Abstract
The butyrophilin-related protein Btn2a2 was up-regulated on murine antigen presenting cells including CD19+ B cells, CD11b+ F4/80+ peritoneal macrophages, and CD11c+ bone marrow-derived dendritic cells after activation with LPS or Pam3CysK4, suggesting a role in modulation of T lymphocytes. Consistent with this, binding of mouse Btn2a2-Fc to CD3+ primary mouse T cells stimulated with anti-CD3 and anti-CD28 reduced the number of proliferating cells and entry of cells into the cell cycle. Binding of Btn2a2-Fc to anti-CD3− stimulated T cells inhibited CD3ε, Zap70, and subsequent Erk1/2 activation. It also interfered with activation of the regulatory subunit of PI3K, p85, and activation of Akt in T cells stimulated with both anti-CD3 and anti-CD28. Inhibition of Akt activation by Btn2a2-Fc was, in contrast to inhibition by PD-L1-Fc, not overcome by anti-CD28 co-stimulation. Using Foxp3-GFP transgenic, naïve T cells, Btn2a2-Fc induced de novo expression of Foxp3 in a dose-dependent manner and Btn2a2-Fc-induced CD4+CD25+Foxp3+ T cells had inhibitory properties. The data indicate an important physiological role for Btn2a2 in inhibiting T cell activation and inducing Foxp3+ regulatory T cells.
Keywords: T cells, Co-stimulation, Suppression, Signal Transduction
Introduction
T lymphocyte activation requires two classes of signals: antigen-specific and co-regulatory. B7 protein family molecules engage ligands on T cells involved in co-regulation and include a number of butyrophilin-related molecules. Butyrophilin, a type I transmembrane glycoprotein, was purified from bovine milk (1). The human BTN1A1 gene mapped to the extended MHC region (2). Nearby, six related genes grouped into three families: BTN2A1, BTN2A2, BTN2A3, BTN3A1, BTN3A2, and BTN3A3 (3; 4). Genes orthologous to BTN1A1 and BTN2A2, Btn1a1 and Btn2a2, respectively, were mapped to mouse chromosome 13. Another butyrophilin-related gene, near HLA-DRA, was named BTNL-II or BTNL2, Btnl2 in mouse (5) and three other butyrophilin-like genes on chromosome 5 were named BTNL3, BTNL8, and BTNL9 (6). Other distant relatives of BTNL2 in mouse are Btnl1, Btnl5, Btnl6, Btnl7, and Btnl9 (7) and the Skint genes (8; 9).
BTN1A1 was expressed predominantly in mammary gland tissue (10; 11), although mouse Btn1a1 was detected in other tissues, including thymic epithelial cells (12). BTN2A1 and 2A2 were detected in many tissues (3; 13). Similarly, mouse Btn2a2 protein was found on the surface of non-activated CD19+ B cells, CD11c+ dendritic cells (DC), CD11b+ F4/80+ peritoneal macrophages, NK1.1+ NK cells and on CD3+T cells, when activated and, by immunofluoresence, on thymic epithelial cells (12). Human BTN3 proteins (BTN3A1, A2, A3) were detected on a variety of cells and tissues (14; 15). Mouse Btnl1 was expressed on bone marrow-derived DC, macrophages, and activated B cells (16) and at high levels in the small intestine, where its expression on enterocytes was increased after treatment with IFN-γ (17). Mouse Btnl2 was also widely expressed (5; 18; 19).
