Abstract
Aims
Magnetic resonance (MR) imaging is widely used for diagnostic imaging in medicine as it is considered a safe alternative to ionizing radiation-based techniques. Recent reports on potential genotoxic effects of strong and fast switching electromagnetic gradients such as used in cardiac MR (CMR) have raised safety concerns. The aim of this study was to analyse DNA double-strand breaks (DSBs) in human blood lymphocytes before and after CMR examination.
Methods and results
In 20 prospectively enrolled patients, peripheral venous blood was drawn before and after 1.5 T CMR scanning. After density gradient cell separation of blood samples, DNA DSBs in lymphocytes were quantified using immunofluorescence microscopy and flow cytometric analysis. Wilcoxon signed-rank testing was used for statistical analysis. Immunofluorescence microscopic and flow cytometric analysis revealed a significant increase in median numbers of DNA DSBs in lymphocytes induced by routine 1.5 T CMR examination.
Conclusion
The present findings indicate that CMR should be used with caution and that similar restrictions may apply as for X-ray-based and nuclear imaging techniques in order to avoid unnecessary damage of DNA integrity with potential carcinogenic effect.
Keywords: Cardiac MRI, DNA damage, γ-H2AX, Flow cytometry, Immunofluorescence microscopy
See page 2337 for the editorial comment on this article (doi:10.1093/eurheartj/eht214)
Introduction
Magnetic resonance (MR) imaging is a widely used and well-established non-invasive medical diagnostic imaging tool. By using a static and a gradient magnetic field in combination with a radiofrequency field (RF), MR provides excellent contrast among different tissues of the body including the brain, musculoskeletal system, and the heart. Although long-term effects on human health from exposure to strong static magnetic fields seem unlikely,1 acute effects such as vertigo, nausea, change in blood pressure, reversible arrhythmia,2 and neurobehavioural effects have been documented from occupational exposition to 1.5 T.3 Cardiac MR (CMR) imaging requires some of the strongest and fastest switching electromagnetic gradients available in MR exposing the patients to the highest administered energy levels accepted by the controlling authorities.4 Studies focusing on experimental teratogenic5–9 or carcinogenic10–12 effects of MR revealed conflicting results. Since CMR is emerging as one of the fastest growing new fields of broad MR application,13 it is of particular concern that a recent in vitro study with CMR sequences has reported on CMR-induced DNA damages in white blood cells up to 24 h after exposure to 1.5 T CMR.4 It is in this context that the European Parliament,14 the International Commission on Non-Ionizing Radiation Protection (ICNIRP),15,16 and the World Health Organization (WHO)17 have urgently called for an action in order to evaluate adverse biological effects of clinical MR scanning.
The aim of the present study was to assess the impact of routine CMR scanning on DNA double-strand breaks (DSBs) of peripheral blood mononuclear cells (PBMCs) as a measure of the carcinogenic potential of this examination.
Methods
Twenty consecutive patients referred for cardiac evaluation were included. After obtaining written informed consent, 10 mL of peripheral blood was drawn before and after undergoing routine contrast (gadobutrolum, Gadovist, Bayer Schering Pharma, Germany) enhanced CMR examination18 on a 1.5 T MR scanner (Philips Achieva, Best, NL, USA) as approved by the local ethics committee (KEK-Nr. 849). PBMCs were obtained using density gradient separation (Histopaque 1077, Sigma-Aldrich) as previously established.19
The clinical CMR protocol used in our daily routine has been recently reported in detail.20 In brief, a commercially available MR scanner (Philips 1.5 T, Achieva, software release 3.2.1) equipped with a maximum gradient strength of 42 mT/m and a maximum gradient speed of 180 mT/m/ms was used. The following standard pulse sequences to generate images were used: gradient echo, steady-state free precession, FastSE, T2-weighted double-inversion black-blood spin-echo sequence for oedema imaging, balanced SSFP sequence for perfusion and inversion recovery segmented gradient echo sequence for late gadolinium enhancement.
DSBs were detected by immunofluorescence microscopy using a rabbit-anti-human phospho-histone γ-H2AX and a goat-anti-rabbit-AlexaFluor-488 antibody (CST Cell Signalling Technology, adapted from May et al.21). Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories) and the γ-H2AX foci per lymphocyte were visualized on an inverse confocal microscope (CLSM-Model SP5, Leica Microsystems) and quantified by a blinded observer.
