Abstract
Context
Maternal obesity is associated with high plasma triglyceride, poor vascular function and increased risk for pregnancy complications. In normal weight pregnant women, higher triglyceride is associated with increased small, dense LDL.
Hypothesis
In obese pregnancy, increased plasma triglyceride concentrations result in triglyceride enrichment of VLDL-1 particles and formation of small dense LDL via lipoprotein lipase.
Design
Women (n=55) of BMI 18-46 kg/m2 were sampled longitudinally at 12, 26 and 35 weeks’ gestation and 4 months post-natal.
Setting
Women were recruited at hospital antenatal appointments and study visits were in a clinical research suite.
Outcome measures
Plasma concentrations of lipids, triglyceride-rich lipoproteins, lipoprotein lipase mass, estradiol, steroid hormone binding globulin, insulin, glucose, leptin and adiponectin were determined.
Results
Obese women commenced pregnancy with higher plasma triglyceride, reached the same maximum and then returned to higher post-natal levels than normal weight women. Estradiol response to pregnancy (trimester 1-3 incremental area under the curve) was positively associated with plasma triglyceride response (r2 adjusted 25%, P<0.001). In the third trimester, the proportion of small, dense LDL-III was 2 fold higher in obese women than normal weight women (mean [SD] 40.7[18.8] vs 21.9[10.9] %, P<0.005), and 35% of obese, 14% of overweight and none of the normal weight women displayed an atherogenic LDL subfraction phenotype. The LDL-III mass response to pregnancy was inversely associated with adiponectin response (17%, P=0.013).
Conclusions
Maternal obesity is associated with an atherogenic LDL subfraction phenotype and may provide a mechanistic link to poor vascular function and adverse pregnancy outcome.
Keywords: pregnancy, lipids, obesity
Introduction
Obesity in the pregnant population has increased by 70-100% over a decade and one in five pregnant women in the UK is obese(1). The consequences include increased risk of metabolic complications such as preeclampsia (PE) and gestational diabetes mellitus (GDM) which also impact on fetal morbidity and mortality(2). Dysregulation of maternal lipid metabolism may be an underlying component in the aetiology of PE and GDM(3-4). Maternal obesity has also been linked to the development of obesity in offspring via an fetal over-supply of glucose and/or lipids in utero(5).
Maternal adaptation to pregnancy includes increased storage of fatty acids in adipose tissue in early gestation, and mobilisation of fatty acids from adipose tissue in response to maternal insulin resistance induced by pregnancy hormones, in mid-to-late gestation(6). Obese mothers have higher fasting insulin, plasma triglycerides (TG) and lower high density lipoprotein concentrations in the third trimester of pregnancy in combination with increased markers of inflammation(7). Thus, the physiologically normal insulin resistance is much more pronounced in overweight and obese women. Obese women also have a compromised vascular adaptation to pregnancy and vascular function fails to show the same extent of improvement as that of normal weight pregnant women(8). The combination of an adverse metabolic/inflammatory profile and vascular dysfunction may impact on both placental development and the maternal response to pregnancy, leading to adverse outcomes such as GDM and PE(9).
The major TG-carrying particle in the circulation is VLDL, which can be fractionated into two structurally and metabolically distinct subfractions; large TG–rich VLDL-1 and smaller VLDL-2. Triglyceride in these particles is hydrolysed by lipases resulting in the formation of smaller particles(10). Non-pregnant obesity is characterised by an increase in plasma VLDL concentration, predominately due to VLDL-1, an increase in IDL and a shift in LDL size distribution towards smaller denser lipoproteins(11). An increased proportion of small dense LDL (LDL-III) is indicative of an atherogenic lipoprotein phenotype(12) and has been associated with hepatic steatosis(13-14). In a study of 10 normal weight pregnant women, VLDL-1 and VLDL-2 subfractions increased in parallel by up to 5 fold throughout gestation(15). Plasma LDL concentration rose by up to 70% and in some women there was a shift towards smaller denser LDL particles which was associated with TG concentration at 10 weeks’ gestation and the rate of change in TG for a given increment in estrogen(15).
We have previously suggested that GDM and PE may result from inappropriate handling of the increased TG turnover of pregnancy, leading to an ectopic accumulation of fat, particularly in the liver(9, 16), and formation of an atherogenic lipoprotein profile. While plasma TG levels and the proportion of small dense LDL have been shown to be higher in GDM(17) and PE(18) compared to normal weight pregnant women, there are no data on small dense LDL in obese pregnancy. We hypothesised that in obese pregnancy, increased plasma TG concentrations result in TG-enrichment of large VLDL-1 particles, which are a precursor for the formation of small dense LDL via the action of plasma lipoprotein lipase (LPL). In this study, we followed gestational changes in maternal VLDL and LDL concentrations and subfraction distribution in women of different BMI. LPL mass and hormone/adipokine concentrations were also assessed in relation to the lipid and lipoprotein changes.
Methods
Patient recruitment
Women (n=55) registered for obstetric care at the Princess Royal Maternity Unit, Glasgow, who were healthy and normotensive with no significant past medical history were recruited. Normal weight individuals were those with a booking BMI<25kg/m2, overweight with BMI≥25kg/m2 but <30kg/m2, and obese with BMI≥30kg/m2. The study was performed according to the Declaration of Helsinki, approval was granted by the Research Ethics Committee of North Glasgow University NHS Trust, and each subject gave written informed consent. The women attended after an overnight fast (>10 hours). Blood samples were collected into 1mg/mL EDTA at a mean of 12.5 (range 8–14) [T1], 26.1 (24–28) [T2] and 35.5 (33–38) [T3] weeks’ gestation and plasma collected by low-speed centrifugation. Post-natal samples were collected 17.1 (12–26) [PN] weeks after delivery. Maternal booking (i.e. first antenatal hospital appointment) characteristics and delivery details were recorded from the patients’ notes. Birth weights were normalised by gestation at birth, fetal sex and maternal parity. Deprivation category (DEPCAT score), a measure of socioeconomic status, was assigned using the Scottish Area Deprivation Index for Scottish postcode sectors, 1998(19).