It has been suspected that butyrophilin family molecules would have a co-receptor role, with the possible exception of BTN1A1, which, through homotypic interaction facilitates milk droplet secretion (20). However, exosomes in human breast milk, containing BTN1A1, inhibited cytokine production by PBMC and led to an expansion of CD4+ Foxp3+ T cells (21). In support of a co-receptor role, mouse Btn1a1-Fc or Btn2a2-Fc fusion proteins inhibited T cell proliferation, and IL-2 and IFN-γ production by CD4+ or CD8+ T cells, activated with anti-CD3 or anti-CD3 and anti-CD28 (12). A dose-dependent inhibition of anti-CD3 and anti-CD28-induced T cell proliferation was also observed with plate-bound mouse Btnl2-Fc (18; 19). In addition, inhibition of IL-2 production by Btnl2-Fc was detected (19). Btnl2 engagement overcame the effects of the positive co-regulatory molecule ICOSL on T cell proliferation and reduced secretion of cytokines such as TNF-α, GM-CSF, IL-2, IL-4, IL-6, IL-17, IFN-γ but not IL-10 (18). Btnl1 also affected T cell proliferation through inhibition of cell cycle entry (16). For BTN3A1, also called BTN3A, a stimulatory role in stress sensing by γδ-T cell was demonstrated when bound by a specific antibody (22; 23).
In an EAE mouse model, a blocking anti-Btnl1 antibody led to induction of EAE after vaccination with low doses of MOG (16). The antibody led to increased Th17 cells and IL-17 cytokine levels, suggesting a protective role for Btnl1 in the pathogenesis of EAE by preventing Th17 polarization (16). Using a model system for the interaction of intra epithelial lymphocytes (IEL) it was shown that Btnl1 on enterocytes inhibited IL-6 and IFN-γ production by these cells (17).
We set out to investigate the function of Btn2a2 in relation to T lymphocyte regulation.
Materials and methods
C57BL/6 mice at age 6 wk were from Harlan Laboratories (Loughborough, UK). C57BL/6 and NOD Foxp3-GFP transgenic mice were housed in specific pathogen-free conditions according to Home Office requirements. Experiments were approved by Ethical Review Committee.
Cells
Mouse cells from were harvested as described (12). CD3+ or CD4+ T cells were enriched by negative-selection (Stem Cell Technologies, London, UK). When naïve T cells of NOD Foxp3-GFP mice were purified, negatively enriched CD4+ T cells were stained with anti-CD4, anti-CD25, and anti-CD62L antibodies at 1 μg/ml (eBioscience, Hatfield, UK) and sorted for CD4+ CD25− CD62Lhigh Foxp3-GFP− cells. CD19+ B cells were enriched from spleen by positive selection. Macrophages were from peritoneal lavage. Bone marrow-derived DC were prepared as described (24). CD3+ 2B4 cells (25) were maintained in complete RPMI, 5% FCS.
Professional APCs were activated in RPMI with 10% FCS, 100 ng/ml LPS (Sigma) or 1 μg/ml Pam3CysK4 for 16h. For T cell signaling and activation, plates were coated with 1 μg/ml anti-CD3 clone 145-2C11, 1-5 μg/ml anti-CD28 clone 37.51 (eBioscience), 10 μg/ml anti-human Fc (Newmarket Scientific, Kennett, UK) and 10 μg/ml Fc fusion protein (12).
For signaling assays, CD3+ primary T cells, or 2B4 cells, were seeded at 0.5× 106 /ml, centrifuged and incubated at 37°C and 5% CO2. Stimulation was stopped using ice cold PBS. For analysis of cell cycle progression or Foxp3-GFP expression, cells were incubated in RPMI with 10% FCS, 50 μM β-mercaptoethanol, and 2 mM L-Glutamine (both Sigma-Aldrich) for 3 to 7 d at 37°C and 5% CO2. Human rTGF-β1 (TGF–β) was from R&D Systems (Abingdon, UK).