With flow cytometry (FACScanto, BD Bioscience), DSBs were additionally quantified in T-lymphocytes22,23 previously identified by a mouse-anti-human CD3-APC antibody (Life Technologies). Based on forward and side light scattering, PBMCs were gated for viable single-cell events and proper compensation controls were used in flow cytometric analyses to correct for spectral overlap. Data from flow cytometric quantification (MFI, geometric mean of fluorescence intensity of γ-H2AXpositive T-lymphocytes) was evaluated using FlowJo software (V10.0.2, Tree Star, Inc.).
Based on a variation of γ-H2AX assessment at 20% as reported by Muslimovic et al.,22 an average difference in γ-H2AX findings reported in ex vivo experiments,4 aiming at alpha = 0.05 and a power (1 − β) of 0.8, the number of patients necessary was calculated between 10 and 15.
SPSS 20.0 (SPSS, Chicago, IL, USA) was used for all statistical analysis. The Shapiro–Wilk test was applied to exclude normal distribution of data sets. This was followed by testing for significant differences between DSBs before and after CMR examination by using the Wilcoxon signed-rank test. P-values of <0.05 (two-tailed) were considered statistically significant.
Results
Mean age of patients was 53 ± 13 years and 16 (80%) were males. Ten patients were referred for evaluation of cardiomyopathy and 10 for the assessment of myocardial ischaemia. The mean CMR scan duration was 68 ± 22 min with an average contrast media bolus of 15 ± 4 mL. The patient baseline characteristics are given in Table 1.
Table 1.
Patient baseline characteristics (n = 20)
| Age (years ± SD) | 53 ± 13 |
| BMI (kg/m2 ± SD) | 25 ± 4 |
| Male, n (%) | 16 (80) |
| Cardiovascular risk factors, n (%) | |
| Arterial hypertension | 6 (30) |
| Diabetes mellitus | 4 (20) |
| Dyslipidaemia | 4 (20) |
| Smoking | 2 (10) |
| Positive family history | 1 (5) |
| Medications, n (%) | |
| Aspirin | 7 (35) |
| Beta-blocker | 9 (45) |
| ACE/angiotensin II inhibitor | 8 (40) |
| Statin | 7 (35) |
SD, standard deviation; BMI, body mass index.
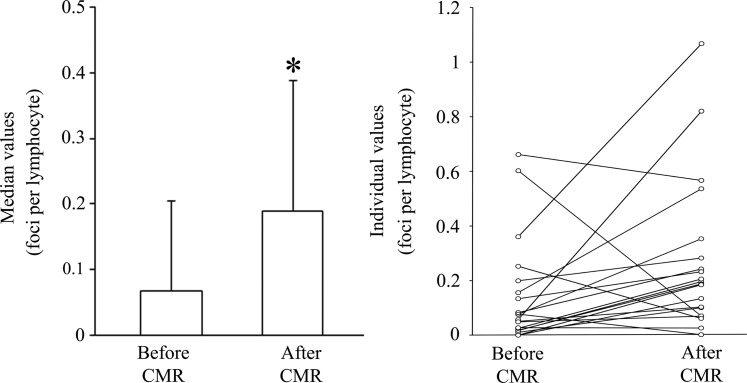
By immunofluorescence microscopy (Figure 1), the median number of DSBs (foci, Table 2) per lymphocyte in baseline samples was 0.066 (range: 0–0.661) and increased significantly (P < 0.05) after CMR exposure to 0.190 (range: 0–1.065, Figure 2).
