Abstract
The history of the development of modern chemotherapy for tuberculosis (TB), largely due to the British Medical Research Council, is first described. There is a current need to shorten the duration of treatment and to prevent and cure drug-resistant disease. These aims will only be achieved if the way in which multidrug treatment prevents resistance from emerging and the reasons for the very slow response to chemotherapy are understood. Consideration of mutation rates to resistance and the size of bacterial populations in lesions makes it very unlikely that resistance would emerge spontaneously, leaving irregularity in drug taking and inadequate dosage as the main reasons for its occurrence. Slow response to treatment seems due to the presence of persister populations whose natural history is only partly known. In the future, we need to explore the persister state in patients and in experimental murine TB, and to take it into account in the design of future mouse experiments. The activity of rifamycins and pyrazinamide is being increased by a rise in rifamycin dosage and the inhalation of pyrazinoic acid. New drugs are gradually being brought into use, initially TMC207 and the nitroimadazoles, PA824 and OPC67683. They will need to be tested in new combination regimens for drug-susceptible and multi- and extensively drug-resistant disease.
Keywords: tuberculosis, treatment, drug resistance, bacterial persistence, new drugs
THE PAST
Initial studies
THE EFFECTIVE TREATMENT of tuberculosis (TB) started in 1946, with the introduction of streptomycin (SM, S). In the first clinical trial with a random allocation of patients to the regimens, undertaken by the Tuberculosis Research Unit (Director Philip Hart) of the British Medical Research Council (BMRC), SM given alone caused a dramatic reduction in immediate mortality and striking improvements in chest radiology and bacteriology,1 but the 5-year assessment showed that the patients who received SM eventually died in almost the same proportion and speed as those who did not receive it, due to the frequent emergence of SM resistance.2 A second BMRC clinical trial then showed that combined treatment with SM and para-aminosalicylic acid (PAS, P) greatly reduced the incidence of SM resistance.3,4 In 1952, isoniazid (INH, H) was introduced as a new wonder drug. Its efficacy stemmed from its low minimum inhibitory concentration (MIC) against Mycobacterium tuberculosis and its low toxicity. The usual dose of 200 mg daily at that time was at least 10 times greater than the minimal effective dose.5 Following the experience with PAS and SM, the activity of INH was explored in regimens containing INH alone, or INH with SM or PAS, in BMRC studies organised by Wallace Fox, who thereafter led the BMRC effort.6,7 Shortly after this period, the BMRC organised the first national survey of drug resistance in the United Kingdom to find that resistant strains were almost always resistant to only one of the three available drugs.8,9 This led John Crofton to explore a regimen starting with the three drugs, SM, PAS and INH (SPH/PH) so that two would be available for almost any resistant strain in the community, followed by a continuation phase of the two oral drugs, PAS and INH.10,11 Crofton’s experience led to a clinical trial under the auspices of the International Union Against Tuberculosis to assess this regimen.12 As there was a high drop-out rate, only 352 of 581 patients admitted completed their year of treatment, but among these there were no failures. The regimen of a year’s treatment in hospital with PAS and INH supplemented initially with SM (3SPH/9PH) was then widely adopted in Europe. However, as it required at least 1 year of treatment in hospital with very expensive drug bills due to the large amounts of PAS, this meant that the regimen could not be used widely in any but the richer countries.
Modern regimens
The further period of development, from the 1960s to 1986, when modern regimens were first clearly delineated, provided solutions for the major difficulties of the 3SPH/9PH regimen. BMRC studies in East Africa established that the far cheaper thioacetazone could be substituted for PAS.13 In 1960, the classic study at the Tuberculosis Chemotherapy Centre, Madras, India, under the direction of Fox, showed that domiciliary chemotherapy could be as effective as treatment in expensive hospitals or sanatoria.14,15 This study immediately raised the issue of how to ensure regular drug taking during a year of domiciliary treatment,16 an issue that led many years later to the DOTS strategy by the World Health Organization (WHO). The first programme to address this issue was the development of fully supervised intermittent regimens, mainly at Madras. A more fruitful approach lay in shortening the treatment period. During the 1950s and 1960s, the technique of long-term experiments on the treatment of TB in mice was explored at Cornell University, Ithaca, NY, USA, and established the remarkable ability of pyrazinamide (PZA, Z) to kill bacilli that persisted in organs after treatment with INH and SM.17 Later experiments at the Pasteur Institute in Paris established that rifampicin (RMP, R) could accelerate the killing of tubercle bacilli in mouse organs.18 These mouse experiments led to one of the most important BMRC clinical trials, which demonstrated that the addition of RMP or PZA to a basic 6-month regimen of SM+INH could radically reduce the relapse rate (Table 1).19 From this finding stemmed the development of modern short-course treatment in a series of clinical trials in East Africa, Hong Kong and Singapore, with a few later studies in Madras, Prague and Algeria.20 Among the more important findings of this series of trials were 1) the demonstration that there was bactericidal synergism between RMP and PZA, so that both were necessary for the most rapid sterilisation of lesions;21-23 2) the finding in three trials that RMP was an effective sterilising drug throughout treatment, whereas PZA was only sterilising during the initial intensive phase,24-26 which was assumed to be due to a change in lesional pH from mildly acidic during acute inflammation to neutral as inflammation died down;27 and 3) that the initial intensive phase should last 2 months. Treatment regimens with a continuation phase consisting of 4 months of RMP+INH were extensively trialled in Singapore, while those with an alternative continuation phase of 6 months of thioacetazone+INH were trialled in East Africa. When human immunodeficiency virus infection led to an increase in the toxicity of thioacetazone as to render it unusable, ethambutol (EMB, E) was given in its place. The 6-month regimen of 2HRZE/4RH was later shown to be much more effective than an 8-month regimen with a continuation phase of EH (2HRZE/6EH), particularly in patients whose organisms were initially resistant to INH.28 The WHO now recommends only the 6-month regimen.
Table 1.