Maternal plasma lipids and lipoproteins
Plasma total cholesterol and TG concentrations, and very low density lipoprotein-1 (VLDL-1) (Sf 60-400), VLDL-2 (Sf 20-60), IDL (Sf 12-20) and LDL (Sf 0-12) concentration and composition were determined as described(15). Plasma non-esterified fatty acid concentration (NEFA) was quantitated by colorimetric assay (Wako, Alpha Laboratories). The mgTG/mgCE ratio was used as an index of the TG-carrying capacity of each particle. Isolation of LDL subfractions from plasma was achieved by density gradient ultracentrifugation using a discontinuous salt gradient(15, 20) and reported as mass concentration and percentage abundance. Subjects were classified as having the atherogenic LDL subfraction profile, Pattern B, when LDL-III >50%(21).
Hormone and lipoprotein lipase measurement
Plasma estradiol was estimated using the Immunulite semi-automated assay system (DPC). Insulin (Mercodia), leptin and adiponectin (R&D Systems) and SHBG (IBL) analyses were performed by ELISA. HOMA was calculated as [fasting insulin (mU/L) x fasting glucose (mmol/L)]/22.5]. LPL mass was determined by ELISA using bovine LPL as standard(22-23). LPL genotype was determined by allelic discrimination analysis on a 7900 HT sequence detection system (Applied Biosystems) using Taqman SNP Genotyping Assay (C_901792_1).
Statistical analysis
Values for continuous variables are given as mean (standard deviation) and number (%) for categorical variables. Total and incremental areas under the time (T1 to T3 weeks’ gestation) x concentration curve were calculated using the trapezium method with correction for baseline (T1) parameter level(24). Normality testing was carried out using the Ryan–Joiner test and data was log or square root transformed to achieve a normal distribution as necessary. One way ANOVA or chi-squared test was used when assessing the whole group for differences between time (with repeated measures) or BMI group. Simultaneous difference testing between BMI group across time points was carried out using a univariate split-plot approach repeated measures analysis with post hoc ANOVA for specific time /BMI category differences. Associations between variables were examined using univariate regression analysis. Statistical analysis used the JMP statistical analysis program (Version 5.1, SAS Institute).
Results
Patient characteristics
Characteristics of the women at booking visit and offspring characteristics at delivery are shown in Table 1. Normal weight women were, on average, 2 years younger than heavier weight women. As expected the BMI, systolic and diastolic blood pressure was significantly higher in higher BMI categories. Gestation at delivery did not differ between the 3 groups. Placental weight, birth weight and birth weight centile of offspring increased with increasing maternal booking BMI.
Table 1. Maternal antenatal booking characteristics according to WHO BMI category.
Values are mean and standard deviation (SD) for continuous variables or number (%) for categorical variables. ANOVAwas used to test for differences among groups (*on log transformed where appropriate). Chi squared test was used to test for differences among groups for categorical variables. Different superscript letters indicate differences between individual groups using post hoc Tukey-Kramer test or subgroup chi-squared test.
| Normal weight (n=13) |
Overweight (n=15) |
Obese (n=27) |
P value | |
|---|---|---|---|---|
| Booking visit | ||||
| Age* (years) |
27 (4)a | 29 (6)a,b | 29 (6)b | 0.029 |
| Smokers (number (%)) |
7 (54) | 3 (20) | 10 (37) | 0.18 |
| Deprivation Index DEPCAT Score (number (%)) |
||||
| Affluent (1-2) | 1 (8) | 3 (20) | 1 (4) | |
| Intermediate (3-5) | 5 (38) | 3 (20) | 10 (37) | 0.74 |
| Deprived (6-7) | 7 (54) | 9 (60) | 16 (59) | |
| Parity (number (%)) |
||||
| 0 | 6 (46) | 7 (47) | 12 (44) | 0.99 |
| >0 | 7 (54) | 8 (53) | 15 (56) | |
| BMI* (kg/m2) |
21.2 (1.9)a | 26.1 (1.0)b | 33.7 (4.2)c | <0.001 |
| Systolic Blood Pressure* (mmHg) |
107 (12)a | 117 (9)b | 124 (11)b | <0.001 |
| Diastolic Blood Pressure* (mmHg) |
61 (6)a | 68 (7)a, b | 73 (10)b | <0.001 |
| LPL S447X genotype (SS:SX, number (%)) |
7:6 (54:46) a | 14:1 (93:7) b | 22:5 (81:19) a,b | 0.035 |
| At delivery | ||||
| Gestation at delivery (weeks)* |
39.6 (1.3) | 39.9 (0.9) | 40.0 (1.5) | 0.46 |
| Placental weight* (g) |
649 (143)a | 817 (162)b | 790 (177 b | 0.020 |
| Birth weight* (g) |
3246 (521)a | 3829 (533)b | 3659 (512)a, b | 0.015 |
| Birth Weight Centile | 38 (32)a | 61 (34)a, b | 74 (21)b | 0.001 |
Plasma lipids and lipoproteins
Plasma total and LDL cholesterol increased from T1 to T3 and declined in the post-natal period (P<0.001), but maternal BMI category had no impact on these changes (Table 2). Plasma TG increased over gestation and declined post-natally (P<0.001), and the response differed between BMI categories (P=0.030) with overweight and obese women having higher plasma TG concentrations than normal weight women. At a BMI of 20 kg/m2 plasma TG concentration increases by 154% over gestation, whereas the increase is only 66% at a BMI of 40kg/m2 (Supplemental Figure 1). Notably, whilst the maximum plasma TG concentration reached at T3 was similar, irrespective of BMI, obese women expressed higher values at baseline.
Table 2. Maternal plasma lipid and lipoprotein concentrations during and after pregnancy according to WHO BMI category.
Mean (standard deviation) are shown.