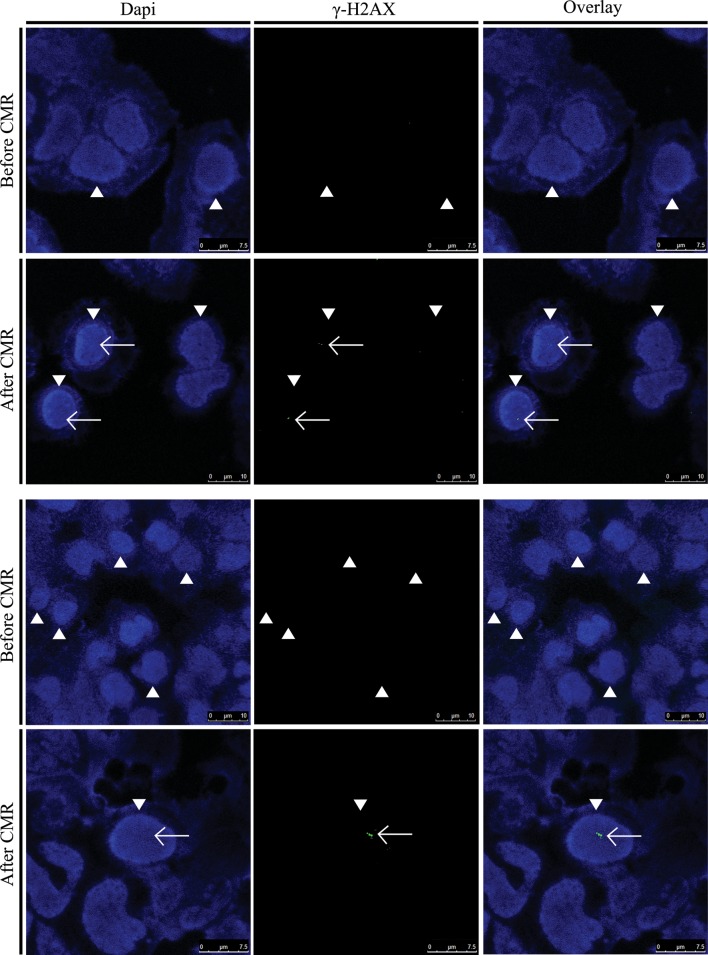
Figure 1.
Visualization of double-strand breaks (DSBs) in nuclei (arrow heads) of human lymphocytes of two patients before and after cardiac magnetic resonance scans by immunofluorescence microscopy. DSBs (foci, white arrows) are detected by γ-H2AX staining (green).
Table 2.
Increase in double-strand breaks after cardiac magnetic resonance assessed by immunofluorescence
| Microscopy foci per lymphocyte |
Flow cytometry MFI |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Mean | 0.143 | 0.270* | 2989 | 3395* |
| SD | 0.191 | 0.227 | 850 | 906 |
| Median | 0.066 | 0.190* | 2758 | 3232* |
| MAD | 0.137 | 0.199 | 640 | 696 |
| IQR | 0.169 | 0.257 | 1133 | 1198 |
IF, immunofluorescence (units are foci per lymphocyte); MFI, geometric mean of T-lymphocyte fluorescence intensity (arbitrary units); γ-H2AX, marker of DSBs; SD, standard deviation; MAD, median absolute deviation; IQR, interquartile range.
*Indicates P < 0.05 vs. before.
Figure 2.
Amount of double-strand breaks before and after cardiac magnetic resonance (CMR) scan by immunofluorescence microscopy. After CMR scanning, there was a significant increase (*P < 0.05) in γ-H2AX foci per lymphocyte by immunofluorescence microscopy. Bars indicate median values with median absolute deviation (left panel) and individual values are interconnected with a line (right panel).
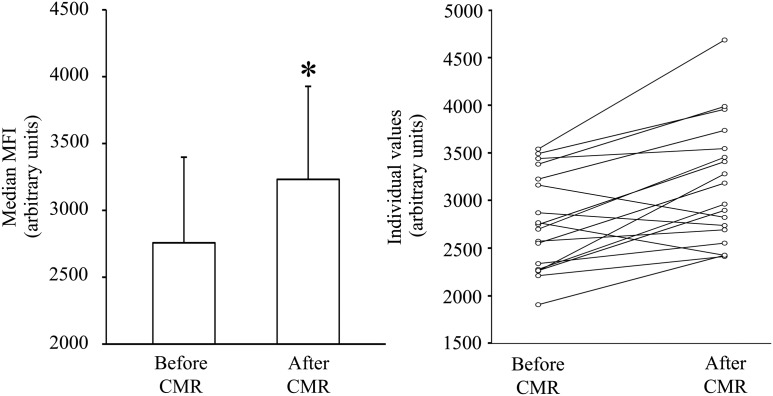
In T-lymphocytes, flow cytometry (Figure 3) revealed a median MFI (arbitrary units) of 2758 (range: 1907–5109) before and 3232 (range: 2413–5484) after CMR (P < 0.005, Table 2 and Figure 4).