Effect of the addition of thioacetazone (control), PZA or RMP to a basic 6-month SM+INH regimen on the relapse rate in a multicentre East African regimen study19
| Patients n |
Relapses % |
|
|---|---|---|
| SM+INH | 112 | 29 |
| SM+INH+thioacetazone | 104 | 22 |
| SM+INH+PZA | 153 | 8 |
| SM+INH+RMP | 152 | 3 |
PZA = pyrazinamide; RMP = rifampicin; SM = streptomycin; INH = isoniazid
THE PRESENT
The WHO estimates that TB is just beginning to decline under the effects of case finding and treatment; however, there were still 8.8 million cases and 1.5 million deaths in 2010.29 The main problems with current chemotherapy are first that the 6-month treatment regimen is too long, allowing opportunities for interruptions in drug taking that may lead to the emergence of drug resistance, as well as creating a serious burden for both patients and clinics. The second problem is the increasing prevalence of multidrug-resistant (MDR) strains of M. tuberculosis resistant to RMP and INH, and sometimes to injectables and fluoroquinolones as well (extensively drug-resistant [XDR]).30,31 Any solution to these problems depends on an understanding of the two theoretical issues underlying the success of chemotherapy, namely the prevention of the emergence of drug resistance by the simultaneous use of two or more antibacterial agents, and the reasons for the very slow killing of all M. tuberculosis in the lesions.
Prevention of drug resistance
Resistance arises against each of the anti-tuberculosis drugs by bacterial chromosomal mutations. While these mutations are rare events, a mutation early in multiplication produces a clone of resistant bacilli that are found more frequently. The mutation rates were thus found to be about 2.6 × 10−8 for INH and 2.2 × 10−10 for RMP, while the more useful estimates of the highest proportion of mutants that can be expected in an unselected bacterial population were found to be 3.5 × 10−6 for INH and 3.3 × 10−8 for RMP.32 A doubly resistant mutant might therefore be found in a population of about 1015 bacilli. A recent calculation has made these proportions somewhat greater.33 Given these estimates, it should be possible to estimate the chances of spontaneous resistance arising during simultaneous treatment with INH and RMP, providing we know the size of the bacterial population in the tuberculous lesions. Here we find that investigators refer back to a statement without any supporting evidence in a paper by Shimao34 that a lesion contains about 108 bacilli. We know that a diagnosis of TB may be made when a sputum smear contains bacilli; however, a substantial proportion of patients may have no detectable bacilli in smears but may have a positive culture. Another proportion will have no bacteriological evidence but only radiographic evidence of disease. It is thus clear that there is very wide variation in the size of the bacterial population when patients seek treatment at the start of chemotherapy. How do we estimate the sizes of these populations? Because lesions do not change rapidly in extent, the size of the multiplying population35 should be approximately equal to the number of bacilli being excreted in sputum.36 We can then estimate that a very high value of this population is 1010 bacilli, with 109 being more common as a high value, and with numbers ranging downwards in those with negative smears and even negative cultures. These considerations render it highly unlikely that resistance would ever arise even in large patient populations during continuous treatment with INH and RMP. Furthermore, during the initial phase of treatment, when bacterial populations are large, treatment is almost always with PZA, known to prevent resistance emerging,37 and sometimes EMB as well as RMP and INH. The origins of drug resistance appear to be due to 1) irregularity in drug taking by mechanisms described elsewhere;38 while attempts to simulate the emergence of resistance by irregular drug taking have failed,39 resistance does emerge, although rarely, in relapse cultures;40,41 2) inadequate dosage, particularly of RMP,42 leading to a slow response and eventual resistance; and 3) the prescription of single-drug treatment for financial reasons by private practitioners, a common but regrettable practice in some countries. The increasing prevalence of MDR- and XDR-TB strains appears to be due mainly to limited epidemics with MDR strains and sometimes to superinfections in clinics of patients with drug-susceptible disease;43 resistant strains therefore have their own epidemiology, and are capable of creating disastrous epidemics, with treatment that is expensive and of low efficacy.
Slow killing during treatment
A rapidly growing culture of M. tuberculosis is killed within days by the antibacterial drugs, but it takes 6 months to complete the sterilisation of tuberculous lesions in the lungs of patients. From the very early days, this phenomenon was attributed to the presence of slowly growing or non-multiplying populations of bacilli, in particular those in the stationary phase of growth or existing under anaerobic conditions.44 Those particularly likely to contribute to the survivors of anti-bacterial action, the so-called persisters, have been described in what seem to be different stages in the development of the same population.45 Initially, they go through the non-replicating phases, nrp1 and nrp2, lasting approximately 3 weeks, described by Wayne et al.,46 when they acquire tolerance to INH and minimal tolerance to rifamycins. After further incubation for about 3 months, they become the bacilli of the Hu/Coates model,47 with a failure to grow on solid media and greater tolerance to rifamycins, although they are killed rapidly by PZA.48 After further incubation they require resuscitation-promotion factor (RPF) to recover in liquid medium and have been identified in large numbers in sputum from patients.49 As this process proceeds, the energy resources of the cells measured as ATP (adenosine triphosphate) decreases. Eventually they form small ovoid, thick-walled cells and may become difficult or impossible to resuscitate.50 The overall picture is thus of a population that develops increasing degrees of tolerance to drugs, including RMP, inability to grow on solid medium and dependency on RPF for growth, with something resembling spore formation as the end stage.