For T reg-induction experiments, CD4+CD25−CD62LhighFoxp3− cells were activated with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28, 10 μg/ml anti-human Fc, and 10 μg/ml BTN2A2-Fc at 0.5× 106 cells/ml for 4 d. Induced Treg (CD4+CD25+ Foxp3+), or control cells, were sorted and mixed with non-activated CD3+CD25−Foxp3− T cells 1:1, the latter labeled with 1 μM eFluor647 (eBioscience). Mixed cells were cultured at 1.0× 106 /ml with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28, and 10 μg/ml Fc fusion protein for another 4 d. Co-inhibition was evaluated by dilution of cell proliferation dye in CD8+ T cells by flow cytometry, and secretion of IFN-γ, by ELISA (eBioscience). TGF-β in supernatants was probed by biological assay (26). The TGF-β assay is sensitive to 60-100pg/ml.
Surface expression of Btn2a2 or other molecules was detected by flow cytometry as described previously (12). Cellular DNA content was assessed by propidium iodide or Hoechst 33342 staining (both Sigma-Aldrich) of cells.
Preparation and analysis of cell lysates
Cell pellets were lysed in buffer with protease inhibitor mix and phosphatase inhibitor mix (Sigma) at 1:100. Cell lysates on ice were mixed vigorously before centrifugation at 15.000 rpm and 4°C for 20 min. For protein immunoprecipitation, cells were lysed at 1-4×107/ml in buffer with 20 mM Tris-HCl at pH 8.0, 150 mM NaCl, and 1% NP40, then with low stringency buffer: 10 mM Hepes at pH 7.6, 250 mM NaCl, 0.1% NP40, and 5 mM EDTA. CD3ε was precipitated with clone CD3-12, AbD (Serotec), p85 with antibody from Cell Signaling Technologies. Biotinylated anti-Zap70 antibody (eBiosciences) was used at 1 μg/ml.
Immunoprecipitates were separated in 10% SDS-PAGE and western blotted. The following antibodies were used: Anti-p-Tyr (clone 4G10, Millipore), anti-β-actin-HRP (Sigma), and anti-CD3ε (clone CD3-12, AbD Serotec). From Cell Signaling Technologies: Anti-Zap70 (clone D1C10E), anti-p-Erk1/2 (D3F9), anti-Erk1/2 (clone 137F5), anti-p-p85, anti-p85 (clone 19H8), anti-p-Akt (Ser473, clone D9E) anti-p-Akt (Tyr308, clone C31E5E), anti-Akt (clone C67E7), and anti-p27kip1.
Results
Activation of antigen-presenting cells up-regulates cell-surface Btn2a2
To probe the role of Btn2a2 in regulation of T cells by professional antigen presenting cells (APC), we studied surface expression on CD19+ B cells, bone-marrow-derived CD11c+ DC, and CD11b+ F4/80+ peritoneal macrophages. Cells were activated for 16 h with the Toll-like receptor (TLR) ligands lipopolysaccharide (LPS), which activates TLR4, or the lipoprotein Pam3CysK4, which activates TLR1 and TLR2. Activation of cells was confirmed by cell surface expression of MHC class II, for DC and peritoneal macrophages, or CD86 for B cells. Btn2a2 expression on the surface of activated B cells, bone marrow-derived DC, and peritoneal macrophages was increased compared to non-activated cells (Fig. 1).
Figure 1. Increased cell surface expression of Btn2a2 on APC after activation.
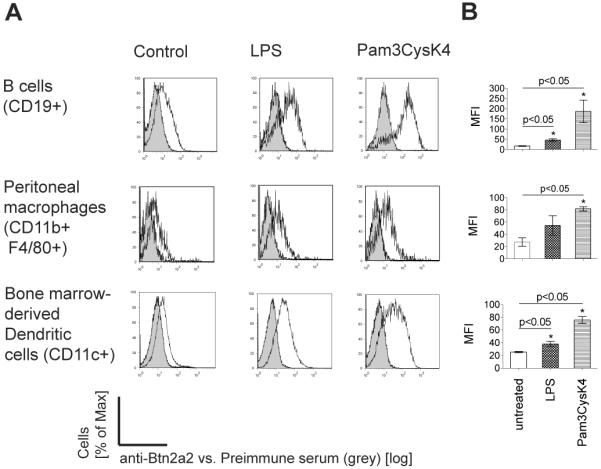
APC: Splenic B cells; bone marrow DC (20 ng/ml GM-CSF, 10 ng/ml IL-4); peritoneal macrophages enriched by adhesion to plastic. Activation was for 16 h with 100 ng/ml LPS or 1 μg/ml Pam3CysK4. (A) Anti-Btn2a2 antibody (black outline), pre-immune serum (grey filled). (A) Data representative of 3 experiments. (B) Significance (p < 0.05) confirmed with paired t-test (*).