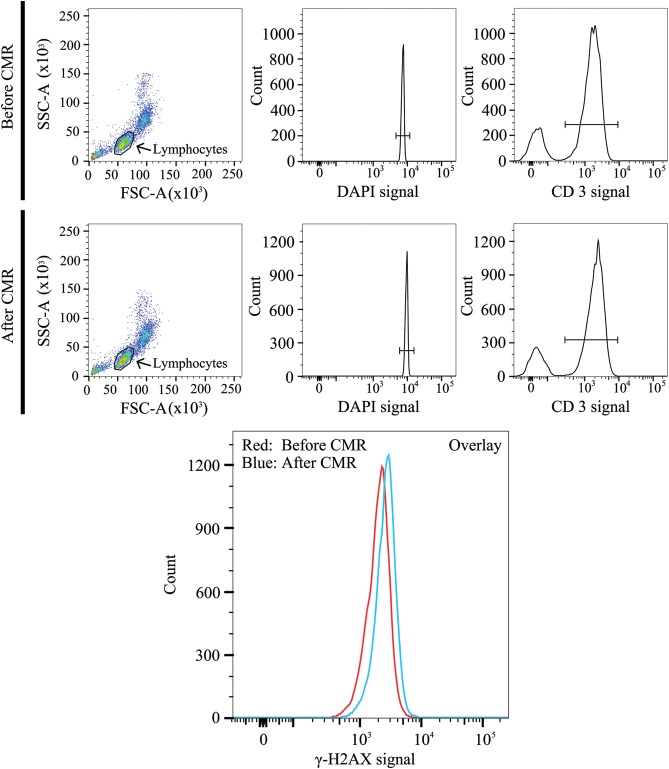
Figure 3.
Flow cytometric analysis of double-strand breaks (γ-H2AXpositive T-lymphocytes) before and after cardiac magnetic resonance (CMR) scan. T-lymphocytes were readily identified by representative dot plots and histograms (lymphocytes, DAPI, and CD3). The shift of the left curve (red, before CMR) to the right curve (blue, after CMR) in the presented overlay indicates an increase in double-strand breaks (γ-H2AXpositive T-lymphocytes). SSC-A: side scatter channel area. FSC-A: forward scatter channel area. DAPI: 4′,6-diamidino-2-phenylindole, counterstaining cell nuclei. CD3: mouse-anti-human CD3-APC antibody counterstaining specifically the T-lymphocytes.
Figure 4.
Amount of double-strand breaks before and after cardiac magnetic resonance scan by flow cytometry of γ-H2AXpositive T-lymphocytes using geometric mean fluorescence intensity (MFI). The median MFI increased significantly after cardiac magnetic resonance scanning (*P < 0.005, left panel). Individual values are interconnected with a line (right panel).
Discussion
We show here that clinical routine CMR scanning exerts genotoxic effects. Although many experimental in vitro studies have suggested DNA damage after exposure to MR imaging, we present the first in vivo results documenting that contrast CMR scanning in daily clinical routine is associated with increased lymphocyte DNA damage.
The different components of the magnetic field during CMR may have contributed to the observed DNA damage. The gradient field generated during MR scanning includes extremely low frequencies (ELF), which have been classified by the International Agency for Research on Cancer (IARC) as possible human carcinogen (group 2B)24 based on a large body of literature on the genotoxic effects of ELF magnetic fields.25–28 The latter seem to be involved directly and indirectly in DNA and chromosomal damage by inducing reactive oxygen species.29 Similarly, DNA damage and chromosome alterations have been discussed after exposure to RF.