Action of the antibacterial drugs
Early bactericidal activity
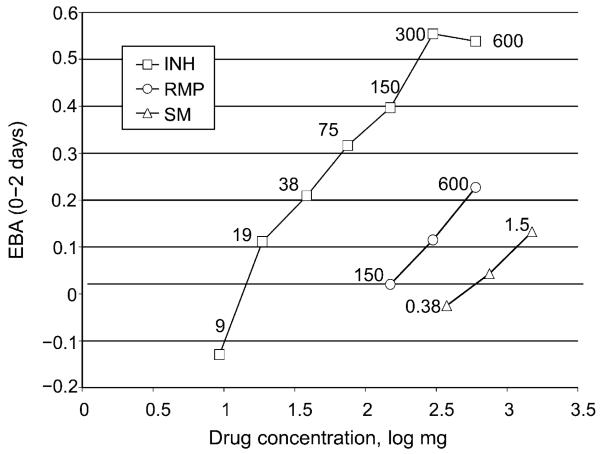
A large study of the action of drugs alone and in combination on the bacterial content of sputum during the first 14 days of treatment was carried out by Jindani et al. in 1980.51,52 Only the kill during the first 2 days was found to distinguish the action of different drugs and different dose sizes of the same drug. In later studies, drugs were given in lower doses to find the size of the dose that just failed to produce a kill (the minimal effective dose [MED]).5 The ratio between the usual therapeutic dose size and the MED was termed the therapeutic margin. Examples of such studies (Figure 1) indicate therapeutic margins of 300/15 = 20 for INH,5 but only 600/150 = 4 for RMP53 and 1.5 for SM.54 A high therapeutic margin (>4) suggests that the drug is capable of penetrating throughout large necrotic lesions, whereas a small margin of <4 may indicate that the drug will not penetrate all lesions, leaving the possibility of local monotherapy.55 While log dose is proportional to the 0–2 day early bactericidal activity (EBA; Figure 1) for many drugs, it does not apply to TMC20756 or PA824.57
Figure 1.
EBA during the first 2 days of treatment with a range of dose sizes of INH, RMP or SM chosen to include low doses that produced no EBA. EBA = early bactericidal activity; INH = isoniazid; RMP = rifampicin; SM = streptomycin.
Relations between area under curve, peak concentration and efficacy
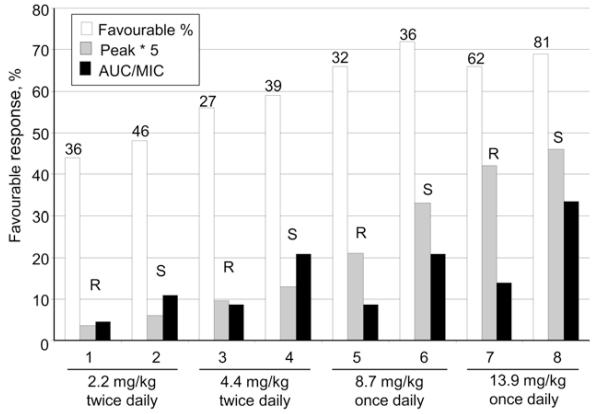
Studies carried out by an Astra-Zeneca team using acute tuberculous infections in mice have shown that efficacy is most closely related to the area under curve (AUC)/MIC for INH,58 RMP59 and fluoroquinolones.60 While these results are clearcut in the relatively simple models used, evidence from studies on intermittent regimens, and in particular from clinical trials on high dosage monotherapy with INH, suggest that this may not be true in the treatment of pulmonary TB, when complex bacterial populations are gradually being eliminated over a far longer time period. Patients can be divided into slow or rapid inactivators according to the rate of acetylation. The rate of acetylation of rapid inactivators is about 2.4 times more rapid than that of slow inactivators, while peak concentrations are only slightly lower, making it possible to separate associations with the AUC from those with peak concentrations. As this rate was measured in patients taking part in studies on the toxicity of a range of high INH doses at Madras, it was possible to see what measures of plasma concentration were best associated with efficacy and toxicity.61-63 The incidence of peripheral neuritis was associated with the duration of the pulse (also the AUC), but surprisingly, efficacy was best related to peak concentration and not to the AUC (Figure 2). This efficacy association probably arose because high peaks gradually killed mutants with low degrees of resistance during several months of treatment. While mutants with low degrees of resistance to RMP do not exist, we have already noted the likely presence of sub-populations of persisters with some degree of tolerance to RMP. These are responsible for prolonging treatment and may only be eliminated by numerous successive exposures to high peak concentrations, similarly to INH, so that peaks rather than AUC could be best associated with the ability of rifamycins to sterilise lesions completely. The absence of persister populations in the acute disease of mice used by the Astra-Zeneca team may explain their failure to demonstrate an association between efficacy and peak rifamycin concentrations.
Figure 2.
The efficacy, peak concentrations and AUCs obtained in rapid and slow acetylators of isoniazid in a series of small trials of treatment with isoniazid alone. AUC = area under curve; MIC = minimum inhibitory concentration; R = rapid; S = slow.
Action of drugs
Isoniazid
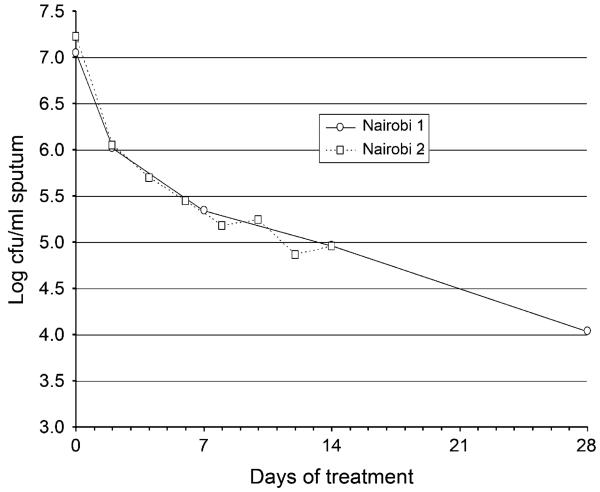
INH is converted to an isonicotinyl-NAD adduct by the bacterial peroxidase katG. This adduct inhibits inhA, a key bacterial enzyme in the FAS II (fatty acid biosynthesis) production of the cell wall mycolic acid. Resistance usually arises by mutation in katG, but less often in inhA, ahpC and ndh.64 While INH is highly bactericidal against dividing bacteria, with an MIC of 0.05 μg/ml and a high therapeutic margin, it has much slower action against non-multiplying cells. At high dosage, it can cause peripheral neuritis, as it quantitatively removes vitamin B6 from the body by combination in the urine.65,66 At about 15 mg/kg it sometimes causes convulsions. When treatment is given with regimens containing INH and the content of M. tuberculosis in sputum is measured by plating on selective culture medium, a typical curve in the log cfu (colony forming unit) counts is obtained which starts with a rapid fall and then levels off as an exponential kill from about day 7 (Figure 3). The initial rapid part of the kill seems to be due to the action of INH on an initially multiplying part of the bacterial population. After the initial period, INH has little further bactericidal effect, as shown from EBA studies, and from the similarity of response of patients with pretreatment strains resistant to INH compared to those with pan-susceptible strains.68 However, it probably plays an important part in maintaining post-antibiotic inhibition of bacterial growth, as several successive short exposures similar to those during treatment result in complete inhibition of multiplication lasting several days.69
Figure 3.