Btn2a2 binding inhibits cell cycle entry of activated T cells
Btn2a2-Fc engagement reduced proliferation and metabolic activity in anti-CD3 or anti-CD3/CD28 stimulated T cells (12). This could indicate a reduced progression of T cells into the cell cycle. To investigate this, enriched, CD3+ primary T cells were stained with cell proliferation dye and activated using 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 and either Btn2a2-Fc or human Fc at 10 μg/ml. Four days after stimulation, cells were analysed for dilution of the cell proliferation dye eFluor® 670. Figure 2 A shows reduced T cell proliferation in the sample activated in presence of Btn2A2-Fc compared to the control. DNA content, analyzed by propidium iodide staining, showed that fewer cells progressed through the cell cycle when stimulated with Btn2a2-Fc (Figure 2 B).
Figure 2. Btn2a2 inhibited entry into the cell cycle of anti-CD3 and anti-CD28-activated CD3+ primary T cells.
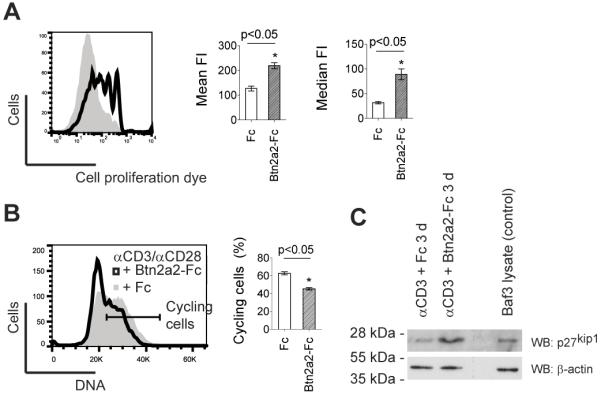
T cells from spleen and lymph nodes were stimulated for 4 d using 1 μg/ml anti-CD3, 1 μg/ml anti-CD28, and 10 μg/ml fusion protein. (A) Cell proliferation of cells 4 d after activation in Btn2A2-Fc (black outline) or Fc control (grey area) visualized with 1 μM eFluor670. (B) DNA content visualized using propidium iodide. % cycling cells (horizontal bar) calculated by subtracting the two-fold percentage of cells in the left hand part of the G0/G1 peak from 100%. (C) 4 d after activation of cells with 1 μg/ml anti-CD3 in presence of Fc control or BTN2A2-Fc, expression of the cell cycle entry inhibitor p27kip1 was analysed by western blotting for p27kip1. 1×105 cells per lane. Mouse Baf/3 cells were positive control for p27kip1. Loading control: β-actin. FACS data representative of 3 experiments with 3 replicates. Statistically significance as for Fig. 1.
To investigate the molecular basis for this, expression of p27kip1, a putative inhibitor of the transition between the G0/G1 phase and S phase of the cell cycle, was studied (27). CD3+ T cells stimulated with anti-CD3 and Btn2a2-Fc fusion protein were analysed by western blotting (Figure 2 C). The activation with anti-CD3 alone was chosen, since it was reported that CD28 co-stimulation inhibits expression of p27kip1(28). High levels of p27kip1 were found by western blotting in T cells stimulated with anti-CD3 and Btn2a2-Fc (Figure 2 B). These results indicate that ligation of Btn2a2-Fc leads to higher p27kip1 levels, which reduce cell cycle entry of T cells.