T1 – trimester 1 [12.5 (1.2) weeks]; T2 – trimester 2 [26.1 (1.3) weeks], T3 – trimester 3 [35.5 (1.3) weeks]; PN – postnatal [17.1 (2.9) weeks post delivery]. P Time for association of lipid/lipoprotein parameters over time, P BMI for association with BMI category and P Time*BMI for the interaction between Time and BMI, using a univariate split-plot approach repeated measures analysis. Where the interaction between Time and BMI was significant (P<0.05), one way ANOVA and Tukey Kramer post-hoc tests were conducted. Different superscript letters indicate differences between BMI categories at a particular time point using post hoc Tukey test. Statistical analysis carried out on *log transformed data
| Time of sampling |
Normal weight |
Overweight | Obese | P Time |
P BMI |
P Time * BMI |
|
|---|---|---|---|---|---|---|---|
| Total Cholesterol* (mmol/L) |
T1 | 4.31 (0.60) | 4.65 (0.77) | 4.75(0.97) | <0.001 | 0.88 | 0.019 |
| T2 | 5.95 (1.10) | 6.09 (0.78) | 5.82 (1.06) | ||||
| T3 | 6.42 (0.84) | 6.07 (1.01) | 5.84 (0.97) | ||||
| PN | 3.93 (0.69) | 4.13 (0.59) | 4.49 (1.08) | ||||
| LDL Cholesterol* (mmol/L) |
T1 | 2.49 (0.43) | 2.77 (0.72) | 2.96 (0.93) | <0.001 | 0.83 | 0.11 |
| T2 | 3.85 (0.89) | 3.85 (0.85) | 3.79 (0.97) | ||||
| T3 | 4.13 (0.68) | 3.86 (1.10) | 3.77 (1.03) | ||||
| PN | 2.34 (0.62) | 2.52 (0.48) | 2.87 (1.02) | ||||
|
| |||||||
| Triglyceride* (mmol/L) |
T1 | 1.07 (0.32)a | 1.34 (0.43)a,b | 1.57 (0.63)b | <0.001 | 0.030 | 0.002 |
| T2 | 2.02 (0.87) | 2.50 (0.66) | 2.45 (0.94) | ||||
| T3 | 3.05 (1.05) | 2.86 (1.02) | 2.80 (0.86) | ||||
| PN | 0.82 (0.28)a | 1.16 (0.42)a,b | 1.59 (0.82)b | ||||
| NEFA* (mmol/L) |
T1 | 0.27 (0.42) | 0.12 (0.15) | 0.25 (0.27) | 0.26 | 0.59 | 0.74 |
| T2 | 0.15 (0.19) | 0.19 (0.14) | 0.24 (0.34) | ||||
| T3 | 0.21 (0.23) | 0.21 (0.14) | 0.21 (0.13) | ||||
| PN | 0.26 (0.17) | 0.18 (0.20) | 0.22 (0.21) | ||||
| VLDL* (mg/dL) |
T1 | 68 (26)a | 86 (33)a,b | 113 (66)b | <0.001 | 0.016 | 0.013 |
| T2 | 173 (101) | 176 (54) | 202 (91) | ||||
| T3 | 202 (123) | 220 (103) | 205 (80) | ||||
| PN | 55 (27)a | 83 (40)a,b | 118 (69)b | ||||
| IDL* (mg/dL) |
T1 | 58 (19) | 71 (29) | 85 (63) | <0.001 | 0.44 | 0.30 |
| T2 | 126 (54) | 116 (29) | 136 (52) | ||||
| T3 | 146 (51) | 153 (59) | 157 (101) | ||||
| PN | 70 (29) | 90 (21) | 82 (32) | ||||
| LDL (mg/dL) |
T1 | 74 (22) | 80 (24) | 89 (34) | <0.001 | 0.98 | 0.06 |
| T2 | 140 (46) | 109 (33) | 117 (47) | ||||
| T3 | 111 (19) | 121 (59) | 101 (37) | ||||
| PN | 74 (19) | 80 (26) | 84 (30) | ||||
Plasma NEFA levels did not change over gestation and were unrelated to maternal BMI. The mass concentrations of VLDL, IDL and LDL increased during pregnancy and declined post-natally (all P<0.001) (Table 2). However, plasma VLDL concentration was significantly higher in maternal obesity (P=0.016), with normal weight women having the lowest concentrations. There was an interaction between trimester and BMI category for total cholesterol (P=0.026), but differences did not persist on post hoc testing. Compositional analysis of IDL and LDL (Supplemental Table 1) indicated that measures of TG-enrichment increased over gestation and declined in the post-natal period, but there was no effect of maternal BMI. Time*BMI interactions were evident for plasma TG (P=0.002) and VLDL mass (P=0.013) concentrations (Table 2) with normal weight and obese groups significantly different at the nadirs in T1 and in the post-natal period, but reaching similar peak levels in late gestation.
VLDL subfraction concentration and composition
Plasma VLDL-1 and VLDL-2 increased from T1 to T3 and declined in the post-natal period (P<0.001) (Table 3). Maternal obesity status had a significant impact on these changes (P=0.044 and P=0.044 respectively). The ratio of VLDL-1/VLDL-2 was not influenced by either time of sampling or BMI. There was an interaction between time and BMI for VLDL-1 with a 3-fold higher post-natal level of VLDL-1 in obese compared with normal weight women. The TG-carrying capacity of VLDL-1 and VLDL-2 also increased over gestation and this remained high in the post-natal period (P<0.001 compared to T1 levels) and there was no impact of maternal obesity (Supplemental Table 1).
Table 3. Maternal VLDL and LDL subfraction concentrations during and after pregnancy according to WHO BMI category.
Mean (standard deviation) are shown.
T1 – trimester 1 [12.5 (1.2) weeks]; T2 – trimester 2 [26.1 (1.3) weeks], T3 – trimester 3 [35.5 (1.3) weeks]; PN – postnatal [17.1 (2.9) weeks post delivery]. P Time for association of lipid/lipoprotein parameters over time, P BMI for association with BMI category and P Time*BMI for the interaction between Time and BMI, using a univariate split-plot approach repeated measures analysis. Where the interaction between Time and BMI was significant (P<0.05), one way ANOVA and Tukey Kramer post-hoc tests were conducted. Different superscript letters indicate differences between BMI categories at a particular time point using post hoc Tukey test. Statistical analysis carried out on *log or #square root transformed data.