The cfu count of viable M. tuberculosis in sputum in patients receiving treatment with isoniazid-containing regimens. Data from Brindle et al.67 cfu = colony-forming unit.
Rifampicin
RMP is derived by fermentation from Streptomyces mediteranei, renamed as Amycolatopeus rifamycinica. It binds to the rpoB portion of the bacterial polymerase, thereby preventing formation of new proteins.64 It is highly bactericidal against M. tuberculosis throughout treatment, with an MIC of 0.5 μg/ml, but its therapeutic margin is only 4;53 the standard dose of 450–600 mg (10 mg/kg) is therefore marginal. Nevertheless, it is responsible for the major part of the bactericidal activity of the regimen.68 It has been suggested that the main reason why it has such a small therapeutic margin is that only the 15% of the circulating drug that is unbound to plasma proteins is available in lesions.70
Pyrazinamide
PZA is a synthetic pro-drug converted by the amidase of M. tuberculosis, a product of the pncA gene, to pyrazinoic acid, the active moiety.71,72 Drug resistance usually arises by mutation in pncA. Pyrazinoic acid reaches the exterior of the bacilli, where it is reabsorbed by passive diffusion in a highly pH-dependent manner. It is this dependency on an acid environment that accounted for the initial failure to observe any in vitro PZA activity, and indeed, it also provides the main evidence for the assertion that the pH of acute tuberculous inflammation is mildly acidic, at about pH 5.8. Once inside the bacilli the pyrazinoic acid can only be excreted by an inefficient efflux pump that requires energy. As a result, the pyrazinoic acid accumulates within the bacilli, acidifying the interior, and is probably lethal by membrane damage or by inhibiting trans-translation in persisting cells.73 It should be noted that the absorption of pyrazinoic acid into the cell in an acid environment is a passive process that does not utilise cellular energy, whereas its removal by an efflux pump requires energy. As a result, the lower the energy resource of the cell, measured as its ATP content, the greater the bactericidal activity of PZA. For this reason, it is particularly effective in killing dormant,72 or near dormant bacterial populations which are just the bacilli that are tolerant to other antibacterials, such as the rifamycins. PZA is thus uniquely effective as a scavenger of persistent bacilli, and is likely to remain a key drug in future regimens.
Ethambutol
EMB is an inhibitor of cell-wall synthesis, acting on the arabinosyl transferase embcAB.64 Drug resistance usually arises in embB. Due to the risk of optic neuritis during protracted continued treatment, EMB dosage has been reduced successively to a level at which its efficacy is questionable. While it is often recommended for use when INH-resistant strains are prevalent, there is no evidence to suggest that it reduces the risk of failure.
Fluoroquinolones
The fluoroquinolones interfere with the action of the A subunit of bacterial topoisomerase which is responsible for supercoiling DNA and thus packing it within the cell.64 Mutation to resistance usually occurs in the gyrA component of the topoisomerase. The most active fluoroquinolones against M. tuberculosis are the chemically related moxifloxacin (MFX) and gatifloxacin (GTX), with levofloxacin only slightly less active. A Phase IIB clinical trial has shown that MFX and GTX, but not ofloxacin (OFX), are able to accelerate the elimination of viable bacilli from sputum.40 The fluoroquinolones are particularly useful in the treatment of MDR-TB.
Injectables: aminoglycosides and capreomycin
Second only to the fluoroquinolones, the injectables are particularly effective for treating MDR-TB disease. Aminoglycosides act to inhibit protein formation in the ribosomes.64 Long before genetic analysis was possible, three different mutants resistant to SM were identified.74 Of these, one is in the rpsL gene encoding the ribosomal protein S12 and another is in 16S rRNA, which interacts with S12.64 The genetic origin of the third and most common type of low-level resistance is unknown. Capreomycin is a cyclic peptide antibiotic that binds across the face of 23S and 16S ribosomal fragments. All aminoglycosides have very low EBAs,54,75 which are sometimes so low as to be undetectable. They do not reach their possible potential, perhaps because their activity is greatly influenced by pH, being low in the mildly acid conditions of acute tuberculous inflammation. From studies on the intra-cellular activity of SM, it seems probable that it is less effective against intra-cellular bacilli than against extra-cellular bacilli.76
THE FUTURE
Exploring the persister state
One of the most important areas of research is to define the life cycle and occurrence of persister populations, for example by looking at isolates for better growth on solid than in liquid medium, at tolerance to rifamycins, survival at 51°C77 and at the requirement for RPF to initiate multiplication. We also need to know which drugs are most useful in the elimination of such populations. These characteristics should be examined in patients and also in chronic murine TB.
Modernising the experimental system of the murine model for the treatment of established disease
The interval between infection and the start of treatment in the established disease model is usually 13–18 days, sufficient to allow immunity to develop but not for the appearance of persisters. Their presence could be ensured by prolonging the chronic TB that develops after infection for a period of several weeks or even months before treatment is started.78 It would be important, in using this chronic disease model, to re-examine experiments whose results did not eventually agree with the experience in patients,79,80 such as the effect of substituting MFX for INH,81-83 and the effect of moving from once weekly to daily rifapentine (RPT).84,85
Shortening treatment with current drugs
Regimens with new drugs at sufficiently low cost to be widely affordable will only be available after many years. Attention has therefore turned to increasing the activity of RMP and PZA, which together are responsible for almost all of the bactericidal activity of the current four-drug regimen.68 One line of research, currently under exploration by InterTB at St Georges, the HIRIF (High Rifampicin Dosage) consortium centred at Harvard and the Panacea (Pan African Consortium for Evaluation of Antituberculosis Antibiotics) consortium in the Netherlands, is to explore the use of high-dosage RMP. Plasma concentrations vary greatly between individuals on the current dosage,86,87 which means that ineffective dosing may be common. Single-dose sizes of 1800 mg, some three times the usual daily dose size, have been given intermittently without toxicity.88 With the development of serial sputum colony counting (SSCC),43 smaller numbers of patients can yield evidence on the efficacy of higher dosage; however, the evidence thus provided on the toxicity of higher dose sizes needs supplementing in larger numbers, so that Phase IIB trials are now being split into SSCC efficacy studies (HIRIF and Panacea) and separate toxicity studies (e.g., Rifatox by InterTB) to add additional evidence on toxicity. Not only may high-dosage RMP shorten treatment, it may also eliminate the risk of resistance due to inadequate RMP dosage.