Our results do not allow commenting on the persistence of the induced DNA damage, although this is a key issue of genetic risk assessment, because damage can trigger DNA instability and exert tumourigenic effects. Due to the long time delay between DSB induction and resulting cancer development, our study cannot quantify such long-term effects as this was beyond the scope of the present study. This, however, is true in principle for any observation of DSB induction from any diagnostic radiation exposure including ionizing radiation, for which no direct observational proof of its adverse impact on outcome is available due to the small scale of damage and the long delay between exposure and event. In view of the growing use of new generation MR scanners with increasing magnetic field strength (higher Tesla), our results seem to support the suggestions of the ICNIRP for an urgent need of monitoring workers and for epidemiologic studies on subjects with high levels of exposure or particular conditions such as for example pregnant occupational workers.30
Despite activation of repair mechanisms, persistence of DNA damage has been found in human lymphocytes more than 24 h after exposing patients and blood samples to CMR scanning.4 Co-genotoxic effects of MR in combination with the administered gadolinium-based contrast material may further have contributed to DNA damage due to the potentiating effect of gadolinium-based contrast material and MR exposure.31 As in our study all patients underwent contrast enhanced CMR, reflecting widely used clinical practice,32 we cannot differentiate the precise contribution of the known genotoxic effect of the gadolinium-based contrast material from the effects of the magnetic field. However, the use of contrast material is generally an integrated part of CMR scanning and therefore our results may appropriately represent the effect of a routine CMR scan. The absolute amount of DNA damage is certainly larger in our study compared with previous in vitro studies, as the entire blood of each patient rather than a blood sample was exposed during CMR. According to the assumptions used in the field of radiation protection, an increased number of DNA damages confer a linearly increased risk of cancer. Conversely, even a low number of DSBs may represent a carcinogenic risk according to the linear-no threshold theory. Our results compare well to the more than two-fold increase in DSBs induced by CMR and assessed by immunofluorescence microscopy as reported by Simi et al.,4 which was substantially less pronounced than the almost six-fold increase observed after cardiac CT by Kuefner et al.33 Although only a few data are available using FACS analyses for this low scale of signal, the excellent agreement between microscopy and FACS over a large range of signal including the present study strengthens the validity of our results.34
Of note, observations in several subsets of patients seem to suggest increased sensibilities to MRI exposition, as higher susceptibility for DNA damage by MRI has been found for example in lymphocytes of patients with Turner's syndrome.35 Thus, inappropriate examinations should be avoided and CMR should be used with caution and similar restrictions may apply as for X-ray-based and nuclear imaging techniques where the potential harm is carefully weighted against the obvious benefit offered by each examination in order to avoid unnecessary damage of DNA integrity with potential carcinogenic effect.
Funding
Grants from the Swiss National Science Foundation to P.A.K. and to M.F. are gratefully acknowledged.
Acknowledgements
We thank Christiane Koenig, Jose Maria Mateos, PhD, Stefano Ferrari, PhD, and Florian Mair, MSc, from the University of Zurich, Zurich, Switzerland, and Arnold von Eckardstein, MD, Institute for Clinical Chemistry, University Hospital Zurich, Zurich, Switzerland, for their invaluable advice and excellent technical support for the immunofluorescence analysis.
Conflict of interest: none declared.
References
- 1.Kangarlu A, Robitaille P. Biological effects and health implications in magnetic resonance imaging. Concepts Magn Reson. 2000;12:321–359. [Google Scholar]
- 2.Franco G, Perduri R, Murolo A. Health effects of occupational exposure to static magnetic fields used in magnetic resonance imaging: a review. Med Lav. 2008;99:16–28. [PubMed] [Google Scholar]