| Time of sampling |
Normal weight |
Overweight | Obese | P Time |
P BMI |
P Time * BMI |
|
|---|---|---|---|---|---|---|---|
| VLDL-1# (mg/dL) |
T1 | 15 (8) | 21 (12) | 26 (15) | <0.001 | 0.044 | 0.010 |
| T2 | 43 (29) | 44 (21) | 52 (26) | ||||
| T3 | 51 (30) | 51 (29) | 49 (24) | ||||
| PN | 13 (9)a | 20 (10)a,b | 40 (29)b | ||||
| VLDL-2* (mg/dL) |
T1 | 53 (18) | 66 (24) | 87 (61) | <0.001 | 0.044 | 0.12 |
| T2 | 130 (74) | 132 (39) | 150 (77) | ||||
| T3 | 152 (97) | 170 (83) | 156 (72) | ||||
| PN | 41 (23) | 64 (36) | 77 (47) | ||||
|
| |||||||
| VLDL-1/ VLDL-2* Ratio |
T1 | 0.27 (0.10) | 0.31 (0.15) | 0.34 (0.19) | 0.13 | 0.28 | 0.46 |
| T2 | 0.33 (0.17) | 0.33 (0.12) | 0.39 (0.22) | ||||
| T3 | 0.36 (0.14) | 0.33 (0.19) | 0.37 (0.22) | ||||
| PN | 0.38 (0.27) | 0.38 (0.26) | 0.55 (0.25) | ||||
|
| |||||||
| LDL-I# (mg/dL) |
T1 | 22 (10) | 14 (12) | 17 (12) | <0.001 | 0.15 | 0.34 |
| T2 | 20 (12) | 19 (17) | 20 (13) | ||||
| T3 | 18 (7) | 20 (70) | 19 (11) | ||||
| PN | 48 (25) | 42 (25) | 29 (16) | ||||
| LDL-II# (mg/dL) |
T1 | 81 (34) | 71 (26) | 68 (24) | 0.053 | 0.070 | 0.19 |
| T2 | 92 (29) | 70 (42) | 80 (32) | ||||
| T3 | 107 (27) | 84 (34) | 70 (27) | ||||
| PN | 101 (47) | 76 (44) | 104 (54) | ||||
| LDL-III# (mg/dL) |
T1 | 35 (22) | 32 (20) | 40 (26) | <0.001 | 0.12 | 0.20 |
| T2 | 50 (35) | 62 (37) | 64 (34) | ||||
| T3 | 35 (21) | 49 (20) | 60 (28) | ||||
| PN | 28 (13) | 25 (33) | 62 (68) | ||||
LDL subfraction concentration and distribution
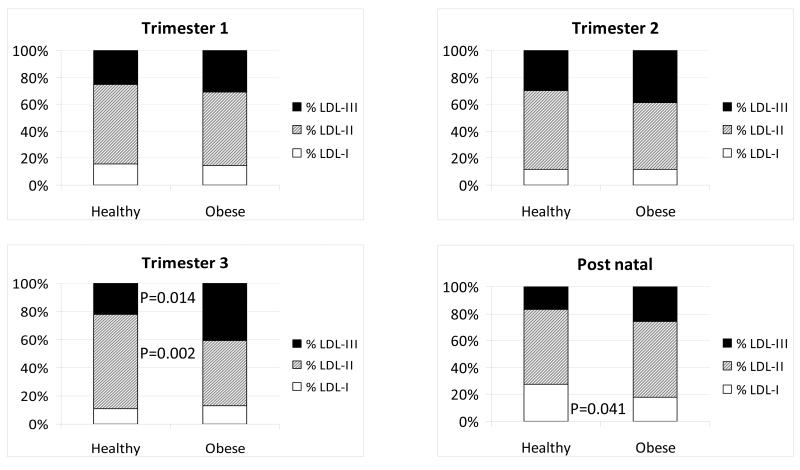
The mass of large, light LDL-I and small, dense LDL-III changed over gestation, whereas LDL-II mass was unchanged (Table 3). LDL-I mass was lowest at T1 (P<0.001) and LDL-III mass was higher at T2 (P<0.001) and T3 (P=0.07) compared to both T1 and post-natal samples (one way ANOVA plus post hoc testing using the whole group, Table 3). There was no overall impact of maternal obesity on LDL subfraction concentrations and no interaction between LDL subfraction concentration and obesity. Figure 1 shows the percentage distribution of LDL subfractions over pregnancy and post-natally. By the end of T3, obese women had a significantly higher proportion of small, dense LDL-III (P=0.014) and a significantly lower proportion of intermediate LDL-II (P=0.002) than normal weight women. Overweight women had LDL subfraction distributions between those of the normal and obese women. In T3 35% of obese, 14 % of overweight and none of normal weight women had an atherogenic LDL subfraction profile, “Pattern B” (greater than 50% small, dense LDL-III). This shift in distribution had resolved by three months post-natal, but there was still a higher proportion of LDL-I particles in normal weight compared to obese women (Figure 1).
Figure 1. LDL subfraction distribution during pregnancy.
Percentage abundance of LDL-I, LDL-II and LDL-III in normal weight and obese individuals in each trimester of pregnancy and three months post-natal. Differences in %LDL subfraction between normal weight and obese mothers at each sampling point was carried by one way ANOVA and post hoc Tukey Kramer tests.