An alternative to RMP for the rifamycin component of treatment is the long half-life RPT. Experiments in murine TB have suggested that treatment could be shortened to about 3 months by the use of daily RPT at a dose of 10 mg/kg.84 However, the TBTC (Tuberculosis Trials Consortium) Study 29 has found almost identical rates of sputum conversion in patients on this daily regimen as compared to daily treatment with the standard RMP-containing regimen.85 This negative result was obtained despite the AUC0–24 of RPT being many times higher with RPT than with RMP, and with the presence of a large extra amount of the microbiologically active desacetyl derivative. The increase in the AUC was, however, due to lengthening of the exposure period and not to an increase in peak concentrations. This result is explicable if we are correct in assuming that long-term efficacy is related to peak concentrations and not to the AUC (see Figure 2). The TBTC Study 29X is supplementing the RPT dose with a meal to substantially increase absorption, but there are serious practical problems in providing meals for all daily drug doses in resource-poor countries. Furthermore, the EBA found with increasing dose sizes of RPT reached a maximum at a dose of about 1200 mg,53 and pharmacological studies also demonstrated maximum plasma levels at a dose of about 15 mg/kg;89 it may therefore not be possible to obtain high RPT peak concentrations by increasing the dose size.
An increase in dose size of PZA seems unlikely to be feasible: when early US Veterans studies on a PZA+ INH regimen administered 3.0 g PZA daily, a substantially higher dose size than the current 1600 mg for 55–70 kg patients, hepatotoxicity resulted in 13% of the patients.34 Furthermore, the bactericidal activity of PZA on culture is only slightly increased by large increases in PZA concentrations. The only useful method seems likely to be the administration of pyrazinoic acid, the active moiety of the pro-drug PZA, by inhalation as a supplement to oral dosage.90 This is postulated to act by providing more active drug even in the presence of drug resistance and, possibly, prolonging activity by acidification of the lesions.
New drugs
Current drugs in the forefront of development are shown in Table 2. Of the new drugs, TMC 207 and PA824 have been most thoroughly explored. TMC207 kills by inhibiting the ATP synthase that is located in the bacterial cell wall and thereby preventing upkeep of the cell membrane.91 When the ATP concentration of M. tuberculosis is high at the onset of treatment, it has first to gradually reduce ATP concentration, which takes several days, before killing starts, accounting for its so-called time-dependent killing91 and for its slow initial activity in the EBA study.56 However, as treatment continues or with intra-cellular bacilli, the persister population has much lower ATP concentrations; killing is therefore now more rapid and dependent on concentration.92 For this reason, it is effective against persister populations93 and works well with PZA.94 It is now available on a named p atient basis for effective treatment of MDR- and XDR-TB disease.95 The mode of action of PA824, and of the related OPC67683, is not fully understood. When bacteria are in an hypoxic non-replicating state, PA824 kills as a nitrous oxide donor.96 It also causes a drop in bacterial ATP levels, and, for this reason, joins PZA and TMC207 in killing by reducing the energy necessary to maintain bacterial cell membranes. This suggests that this group of drugs that reduces bacterial ATP and therefore target membrane function might be particularly effective against persisters.97 The mode of killing of PA824 under aerobic conditions is less clear, but seems to be associated with inhibition of cell-wall mycolic acid synthesis. TMC207, PA824 and OPC67683 have all been developed through Phase IIA (EBA) studies followed by Phase IIB studies in MDR-TB patients,56,57,98 as the response obtained in these patients by the addition is considerably better than can be obtained with a standard basic drug combination. This advantage will, however, be lost for future drugs as it becomes less ethical to treat MDR-TB disease without either TMC207 or PA824.
Table 2.
Pharmokinetic characteristics of current anti-tuberculosis drugs
| Drug | Dose mg |
Peak mg/l |
Half-life h |
AUC mg.h/l |
Protein binding % |
MIC for M. tuberculosis mg/l |
EBA (0–2 days) log cfu/day |
|---|---|---|---|---|---|---|---|
| Isoniazid slow | 300 | 5 | 3 | 18 | 20 | 0.05 | 0.58 |
| Isoniazid rapid | 4 | 1.5 | |||||
| Rifampicin | 600 | 10 | 3 | 65 | 85 | 0.5 | 0.21 |
| Rifapentine | 600 | 13 | 16 | 940 | 98 | 0.2 | 0.24 |
| Pyrazinamide | 2 000 | 40 | 8 | 410 | 20 | 20 | 0.02 |
| Ethambutol | 1 200 | 3 | 2.6 | 500 | 0 | 1.5 | 0.29 |
| Streptomycin | 750 | 40 | 3–5 | 900 | 35 | 1 | 0.07 |
| Amikacin | 750 | 40 | 3–5 | 240 | 4 | 0.5 | 0.05 |
| Capreomycin | 1 000 | 30 | 3–5 | 250 | ? | 2 | ? |
| Moxifloxacin | 400 | 2.5 | 16 | 30 | 40 | 0.25 | 0.53 |
| Levofloxacin | 750 | 9.3 | 7.5 | 101 | 23 | 0.5 | 0.39 |
| Ethionamide | 500 | 3 | 2 | 10 | 20 | 0.6 | ? |
| PAS | 12 000 | 250 | 1 | ? | 60 | 0.5 | 0.26 |
| TMC 207 | 400 | 3.3 | — | — | 99 | 0.06 | — |
AUC = area under curve; MIC = minimum inhibitory concentration; EBA = early bactericidal activity; cfu = colony forming units; ? = uncertain; PAS = para-aminosalicylic acid.