- 3.de Vocht F, van-Wendel-de-Joode B, Engels H, Kromhout H. Neurobehavioral effects among subjects exposed to high static and gradient magnetic fields from a 1.5 Tesla magnetic resonance imaging system—a case-crossover pilot study. Magn Reson Med. 2003;50:670–674. doi: 10.1002/mrm.10604. [DOI] [PubMed] [Google Scholar]
- 4.Simi S, Ballardin M, Casella M, De Marchi D, Hartwig V, Giovannetti G, Vanello N, Gabbriellini S, Landini L, Lombardi M. Is the genotoxic effect of magnetic resonance negligible? Low persistence of micronucleus frequency in lymphocytes of individuals after cardiac scan. Mutat Res. 2008;645:39–43. doi: 10.1016/j.mrfmmm.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Carnes KI, Magin RL. Effects of in utero exposure to 4.7 T MR imaging conditions on fetal growth and testicular development in the mouse. Magn Reson Imaging. 1996;14:263–274. doi: 10.1016/0730-725x(95)02099-f. [DOI] [PubMed] [Google Scholar]
- 6.High WB, Sikora J, Ugurbil K, Garwood M. Subchronic in vivo effects of a high static magnetic field (9.4 T) in rats. J Magn Reson Imaging. 2000;12:122–139. doi: 10.1002/1522-2586(200007)12:1<122::aid-jmri14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Rodegerdts EA, Gronewaller EF, Kehlbach R, Roth P, Wiskirchen J, Gebert R, Claussen CD, Duda SH. In vitro evaluation of teratogenic effects by time-varying MR gradient fields on fetal human fibroblasts. J Magn Reson Imaging. 2000;12:150–156. doi: 10.1002/1522-2586(200007)12:1<150::aid-jmri16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Suzuki H, Suzuki K. Teratogenic effects of static magnetic field on mouse fetuses. Reprod Toxicol. 2006;22:118–124. doi: 10.1016/j.reprotox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Schiffer IB, Schreiber WG, Graf R, Schreiber EM, Jung D, Rose DM, Hehn M, Gebhard S, Sagemuller J, Spiess HW, Oesch F, Thelen M, Hengstler JG. No influence of magnetic fields on cell cycle progression using conditions relevant for patients during MRI. Bioelectromagnetics. 2003;24:241–250. doi: 10.1002/bem.10097. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S, Sheppard AR, Kaune WT, Poole C, Kelsh MA. A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Childhood Leukemia-EMF Study Group. Epidemiology. 2000;11:624–634. doi: 10.1097/00001648-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Repacholi MH, Greenebaum B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics. 1999;20:133–160. doi: 10.1002/(sici)1521-186x(1999)20:3<133::aid-bem1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Vijayalaxmi OG. Controversial cytogenetic observations in mammalian somatic cells exposed to extremely low frequency electromagnetic radiation: a review and future research recommendations. Bioelectromagnetics. 2005;26:412–430. doi: 10.1002/bem.20111. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattsson MO, Auvinen A, Bridges J, Norppa H, Schütz J. European Parliament and the Council. Research needs and methodology to address the remaining knowledge gaps on the potential health effects of EMF. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR); http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_024.pdf. (23 February 2013) [Google Scholar]
- 15.Vecchia P, Hietanen M, Ahlbom A, Anderson LE, Breitbart E, de Gruijl FR, Lin JC, Matthes R, Peralta APT, Söderberg P, Stuck BE, Swerdlow AJ, Taki M, Saunders R, Veyret B. International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines on limits of exposure to static magnetic fields. Health Phys. 2009;96:504–514. doi: 10.1097/01.HP.0000343164.27920.4a. [DOI] [PubMed] [Google Scholar]
- 16.Vecchia P, Hietanen M, Ahlbom A, Anderson LE, Breitbart E, de Gruijl FR, Lin JC, Matthes R, Peralta APT, Söderberg P, Stuck BE, Swerdlow AJ, Taki M, Saunders R, Veyret B. International Commission on Non-Ionizing Radiation Protection (ICNIRP). Amendment to the ICNIRP ‘Statement on medical magnetic resonance (MR) procedures: protection of patients’. Health Phys. 2009;97:259–261. doi: 10.1097/HP.0b013e3181aff9eb. [DOI] [PubMed] [Google Scholar]
- 17.Belyaev I. Static Fields Environmental Health Criteria No. 232. Geneva, Switzerland: World Health Organization, WHO; http://www.who.int/pehemf/publications/EHC_232_Static_Fields_full_document.pdf. (23 February 2013) [Google Scholar]
- 18.Myerson S, Francis J, Neubauer S. Cardiovascular Magnetic Resonance. 2nd ed. Oxford: Oxford University Press; 2010. [Google Scholar]