Associations between plasma lipoprotein lipase mass, hormone and lipoprotein response to pregnancy
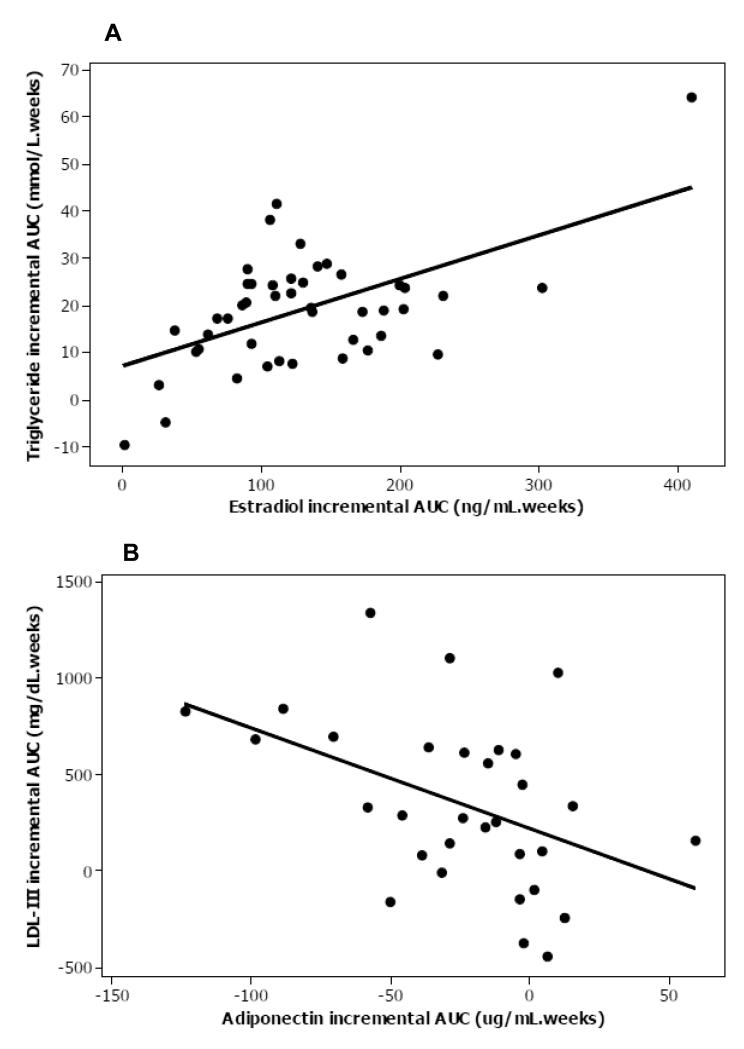
Plasma LPL mass and hormone concentrations are shown (Table 4). S447X LPL genotype was significantly associated with LPL mass (P<0.0001) as described (25). LPL mass declined over gestation, independent of genotype, reaching a nadir in T3 but there was no impact of maternal BMI. Plasma estradiol, SHBG, insulin, HOMA and leptin (Supplemental Figure 2) increased over pregnancy and declined in the post-natal period (P<0.001), whereas plasma adiponectin decreased during pregnancy (P<0.001) (Supplemental Figure 2). Plasma glucose increased during pregnancy and remained elevated in the post-natal period (P<0.001), and there was an impact of BMI, with normal weight women having lower T1 glucose levels (P=0.038). Higher BMI was associated with lower plasma estradiol (P=0.017), SHBG (P=0.038) and adiponectin (P=0.002), but higher leptin (P<0.001). There was a time*BMI interaction for estradiol and leptin but not adiponectin. Plasma LPL mass was inversely associated with TG across all time points in the total group (r=−0.71, r2 adjusted 50.1%, P<0.001); an association that was strengthened by the inclusion of LPL S447X genotype in the model (r2 adjusted 84.6%, P<0.001). The LPL response to pregnancy was not associated with the TG response to pregnancy (independent of S447X genotype). Obese mothers had lower estradiol concentrations than normal weight women in T2 and T3 and higher leptin levels at all time points. Incremental AUC total TG (r2 adjusted 25%, P<0.001) (Figure 2A), VLDL cholesterol (15%, P=0.006), VLDL-1 (13%, P=0.015) and VLDL-2 (22%, P=0.002) were positively associated with incremental AUC plasma estradiol. Incremental AUC insulin (or HOMA) did not explain the variation in TG-rich lipoprotein concentrations, nor in incremental AUC leptin or adiponectin. Incremental AUC adiponectin was negatively associated with incremental AUC LDL-III mass (17%, P=0.013) (Figure 2B). Maternal booking BMI (P=0.004), plasma leptin in T3 (P=0.003) and adiponectin (P=0.010) explained a total of 31% of variance in the relative proportion of small, dense LDL. None of the other measured variables contributed to these associations in multivariate analysis.
Table 4. Maternal plasma LPL and hormone levels during and after pregnancy according to WHO BMI category.
Mean (standard deviation) are shown.
T1 – trimester 1 [12.5 (1.2) weeks]; T2 – trimester 2 [26.1 (1.3) weeks], T3 – trimester 3 [35.5 (1.3) weeks]; PN – postnatal [17.1 (2.9) weeks post delivery]. P Time for association of lipid/lipoprotein parameters over time, P BMI for association with BMI category and P Time*BMI for the interaction between Time and BMI, using a univariate split-plot approach repeated measures analysis. Where the interaction between Time and BMI was significant (P<0.05), one way ANOVA and Tukey Kramer post-hoc tests were conducted. Different superscript letters indicate differences between BMI categories at a particular time point using post hoc Tukey test. Statistical analysis carried out on *log or #square root transformed data.
| Time of sampling |
Normal weight |
Overweight | Obese | P Time |
P BMI |
P Time * BMI |
|
|---|---|---|---|---|---|---|---|
| Lipoprotein lipase mass* (ng/mL) |
T1 | 43 (16) | 32 (10) | 35 (14) | <0.001 | 0.14 | 0.32 |
| T2 | 29 (12) | 22 (8) | 21 (8) | ||||
| T3 | 23 (11) | 19 (5) | 21 (7) | ||||
| PN | 46 (11) | 47 (11) | 45 (14) | ||||
|
| |||||||
| Estradiol# (ng/mL) |
T1 | 3.1 (1.8) | 2.8 (1.4) | 2.5 (1.4) | <0.001 | 0.017 | 0.023 |
| T2 | 12.2 (4.0)a | 10.0 (3.3)a,b | 8.4 (3.3)b | ||||
| T3 | 20.0 (13.6)a | 13.4 (3.1)a,b | 11.5 (3.2)b | ||||
| PN | 0.05 (0.03) | 0.03 (0.02) | 0.11 (0.21) | ||||
| SHBG (mmol/L) |
T1 | 221 (38) | 218 (34) | 191 (36) | <0.001 | 0.038 | 0.23 |
| T2 | 309 (99) | 251 (33) | 252 (63) | ||||
| T3 | 285 (66) | 259 (23) | 253 (26) | ||||
| PN | 59 (27) | 54 (11) | 68 (52) | ||||
|
| |||||||
| Insulin* (mU/L) |
T1 | 16 (7) | 20 (23) | 25 (22) | <0.001 | 0.17 | 0.85 |
| T2 | 32 (29) | 38 (26) | 58 (41) | ||||
| T3 | 54 (46) | 44 (45) | 48 (29) | ||||
| PN | 19 (12) | 31 (52) | 27 (18) | ||||
| Glucose* (mmol/L) |
T1 | 4.0 (0.9) | 4.8 (1.1) | 5.0 (0.7) | <0.001 | 0.038 | 0.069 |
| T2 | 4.6 (0.7) | 5.5 (1.2) | 5.6 (1.2) | ||||
| T3 | 5.2 (0.8) | 5.2 (0.9) | 5.4 (1.2) | ||||
| PN | 5.2 (1.0) | 5.3 (0.7) | 5.4 (0.6) | ||||
| HOMA* | T1 | 2.9 (1.6) | 5.0 (0.3) | 6.0 (1.0) | <0.001 | 0.12 | 0.77 |
| T2 | 6.8 (5.9) | 10.5 (10.0) | 15.4 (11.6) | ||||
| T3 | 14 (13) | 11 (14) | 13 (11) | ||||
| PN | 4.5 (3.7) | 7.4 (11.9) | 6.9 (5.1) | ||||
| Leptin* (ng/mL) |
T1 | 12 (5)a | 18 (6)b | 40 (13)c | <0.001 | <0.001 | 0.010 |
| T2 | 17 (12)a | 22 (10)a | 44 (20)b | ||||
| T3 | 18 (18)a | 24 (14)a | 43 (21)b | ||||
| PN | 12 (8)a | 11 (5)a | 39 (14)b | ||||
| Adiponectin* (ug/mL) |
T1 | 10.9 (3.1) | 10.2 (2.5) | 7.6 (3.4) | <0.001 | 0.002 | 0.42 |
| T2 | 9.3 (3.2) | 8.3 (2.4) | 6.3 (2.8) | ||||
| T3 | 9.1 (3.4) | 8.0 (2.4) | 6.8 (2.3) | ||||
| PN | 10.1 (5.0) | 8.2 (1.7) | 6.5 (2.8) | ||||
Figure 2. Univariate association between plasma response to pregnancy of plasma triglyceride and estradiol and LDL-III mass and adiponectin.