New drugs are undoubtedly needed for treating MDR- and XDR-TB disease. They will only be fully utilised when they have been screened for the most rapidly sterilising combination (not drug alone), as recently promoted by a number of organisations (Global Alliance for TB Drug Development, Critical Path to TB Regimens and PreDiCT-TB). This is unfortunately many years or even decades in the future. In the mean-time, if effective and rapid drug susceptibility testing, preferably by genetic methods, can be made widely available, the addition of a single new drug such as TMC207 in the drug-susceptible standard regimen will prevent a super-infection with a resistant strain occurring in the clinic during the treatment of initially drug-susceptible infections. Effective deployment of completely novel combinations of drugs promises to usher in a new era in the treatment of TB.
References
- 1.Medical Research Council Streptomycin treatment of pulmonary tuberculosis. BMJ. 1948;2:769–782. [PMC free article] [PubMed] [Google Scholar]
- 2.Fox W, Sutherland I, Daniels M. A five-year assessment of patients in a controlled trial of streptomycin in pulmonary tuberculosis. Q J Med. 1954;23:347–366. [PubMed] [Google Scholar]
- 3.Medical Research Council Treatment of pulmonary tuberculosis with streptomycin and para-amino-salicylic acid. BMJ. 1950;2:1073–1085. [PMC free article] [PubMed] [Google Scholar]
- 4.Fox W, Sutherland I. A five-year assessment of patients in a controlled trial of streptomycin, para-aminosalicylic acid and streptomycin plus para-aminosalicylic acid, in pulmonary tuberculosis. Q J Med. 1956;25:221–243. [PubMed] [Google Scholar]
- 5.Donald PR, Sirgel FA, Botha FJ, et al. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156(3 Pt 1):895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 6.Medical Research Council The treatment of pulmonary tuberculosis with isoniazid. BMJ. 1952;2:735. [PMC free article] [PubMed] [Google Scholar]
- 7.Medical Research Council Isoniazid in the treatment of pulmonary tuberculosis. Second Report. BMJ. 1953;1:521–536. [PMC free article] [PubMed] [Google Scholar]
- 8.Fox W, Wiener A, Mitchison DA, Selkon JB, Sutherland I. The prevalence of drug-resistant tubercle bacilli in untreated patients with pulmonary tuberculosis: a national survey, 1955–56 Tubercle. 1957;38:71–84. doi: 10.1016/s0041-3879(57)80001-4. [DOI] [PubMed] [Google Scholar]
- 9.Mitchison DA, Selkon JB. Bacteriological aspects of a survey of the incidence of drug-resistant tubercle bacilli among untreated patients. Tubercle. 1957;38:85–98. doi: 10.1016/s0041-3879(57)80002-6. [DOI] [PubMed] [Google Scholar]
- 10.Crofton J. Chemotherapy of pulmonary tuberculosis. BMJ. 1959;1:1610–1614. doi: 10.1136/bmj.1.5138.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crofton J. Sputum conversion and the metabolism of isoniazid. Am Rev Tuberc. 1958;77:869–871. doi: 10.1164/artpd.1958.77.5.869. [DOI] [PubMed] [Google Scholar]
- 12.International Union Against Tuberculosis An international investigation of the efficacy of chemotherapy in previously untreated patients with pulmonary tuberculosis. Bull Int Union Tuberc. 1964;34(2):83–191. [Google Scholar]
- 13.East African/British Medical Research Council Isoniazid with thiacetazone (thioacetazone) in the treatment of pulmonary tuberculosis in East Africa—fifth investigation. Tubercle. 1970;51:123–151. [PubMed] [Google Scholar]
- 14.Tuberculosis Chemotherapy Centre, Madras A concurrent comparison of home and sanatorium treatment of pulmonary tuberculosis in South India. Bull World Health Organ. 1959;21:51–144. [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews RH, Devadatta S, Fox W, Radhakrishna S, Ramakrishnan CV, Velu S. Prevalence of tuberculosis among close family contacts of tuberculous patients in South India, and influence of segregation of the patient on the early attack rate. Bull World Health Organ. 1960;23:463–510. [PMC free article] [PubMed] [Google Scholar]
- 16.Fox W. The problem of self-administration of drugs; with particular reference to pulmonary tuberculosis. Tubercle. 1958;39:269–274. doi: 10.1016/s0041-3879(58)80088-4. [DOI] [PubMed] [Google Scholar]
- 17.McCune RM, Jr, McDermott W, Tompsett R. The fate of M ycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grumbach F. Activity of rifampicin on experimental tuberculosis in mice. The development of resistance to rifampicin. Therapeutic effects of combinations of different drugs with rifampicin. Antibiot Chemother. 1970;16:392–405. French. [PubMed] [Google Scholar]
- 19.East African/British Medical Research Council Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1972;299:1079–1085. [PubMed] [Google Scholar]
- 20.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis Int. 1999;3(Suppl 2):S231–S279. [PubMed] [Google Scholar]
- 21.East African/British Medical Research Council Controlled clinical trial of four short-course regimens of chemotherapy for two durations in the treatment of pulmonary tuberculosis. Third study. Second report. Tubercle. 1980;61:59–69. doi: 10.1016/0041-3879(80)90012-4. [DOI] [PubMed] [Google Scholar]
- 22.East African/British Medical Research Council Controlled clinical trial of four 6-month regimens of chemotherapy for pulmonary tuberculosis. Second report. Am Rev Respir Dis. 1976;114:471–475. doi: 10.1164/arrd.1976.114.3.471. [DOI] [PubMed] [Google Scholar]
- 23.Santha T, Nazareth O, Krishnamurthy S, et al. Treatment of pulmonary tuberculosis with short course chemotherapy in South India—5-year follow-up. Tubercle. 1989;70:229–234. doi: 10.1016/0041-3879(89)90016-0. [DOI] [PubMed] [Google Scholar]
- 24.East African/British Medical Research Councils Controlled clinical trial of five short-course (4-month) chemotherapy regimens in pulmonary tuberculosis: second report of the fourth study. Am Rev Respir Dis. 1981;123:165–170. doi: 10.1164/arrd.1981.123.2.165. [DOI] [PubMed] [Google Scholar]
- 25.East and Central African/British Medical Research Council (5th Collaborative Study) Controlled clinical trial of four short-course regimens of chemotherapy (three 6-month and one 8-month) for pulmonary tuberculosis: Final report. Tubercle. 1986;67:5–15. doi: 10.1016/0041-3879(86)90027-9. [DOI] [PubMed] [Google Scholar]