- 19.Winchester R, Ross G. Methods for enumerating lymphocyte populations. In: Rose NR, Friedman H, editors. Manual of Clinical Immunology. 1st ed. Washington, DC: American Society for Microbiology; 1976. pp. 64–76. [Google Scholar]
- 20.Fiechter M, Fuchs TA, Gebhard C, Stehli J, Klaeser B, Stahli BE, Manka R, Manes C, Tanner FC, Gaemperli O, Kaufmann PA. Age-related normal structural and functional ventricular values in cardiac function assessed by magnetic resonance. BMC Med Imaging. 2013;13:6. doi: 10.1186/1471-2342-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May MS, Brand M, Wuest W, Anders K, Kuwert T, Prante O, Schmidt D, Maschauer S, Semelka RC, Uder M, Kuefner MA. Induction and repair of DNA double-strand breaks in blood lymphocytes of patients undergoing (18)F-FDG PET/CT examinations. Eur J Nucl Med Mol Imaging. 2012;39:1712–1719. doi: 10.1007/s00259-012-2201-1. [DOI] [PubMed] [Google Scholar]
- 22.Muslimovic A, Ismail IH, Gao Y, Hammarsten O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protoc. 2008;3:1187–1193. doi: 10.1038/nprot.2008.93. [DOI] [PubMed] [Google Scholar]
- 23.Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int J Radiat Biol. 2009;85:369–376. doi: 10.1080/09553000902781147. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO), International Agency for Research on Cancer (IARC), Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-ionizing radiation, Part 1: static and extremely low-frequency (ELF) electric and magnetic fields. IARC Monogr Eval Carcinog Risks Hum. 2002;80:1–395. [PMC free article] [PubMed] [Google Scholar]
- 25.Jian W, Wei Z, Zhiqiang C, Zheng F. X-ray-induced apoptosis of BEL-7402 cell line enhanced by extremely low frequency electromagnetic field in vitro. Bioelectromagnetics. 2009;30:163–165. doi: 10.1002/bem.20461. [DOI] [PubMed] [Google Scholar]
- 26.Juutilainen J. Do electromagnetic fields enhance the effects of environmental carcinogens? Radiat Prot Dosimetry. 2008;132:228–231. doi: 10.1093/rpd/ncn258. [DOI] [PubMed] [Google Scholar]
- 27.Nordenson I, Mild KH, Andersson G, Sandstrom M. Chromosomal aberrations in human amniotic cells after intermittent exposure to fifty hertz magnetic fields. Bioelectromagnetics. 1994;15:293–301. doi: 10.1002/bem.2250150404. [DOI] [PubMed] [Google Scholar]
- 28.Winker R, Ivancsits S, Pilger A, Adlkofer F, Rudiger HW. Chromosomal damage in human diploid fibroblasts by intermittent exposure to extremely low-frequency electromagnetic fields. Mutat Res. 2005;585:43–49. doi: 10.1016/j.mrgentox.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JL, Singh NP, Lai H. Electromagnetic fields and DNA damage. Pathophysiology. 2009;16:79–88. doi: 10.1016/j.pathophys.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Bassen H, Bernhardt JH, Brix G, Lejeune JJ, Owen RD, de Seze R, Saunders R, Ueno S, Veyret B, Zaremba L. Medical magnetic resonance (MR) procedures: protection of patients. Health Phys. 2004;87:197–216. doi: 10.1097/00004032-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz S, Cece H, Kaya I, Celik H, Taskin A, Aksoy N, Kocyigit A, Eren MA. Impact of contrast enhanced MRI on lymphocyte DNA damage and serum visfatin level. Clin Biochem. 2011;44:975–979. doi: 10.1016/j.clinbiochem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Bruder O, Schneider S, Nothnagel D, Dill T, Hombach V, Schulz-Menger J, Nagel E, Lombardi M, van Rossum AC, Wagner A, Schwitter J, Senges J, Sabin GV, Sechtem U, Mahrholdt H. EuroCMR (European Cardiovascular Magnetic Resonance) registry: results of the German pilot phase. J Am Coll Cardiol. 2009;54:1457–1466. doi: 10.1016/j.jacc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Kuefner MA, Hinkmann FM, Alibek S, Azoulay S, Anders K, Kalender WA, Achenbach S, Grudzenski S, Lobrich M, Uder M. Reduction of X-ray induced DNA double-strand breaks in blood lymphocytes during coronary CT angiography using high-pitch spiral data acquisition with prospective ECG-triggering. Invest Radiol. 2010;45:182–187. doi: 10.1097/RLI.0b013e3181d3eddf. [DOI] [PubMed] [Google Scholar]
- 34.MacPhail SH, Banath JP, Yu TY, Chu EH, Lambur H, Olive PL. Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int J Radiat Biol. 2003;79:351–358. doi: 10.1080/0955300032000093128. [DOI] [PubMed] [Google Scholar]
- 35.Scarfi M, Prisco M, Lioi M, Zeni O, Della Noce M, Di Pietro R, Franceschi C, Iafusco D, Motta M, Bersani F. Cytogenetic effects induced by extremely low frequency pulsed magnetic fields in lymphocytes from Turner's syndrome subjects. Bioelectrochem Bioenerg. 1997;43:221–226. [Google Scholar]