A) Incremental AUC total triglyceride was associated with incremental AUC plasma estradiol, r2 adjusted 25%, P<0.001. B) Incremental AUC adiponectin was associated with incremental AUC LDL-III mass r2 adjusted 17%, P=0.013.
Discussion
The concentration of all plasma lipids and lipoprotein fractions increased over pregnancy, with the exception of NEFA. The changes in total cholesterol, total TG, VLDL and LDL represent the physiological hyperlipidaemic adaptation to pregnancy, where lipids are mobilized into the maternal circulation to supply fuel and essential nutrients for the fetus. Maternal obesity was associated with higher plasma levels of TG, VLDL-1 and VLDL-2 predominately at T1 and 17 weeks post delivery. By later gestations, plasma TG and TG-rich lipoproteins appeared to reach the same maximal level. This indicates that obese mothers are less metabolically flexible, in terms of the extent of the metabolic adaptation to pregnancy, than their normal weight counterparts. Estradiol incremental AUC was significantly associated with plasma TG, VLDL-cholesterol, VLDL-1 and VLDL-2 response to pregnancy with no change in VLDL-1 to VLDL-2 total mass ratio during pregnancy. These data indicate that estradiol up-regulates the secretion of both VLDL subfractions equally from the liver to promote the gestational increase in plasma TG. We are unable to assess the relative contributions to the gestational increase in VLDL-2 mass of direct synthesis of VLDL-2 by the liver and conversion of VLDL-1 to VLDL-2 by lipolysis. Kinetic studies in non-pregnant women show that production rates of both large and small VLDL fractions are increased by low dose oral contraceptives(26). Our data are also consistent with the strong relationship observed between the gestational rise in estradiol and the increment in plasma TG in a small group of normal weight pregnant women(15). We found an interaction between BMI and time for estradiol, with obese women having significantly lower estradiol levels in late pregnancy than normal weight women. This is likely due to the sequestration of estradiol in adipose tissue(27). It is notable that measures of insulin resistance did not contribute to gestational change in VLDL concentration. This suggests that the effects of estradiol on VLDL production by the liver may override any insulin effects of VLDL production during pregnancy.
The VLDL compositional data suggest that as pregnancy progresses all TG-rich lipoprotein fractions carry more TG molecules per particle. Although plasma LPL mass was inversely correlated with TG at all time points, LPL incremental AUC (which is negative due to decreasing LPL levels during pregnancy) was not associated with plasma TG response to pregnancy. This finding was independent of the influence of the S447X LPL polymorphism which, for as yet unknown reasons, has a large influence on plasma LPL mass, post-heparin LPL activity and plasma TG levels(25). LPL mass is a poor surrogate of total lipase activity, as it is mostly inactive(22-23). It has been suggested in the non-pregnant, that low LPL mass is a marker of metabolic syndrome and that this reflects the low rate of LPL synthesis by adipocytes in the insulin resistant state(28-29). However, we did not observe a BMI effect on LPL mass in pregnancy. We speculate that steady state plasma levels of TG are determined predominately by increased VLDL production in response to estradiol, rather than by LPL-mediated clearance.
During pregnancy, mass levels of LDL-I and LDL-II were unchanged but the mass of LDL-III increased, particularly in overweight and obese individuals. This was reflected in a significantly higher proportion of LDL-III in obese mothers in T3. Large LDL subfractions are remodelled into smaller, denser LDL-III as plasma TG rises above a threshold of 1.5mmol/L(12), an effect that is facilitated by hepatic lipase and cholesteryl ester transfer protein(30). Obese mothers have raised baseline TG and consequently reach the threshold concentration of TG earlier and more easily than normal weight women. A high proportion of the overweight and obese women had greater than 50% LDL-III in T3. This indicates a shift in their LDL subfraction profile from a healthy “Pattern A” phenotype to an atherogenic “Pattern B” phenotype(21). Small dense LDL-III particles are susceptible to oxidation and oxidised LDL is inversely associated with vascular function in subjects exhibiting cardiovascular risk factors(31). A predominance of LDL-III is a hallmark of conditions in which there is an accumulation of ectopic liver fat, such as non-alcoholic fatty liver disease(32) and type 2 diabetes(14). Thirty five percent of the obese pregnant women had an LDL “Pattern B” profile by T3. This suggests that a subgroup of obese women are less able to cope with the gestational increase in TG, are predisposed to store fat ectopically and are at risk of GDM and PE(9). In theory, fatty liver has been linked to the formation of small, dense LDL through the production and release of TG-enriched VLDL(14). However, there was no evidence in the present study to support a link between the increase in LDL-III and large TG-rich VLDL in obese women. Instead, the relative mass and proportion of LDL-III in T3 were associated with plasma adiponectin levels, and also maternal obesity, leptin and adiponectin, in respective multivariate models. The rise of leptin levels to a T2 peak and the fall of adiponectin to a T3 nadir in pregnancy have previously been reported(33-34). While these changes are suggested to be due to gestational maternal fat accumulation, we found no evidence that maternal insulin resistance played a role, and others suggest that additional factors determine at least leptin levels in pregnancy(35). Adiponectin levels are reduced in obesity and lower still in non-alcoholic fatty liver disease(36). Adiponectin has been reported to reduce fatty acid synthesis via SREBP-1c, activate fatty acid oxidation via AMP kinase and to have anti-inflammatory and anti-oxidative effects at the liver(37). The gestational levels of adiponectin reached by the obese pregnant women (mean 6.8, SD 2.3ug/mL) are in a similar range to obese individuals with nonalcoholic hepatic steatosis (5.6ug/mL), whereas normal weight pregnant women and obese individuals without non-alcoholic hepatic steatosis have similar levels of around 10-11ug/mL(36).