- 26.Hong Kong Chest Service/British Medical Research Council Controlled trial of 2, 4 & 6 months of pyrazinamide in 6-month, 3× weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin and pyrazinamide. Am Rev Respir Dis. 1991;143:700–706. doi: 10.1164/ajrccm/143.4_Pt_1.700. [DOI] [PubMed] [Google Scholar]
- 27.Mitchison DA. The action of anti-tuberculosis drugs in short-course chemotherapy. Tubercle. 1985;66:218–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 28.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364:1244–1251. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization . Global tuberculosis control 2011. WHO; Geneva, Switzerland: [Accessed February 2012]. 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf WHO/HTM/TB/2011.16. [Google Scholar]
- 30.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:53–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 31.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20:810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colijn C, Cohen T, Ganesh A, Murray M. Spontaneous emergence of multiple drug resistance in tuberculosis before and during therapy. PLoS ONE. 2011;8:e18327. doi: 10.1371/journal.pone.0018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimao T. Drug resistance in tuberculosis control. Tubercle. 1987;68:5–16. doi: 10.1016/0041-3879(87)90014-6. [DOI] [PubMed] [Google Scholar]
- 35.Garton NJ, Waddell SJ, Sherratt AL, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen BW, Mitchison DA. Counts of viable tubercle bacilli in sputum related to smear and culure grading. Med Lab Sci. 1992;49:94–98. [PubMed] [Google Scholar]
- 37.Matthews JH. IX. Pyrazinamide and isoniazid used in the treatment of pulmonary tuberculosis. Am Rev Respir Dis. 1960;81:348–351. [Google Scholar]
- 38.Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis. 1998;2:10–15. [PubMed] [Google Scholar]
- 39.Srivastava S, Pasipanodya JG, Meek C, Left R, Gumbo T. Multidrug-resistant tuberculosis not due to non-compliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–1958. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.East and Central African/British Medical Research Council Controlled clinical trial of 4 short-course regimens of chemotherapy (three 6-month and one 8 month) for pulmonary tuberculosis. Tubercle. 1983;64:163–168. [Google Scholar]
- 41.Hong Kong Tuberculosis Treatment Services. East African and British Medical Research Councils First-line chemotherapy in the retreatment of bacteriological relapses of pulmonary tuberculosis following a short-course regimen. Lancet. 1976;1:162–168. [PubMed] [Google Scholar]
- 42.Peloquin C. What is the ‘right’ dose of rifampin? Int J Tuberc Lung Dis. 2003;7:3–5. [PubMed] [Google Scholar]
- 43.Rustomjee R, Lienhardt C, Kanyok T, et al. A Phase II study of the sterilizing activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:128–138. [PubMed] [Google Scholar]
- 44.Mitchison DA, Selkon JB. The bactericidal activities of anti-tuberculous drugs. Am Rev Tuberc. 1956;74(2 Part 2):109–116. doi: 10.1164/artpd.1956.74.2-2.109. [DOI] [PubMed] [Google Scholar]
- 45.Shleeva MO, Bagramyan K, Telkov MV, Mukamolova GV, et al. Formation and resuscitation of ‘non-culturable’ cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology. 2002;148:1581–1591. doi: 10.1099/00221287-148-5-1581. [DOI] [PubMed] [Google Scholar]
- 46.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of non-replicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y, Mangan JA, Dhillon J, et al. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J Bacteriol. 2000;182:6358–6365. doi: 10.1128/jb.182.22.6358-6365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y, Coates AR, Mitchison DA. Sterilising action of pyrazinamide in models of dormant and rifampicin-tolerant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2006;10:317–322. [PubMed] [Google Scholar]
- 49.Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med. 2010;181:174–180. doi: 10.1164/rccm.200905-0661OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shleeva MO, Kudykina YK, Vostroknutova GN, Suzina NE, Mulyukin AL, Kaprelyants AS. Dormant ovoid cells of Mycobacterium tuberculosis are formed in response to gradual external acidification. Tuberculosis (Edinb) 2011;91:146–154. doi: 10.1016/j.tube.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 52.Jindani A, Doré CJ, Mitchison DA. The bactericidal and sterilising activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 53.Sirgel FA, Fourie PB, Donald PR, et al. The early bactericidal activities of rifampicin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:128–135. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- 54.Donald PR, Sirgel FA, Venter A, et al. The early bactericidal activity of streptomycin. Int J Tuberc Lung Dis. 2002;6:693–698. [PubMed] [Google Scholar]
- 55.Elliott AM, Berning SE, Iseman MD, Peloquin CA. Failure of drug penetration and acquisition of drug resistance in chronic tuberculous empyema. Tubercle Lung Dis. 1995;76:463–467. doi: 10.1016/0962-8479(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 56.Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:2831–2835. doi: 10.1128/AAC.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diacon AH, Dawson R, Hanekom M. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54:3402–3407. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47:2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jayaram R, Shandil RK, Gaonkar S, et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2004;48:2951–2957. doi: 10.1128/AAC.48.8.2951-2957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shandil RK, Jayaram R, Kaur P, et al. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 2007;51:576–582. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchison DA. Plasma concentrations of isoniazid in the treatment of tuberculosis. In: Davies DS, Pritchard BNC, editors. Biological effects of drugs in relation to their plasma concentrations. Macmillan; New York, NY, USA: 1973. pp. 169–182. [Google Scholar]
- 62.Mitchison DA. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Seminars Respir Crit Care Med. 2004;25:307–315. doi: 10.1055/s-2004-829503. [DOI] [PubMed] [Google Scholar]
