the LV mass to the development of atherosclerotic cardiovascular events in our study.
Potential mechanisms of progression to heart failure
The LV myocardium is largely composed of fibres oriented in the longitudinal and circumferential directions.1 These fibres generate circumferential and longitudinal shortening, radial thickening, and LV torsion to determine SV. Among these fibres, circumferential fibres are predominant and circumferential shortening is the main determinant of SV.1 Regional circumferential myocardial dysfunction may represent a response to increased myocardial wall stress and/or reflect local alterations of myocardial material properties such as fibrosis or ischaemia due to macro- or microvascular disease. In our analysis, Ecc was significantly related to LVESWS. Elevated afterload or LV wall tension has been suggested to contribute to progressive myocardial dysfunction, including systolic and diastolic dysfunction.21,22 This concept is further supported by the fact that the relationship between Ecc and incident HF was attenuated after adjustment of LVESWS in this study. Therefore, the relationship between Ecc and future development of HF at the population level might be partly mediated by increased LVESWS. However, because such concept has been derived from associations measured in a population. Experimental clinical and/or subclinical studies are needed to further investigate this concept. Secondly, the finding that Ecc is significantly correlated to the LV mass index provides additional evidence that the relationship between reduced Ecc and incident HF is mediated by the adverse ventricular remodelling, which in turn might be related to reduced pre-load reserve and interstitial fibrosis.22,23 The remodelling process is thought to be closely related with neurohormonal activation in response to local alteration of myocardial stress and/or material projections such as interstitial fibrosis.24 However, in our study Ecc predicted HF independent of the LV mass index, suggesting that processes other than remodelling such as reduced regional myocardial perfusion due to microvascular dysfunction may also have contributed to progression of LV dysfunction, even in the absence of interim clinical MI, as we have previously demonstrated.25
Incremental prognostic value of circumferential strain
Reduced LVEF, SV, or increased LVESWS were each a significant predictor of HF in the univariate analysis. However, the predictive power of each of these indices was not greater than that of the LV mass index. Moreover, LVEF was no longer significant when the LV mass index was added to the multivariable model. In contrast, Ecc provided an incremental prognostic value in addition to the LVEF and LV mass index. Therefore, reduced mid-wall circumferential shortening combined with an increased LV mass may have synergistic effects on the development of HF. This finding may have been caused by the large proportion of our sample that had hypertension (47%) among whom mid-wall mechanics have been reported to be significantly related to prognosis.2 However, due to a small number of events and the absence of generally accepted risk probability category of HF, we used rather category-less NRI as a continuous analysis to calculate the incremental prognostic information of Ecc. Our results support the hypothesis that Ecc can be used as an additional parameter for the risk stratification of subclinical HF among asymptomatic individuals without previous history of heart disease.
Limitations
We did not independently study the effect of diastolic dysfunction, but instead focused on systolic myocardial deformation in this analysis. Also, the number of events related to HF was small in this cohort of asymptomatic individuals free of cardiovascular disease at baseline. Therefore, we were not able to separate those with the study HF with preserved vs. reduced EF due to a small sample size. Moreover, we included only Ecc in this study due to the lack of longitudinal strain measurements in the MESA imaging acquisition protocol and less reproducibility of the strain rate compared with circumferential shortening by tagged MRI. Finally, we were unable to investigate any variation by ethnicity due to sample size restrictions.
Conclusions
Myocardial Ecc assessed by tagged MRI provides independent and incremental prognostic information on the development of HF over and above traditional risk factors, the LV mass index, and LVEF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the NIH and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- 1.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–2587. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 2.de Simone G, Devereux RB, Koren MJ, Mensah GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265. doi: 10.1161/01.cir.93.2.259. [DOI] [PubMed] [Google Scholar]
- 3.Yeon SB, Reichek N, Tallant BA, Lima JA, Calhoun LP, Clark NR, Hoffman EA, Ho KK, Axel L. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J Am Coll Cardiol. 2001;38:555–561. doi: 10.1016/s0735-1097(01)01397-3. [DOI] [PubMed] [Google Scholar]
- 4.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–624. doi: 10.1016/j.jacc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events; The MESA (Multi-Ethnic Study of Atherosclerosis) Study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JV, McDonagh TA, Davie AP, Cleland JG, Francis CM, Morrison C. Should we screen for asymptomatic left ventricular dysfunction to prevent heart failure? Eur Heart J. 1998;19:842–846. doi: 10.1093/eurheartj/19.6.842. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 10.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Eng J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 11.Yan RT, Bluemke DA, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, McClelland R, Lima JA. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events: MESA (Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2011;57:1735–1744. doi: 10.1016/j.jacc.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Castillo E, Osman NF, Rosen BD, El-Shehaby I, Pan L, Jerosch-Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase(HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 14.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Alter P, Rupp H, Rominger MB, Klose KJ, Maisch B. A new methodological approach to assess cardiac work by pressure–volume and stress–length relations in patients with aortic valve stenosis and dilated cardiomyopathy. Pflugers Arch Eur J Physiol. 2008;455:627–636. doi: 10.1007/s00424-007-0323-2. [DOI] [PubMed] [Google Scholar]
- 16.Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK, Rosen BD, McClelland RL, Bluemke DA, Lima JA. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2010;31:875–882. doi: 10.1093/eurheartj/ehp454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the c-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Yeboah J, Rodriguez CJ, Stacey B, Lima JA, Liu S, Carr JJ, Hundley WG, Herrington DM. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA) Circulation. 2012;126:2713–2719. doi: 10.1161/CIRCULATIONAHA.112.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S, Siebelink HM, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538–2544. doi: 10.1161/CIRCULATIONAHA.110.955542. [DOI] [PubMed] [Google Scholar]
- 21.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 22.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease: insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–991. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 24.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 25.Rosen BD, Lima JA, Nasir K, Edvardsen T, Folsom AR, Lai S, Bluemke DA, Jerosch-Herold M. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;114:289–297. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