Plasma NEFA levels were stable over pregnancy, as has been observed for non-pregnant obesity, insulin resistance and well-controlled type 2 diabetes(38). Although NEFA concentration does not change this provides no information on NEFA flux; it merely suggests that rates of entry of NEFA into the blood via lipolysis and exit by uptake into tissues are equal. There were no associations between maternal obesity and cholesterol concentrations, levels and composition of cholesterol-rich lipoproteins or plasma NEFA. This observation is analogous to non-pregnant obese individuals, where the predominant phenotype is of metabolic syndrome characterised by raised TG, low HDL levels but often normal cholesterol levels.
The majority of lipid changes in response to pregnancy had resolved to baseline levels by three months after pregnancy. However, TG-enrichment of VLDL-1, and VLDL-2 remained high post-natally. Furthermore, LPL mass concentration was significantly higher in the post-natal period than at any time during pregnancy. This may represent an adaptation for breast-feeding, facilitating utilisation of TG by mammary tissue. Unfortunately, we do not have a record of breast-feeding activity in our women. We also showed that VLDL-1 levels remain significantly elevated in obese women post-pregnancy. This could be explained by the relative loss of estradiol and a re-emergence of the predominant effect of insulin resistance, with resulting failure to suppress VLDL-1 secretion from the liver(26).
The strengths of this study are the longitudinal design, wide range of BMI, larger numbers than reported previously and concurrent measurements of lipids and hormones. There are a variety of methods for separating LDL subfractions ranging from the specialist analytical or density gradient ultracentrifugation and nuclear magnetic resonance, to more accessible gradient and tube gel electrophoresis. While a definitive classification of LDL subfractions has not been established, the method used here, density gradient electrophoresis, identifies the “classic” LDL-I, LDL-II and LDL-III subfractions that have an established link to metabolic disease risk(12). A weakness is the lack of direct enzyme activity measurements. We were unable to measure activities of LPL (and hepatic lipase) in plasma as the intravenous injection of heparin to release the functional pool of lipases from the endothelium is ethically not acceptable in pregnant women. Furthermore, we lacked detailed dietary and breast-feeding data, and the absence of a kinetic analysis of lipoprotein metabolism left us unable to comment on the inter-conversion of VLDL-1 to VLDL-2.
Increased plasma levels of TG and cholesterol is an essential adaptation to pregnancy. Normal weight mothers enter pregnancy with a healthy lipid profile and increase levels of cholesterol and TG-rich lipoproteins to a maximum, which decline to pre-pregnancy levels post-natally. Obese mothers begin pregnancy with higher levels of TG-rich lipoproteins and these rise to the same level as normal weight women by late gestation. Although in quantitative terms, the plasma lipids of obese mothers are equivalent to that of their normal weight counterparts, the quality of their lipids may convey greater cardio-metabolic risk due to the formation of small, dense LDL. Not all obese women develop metabolic complications of pregnancy. We speculate that a subset of obese women are susceptible to the accumulation of ectopic fat in the liver, perhaps due to low adiponectin levels, and accompanying adverse changes in LDL composition. Small dense LDL has been observed in both PE and GDM(17-18) and these conditions are susceptible to fatty liver in pregnancy(39). Our data suggest that a subset of obese women, at high risk for PE and GDM, may be identified for targeted intervention. Such intervention may include specific dietary manipulations, such as long chain n-3 polyunsaturated fatty acid supplementation, which are known to exert a favourable impact on the pathways described herein. Response to pregnancy may also reveal at an early stage in a woman’s life her susceptibility to metabolic abnormalities and fatty liver later in life(40).
Supplementary Material
Acknowledgments
We gratefully acknowledge the generous support of: British Heart Foundation (PG/02/167/14801), British Medical Association Research Grant, The Biochemical Society Travel Grant, The Carnegie Trust, The Harold Hyam Wingate Foundation, University of Wollongong Study Leave Assistance Grants.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. International journal of obesity (2005) 2010;34:420–428. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 2.Jarvie E, Ramsay JE. Obstetric management of obesity in pregnancy. Seminars in fetal & neonatal medicine. 2010;15:83–88. doi: 10.1016/j.siny.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology (Cambridge, Mass) 2003;14:368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 5.Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr. 1998;132:768–776. doi: 10.1016/s0022-3476(98)70302-6. [DOI] [PubMed] [Google Scholar]
- 6.Huda SSSN, Freeman DJ. Lipoprotein metabolism and vascular complications in pregnancy. Clinical Lipidology. 2009;4:91–102. [Google Scholar]
- 7.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. The Journal of clinical endocrinology and metabolism. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 8.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. The Journal of clinical endocrinology and metabolism. 2007;92:969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 9.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clinical science (London, England : 1979) 2010;119:123–129. doi: 10.1042/CS20090640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–3556. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 11.Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. International journal of obesity (2005) 2008;32:1655–1664. doi: 10.1038/ijo.2008.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241–253. doi: 10.1016/0021-9150(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 13.Cali AM, Zern TL, Taksali SE, de Oliveira AM, Dufour S, Otvos JD, Caprio S. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes care. 2007;30:3093–3098. doi: 10.2337/dc07-1088. [DOI] [PubMed] [Google Scholar]