- 63.Mitchison DA. PK/PD and the choice of high dosage rifamycins. Int J Tuberc Lung Dis. 2012;16 doi: 10.5588/ijtld.11.0818. in press. [DOI] [PubMed] [Google Scholar]
- 64.Da Silva PEA, Palomino JC. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis classical and new drugs. J Antimicrob Chemother. 2011;66:1417–1430. doi: 10.1093/jac/dkr173. [DOI] [PubMed] [Google Scholar]
- 65.Biehl JP, Vilter RW. Effects of isoniazid on pyridoxine metabolism. J Am Med Assoc. 1954;156:1549–1552. doi: 10.1001/jama.1954.02950170003002. [DOI] [PubMed] [Google Scholar]
- 66.van der Watt JJ, Harrison TB, Benatar M, Heckmann JM. Polyneuropathy, anti-tuberculosis treatment and the role of pyridoxine in the HIV/AIDS era: a systematic review. Int J Tuberc Lung Dis. 2011;15:722–728. doi: 10.5588/ijtld.10.0284. [DOI] [PubMed] [Google Scholar]
- 67.Brindle R, Odhiambo J, Mitchison D. Serial counts of Mycobacterium tuberculosis in sputum as surrogate markers of the sterilising activity of rifampicin and pyrazinamide in treating pulmonary tuberculosis. BMC Pulm Med. 2001;1:2. doi: 10.1186/1471-2466-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4:796–806. [PubMed] [Google Scholar]
- 69.Awaness AM, Mitchison DA. Cumulative effects of pulsed exposures of Mycobacterium tuberculosis to isoniazid. Tubercle. 1973;54:153–158. doi: 10.1016/0041-3879(73)90035-4. [DOI] [PubMed] [Google Scholar]
- 70.Mitchison DA. Rifapentine: the way forward. Int J Tuberc Lung Dis. 1998;2:612–615. [PubMed] [Google Scholar]
- 71.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 72.Mitchison DA, Zhang Y. Recent developments in the study of pyrazinamide: an update. In: Donald P, van Helden PD, editors. Antituberculosis chemotherapy. Progress in respiratory research. Vol. 40. Karger; Cape Town, South Africa: 2011. pp. 32–43. [Google Scholar]
- 73.Shi W, Zhang X, Jiang X, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchison DA. The segregation of streptomycin-resistant variants of Mycobacterium tuberculosis into groups with characteristic levels of resistance. J Gen Microbiol. 1953;5:596–604. doi: 10.1099/00221287-5-3-596. [DOI] [PubMed] [Google Scholar]
- 75.Donald PR, Sirgel FA, Venter A, et al. The early bactericidal activity of amikacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2001;5:533–538. [PubMed] [Google Scholar]
- 76.Crowle AJ, Sbarbaro JA, Judson FN, Douvas GS, May MH. Inhibition by streptomycin of tubercle bacilli within cultured human macrophages. Am Rev Respir Dis. 1984;130:839–844. doi: 10.1164/arrd.1984.130.5.839. [DOI] [PubMed] [Google Scholar]
- 77.Wallace JG. The heat resistance of tubercle bacilli in the lungs of infected mice. Am Res Respir Dis. 1961;83:866–871. doi: 10.1164/arrd.1961.83.6.866. [DOI] [PubMed] [Google Scholar]
- 78.Rees RJW, Hart PD. Analysis of the host-parasite equilibrium in chronic murine tuberculosis by total and viable bacillary counts. Br J Exp Pathol. 1961;41:83–88. [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchison DA, Chang KC. Experimental models of tuberculosis: can we trust the mouse? Am J Respir Crit Care Med. 2009;180:201–202. doi: 10.1164/rccm.200905-0708ED. [DOI] [PubMed] [Google Scholar]
- 80.Almeida D, Nuermberger E, Tasneen R, et al. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2009;53:4178–4184. doi: 10.1128/AAC.00830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169:421–426. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 82.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170:1131–1134. doi: 10.1164/rccm.200407-885OC. [DOI] [PubMed] [Google Scholar]
- 83.Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180:273–228. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- 84.Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dorman SE, Goldberg S, Feng P, et al. A Phase II study of a rifapentine-containing regimen for intensive phase treatment of pulmonary tuberculosis: preliminary results for Tuberculosis Trials Consortium Study 29. Am J Respir Crit Care Med. 2011;183:A6413. [Google Scholar]
- 86.Chigutsa E, Visser ME, Swart EC, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother. 2011;55:4122–4127. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilkins JJ, Savic RM, Karlsson MO, et al. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semi-mechanistic model to describe variable absorption. Antimicrob Agents Chemother. 2008;52:2138–2148. doi: 10.1128/AAC.00461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verbist L. Pharmacological study of rifampicin after repeated high dosage during intermittent combined therapy. I. Variation of rifampicin serum levels (947 determinations) Respiration. 1971;28(Suppl):S7–S16. doi: 10.1159/000194957. [DOI] [PubMed] [Google Scholar]
- 89.Dooley KE, Bliven-Sizemore E, Weiner M, et al. A Phase I dose escalation trial of the pharmacokinetics, safety, and tolerability of rifapentine dosed daily in healthy volunteers: preliminary results from Tuberculosis Trials Consortium Study 29. Am J Respir Crit Care Med. 2011;183:A6341. [Google Scholar]
- 90.Mitchison DA, Fourie PB. The near future: improving the activity of rifamycins and pyrazinamide. Tuberculosis (Edin) 2010;90:177–181. doi: 10.1016/j.tube.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 92.Dhillon J, Andries K, Phillips PP, Mitchison DA. Bactericidal activity of the diarylquinoline TMC207 against Mycobacterium tuberculosis outside and within cells. Tuberculosis (Edinb) 2010;90:301–305. doi: 10.1016/j.tube.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Koul A, Vranckx L, Dendouga N, et al. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem. 2008;283:25273–25280. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- 94.Ibrahim M, Andries K, Lounis N, et al. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother. 2007;51:1011–1015. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 96.Manjunatha U, Boshoff HI, Barry CE. The mechanism of action of PA-824: novel insights from transcriptional profiling. Commun Integr Biol. 2009;2:215–218. doi: 10.4161/cib.2.3.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hurdle JG, O’Neill AJ, Chopra I, Lee RE. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 2011;9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]