- 14.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes care. 2006;29:1845–1850. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 15.Sattar N, Greer IA, Louden J, Lindsay G, McConnell M, Shepherd J, Packard CJ. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small, dense low density lipoprotein. The Journal of clinical endocrinology and metabolism. 1997;82:2483–2491. doi: 10.1210/jcem.82.8.4126. [DOI] [PubMed] [Google Scholar]
- 16.Mackay VA, Huda SS, Stewart FM, Tham K, McKenna LA, Martin I, Jordan F, Brown EA, Hodson L, Greer IA, Meyer BJ, Freeman DJ. Preeclampsia is associated with compromised maternal synthesis of long-chain polyunsaturated Fatty acids, leading to offspring deficiency. Hypertension. 2012;60:1078–1085. doi: 10.1161/HYPERTENSIONAHA.112.197897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo M, Berneis K, Altinova AE, Toruner FB, Akturk M, Ayvaz G, Rini GB, Spinas GA, Arslan M. Atherogenic lipoprotein phenotype and LDL size and subclasses in women with gestational diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2008;25:1406–1411. doi: 10.1111/j.1464-5491.2008.02613.x. [DOI] [PubMed] [Google Scholar]
- 18.Belo L, Caslake M, Gaffney D, Santos-Silva A, Pereira-Leite L, Quintanilha A, Rebelo I. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis. 2002;162:425–432. doi: 10.1016/s0021-9150(01)00734-1. [DOI] [PubMed] [Google Scholar]
- 19.Carstairs V, Morris R. Deprivation and mortality: an alternative to social class? Community medicine. 1989;11:210–219. doi: 10.1093/oxfordjournals.pubmed.a042469. [DOI] [PubMed] [Google Scholar]
- 20.Griffin BA, Caslake MJ, Yip B, Tait GW, Packard CJ, Shepherd J. Rapid isolation of low density lipoprotein (LDL) subfractions from plasma by density gradient ultracentrifugation. Atherosclerosis. 1990;83:59–67. doi: 10.1016/0021-9150(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 21.Davies IG, Graham JM, Griffin BA. Rapid separation of LDL subclasses by iodixanol gradient ultracentrifugation. Clinical chemistry. 2003;49:1865–1872. doi: 10.1373/clinchem.2003.023366. [DOI] [PubMed] [Google Scholar]
- 22.Tornvall P, Olivecrona G, Karpe F, Hamsten A, Olivecrona T. Lipoprotein lipase mass and activity in plasma and their increase after heparin are separate parameters with different relations to plasma lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1086–1093. doi: 10.1161/01.atv.15.8.1086. [DOI] [PubMed] [Google Scholar]
- 23.Vilella E, Joven J, Fernandez M, Vilaro S, Brunzell JD, Olivecrona T, Bengtsson-Olivecrona G. Lipoprotein lipase in human plasma is mainly inactive and associated with cholesterol-rich lipoproteins. Journal of lipid research. 1993;34:1555–1564. [PubMed] [Google Scholar]
- 24.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ (Clinical research ed) 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rip J, Nierman MC, Ross CJ, Jukema JW, Hayden MR, Kastelein JJ, Stroes ES, Kuivenhoven JA. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- 26.Walsh BW, Sacks FM. Effects of low dose oral contraceptives on very low density and low density lipoprotein metabolism. The Journal of clinical investigation. 1993;91:2126–2132. doi: 10.1172/JCI116437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larner JM, Shackleton CH, Roitman E, Schwartz PE, Hochberg RB. Measurement of estradiol-17-fatty acid esters in human tissues. The Journal of clinical endocrinology and metabolism. 1992;75:195–200. doi: 10.1210/jcem.75.1.1619010. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi J, Nohara A, Kawashiri MA, Inazu A, Koizumi J, Nakajima K, Mabuchi H. Serum lipoprotein lipase mass: clinical significance of its measurement. Clin Chim Acta. 2007;378:7–12. doi: 10.1016/j.cca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Saiki A, Oyama T, Endo K, Ebisuno M, Ohira M, Koide N, Murano T, Miyashita Y, Shirai K. Preheparin serum lipoprotein lipase mass might be a biomarker of metabolic syndrome. Diabetes Res Clin Pract. 2007;76:93–101. doi: 10.1016/j.diabres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Watson TD, Caslake MJ, Freeman DJ, Griffin BA, Hinnie J, Packard CJ, Shepherd J. Determinants of LDL subfraction distribution and concentrations in young normolipidemic subjects. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1994;14:902–910. doi: 10.1161/01.atv.14.6.902. [DOI] [PubMed] [Google Scholar]
- 31.Aragones G, Ferre R, Girona J, Plana N, Merino J, Heras M, Masana L. Small artery dilation and endothelial markers in cardiovascular risk patients. European journal of clinical investigation. 2012;42:34–41. doi: 10.1111/j.1365-2362.2011.02553.x. [DOI] [PubMed] [Google Scholar]
- 32.Sugino I, Kuboki K, Matsumoto T, Murakami E, Nishimura C, Yoshino G. Influence of fatty liver on plasma small, dense LDL- cholesterol in subjects with and without metabolic syndrome. Journal of atherosclerosis and thrombosis. 2011;18:1–7. doi: 10.5551/jat.5447. [DOI] [PubMed] [Google Scholar]
- 33.Paradisi G, Ianniello F, Tomei C, Bracaglia M, Carducci B, Gualano MR, La Torre G, Banci M, Caruso A. Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol. 2010;26:539–545. doi: 10.3109/09513591003632084. [DOI] [PubMed] [Google Scholar]
- 34.Sattar N, Greer IA, Pirwani I, Gibson J, Wallace AM. Leptin levels in pregnancy: marker for fat accumulation and mobilization? Acta Obstet Gynecol Scand. 1998;77:278–283. [PubMed] [Google Scholar]
- 35.Misra VK, Trudeau S. The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity (Silver Spring, Md) 2011;19:416–421. doi: 10.1038/oby.2010.172. [DOI] [PubMed] [Google Scholar]
- 36.Targher G, Bertolini L, Scala L, Poli F, Zenari L, Falezza G. Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clinical endocrinology. 2004;61:700–703. doi: 10.1111/j.1365-2265.2004.02151.x. [DOI] [PubMed] [Google Scholar]
- 37.Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World journal of gastroenterology : WJG. 2011;17:2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clinics and research in hepatology and gastroenterology. 2011;35:182–193. doi: 10.1016/j.clinre.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Forbes S, Taylor-Robinson SD, Patel N, Allan P, Walker BR, Johnston DG. Increased prevalence of non-alcoholic fatty liver disease in European women with a history of gestational diabetes. Diabetologia. 2011;54:641–647. doi: 10.1007/s00125-010-2009-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.