Btn2a2 ligation inhibits T cell receptor activation
To investigate whether Btn2a2-Fc co-inhibits activation of T cells at the level of the T cell receptor and CD3 complex, cells of the T cell line 2B4 were stimulated for 4 min using anti-CD3 and Btn2a2-Fc. Activation of the TCR/CD3 complex was assessed by analysis of CD3ε phosphorylation (Figure 3). After stimulation for 4 min, cells were lysed, and the molecule of interest was precipitated. As shown in Figure 3A, interaction of Btn2a2-Fc with the T cells strongly reduced CD3ε phosphorylation. The molecule responsible for transmitting the activation signal from the TCR/CD3 complex to downstream signaling pathways is the tyrosine kinase Zap70, which is activated by tyrosine phosphorylation. Zap70 phosphorylation was reduced when cells were stimulated with anti-CD3 in the presence of Btn2a2-Fc (Fig 3B).
Figure 3. Btn2a2 co-ligation inhibits T cell receptor signaling in anti-CD3 activated T cells.
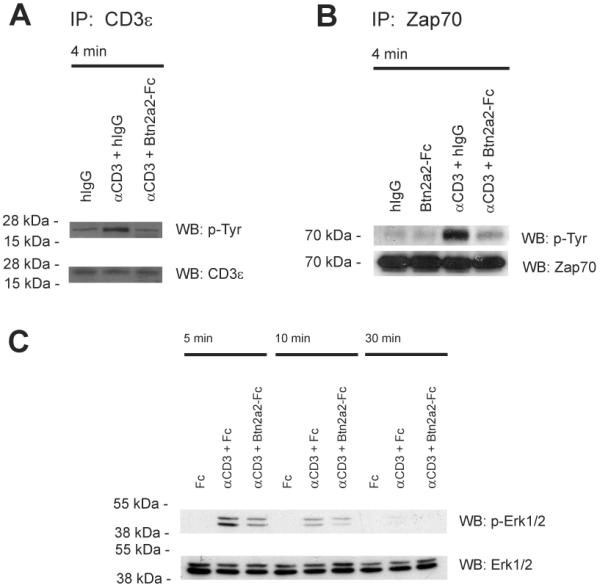
(A) 2B4 cells were stimulated for 4 min with 1 μg/ml anti-CD3 (clone 2c11) in 10 μg/ml Btn2a2-Fc or hIgG. Control: 2B4 cells stimulated with 10 μg/ml hIgG. After immunoprecipition with anti-CD3ε (clone CD3-12) and SDS-PAGE analysis was for tyrosine phosphorylation (4G10). (B) 2B4 cells stimulated 5 min with 1 μg/ml anti-CD3 in 10 μg/ml Btn2a2-Fc or hIgG. After lysis, samples were immunoprecipitated with biotinylated anti-Zap70 antibody, treated as in A and analysed for phosphorylation (4G10). (C) CD3+ primary T cells were stimulated with 1 μg/ml anti-CD3 and 10 μg/ml fusion protein, treated as above, and analysed for phosphorylation of Erk1 and Erk2 (p-Erk1/2). Antibody to total Erk1/2 (Erk1/2) was used for loading. Data from >2 independent experiments.
Erk1 and Erk2, activated by tyrosine phosphorylation, are downstream targets of signals from the TCR, receptors for mitogens, growth factors or cytokines and are critically involved in cell regulation (29). We assessed whether Btn2a2-Fc inhibition influences anti-CD3-induced Erk1/2 activation. CD3+ T cells were stimulated with anti-CD3 (Figure 3 C). Btn2a2 co-ligation inhibited anti-CD3-induced Erk1/2 activation, detected by reduced phosphorylation of Erk1/2. This occurred at different time-points, indicating that the activation of Erk1/2 was significantly reduced, not merely delayed, by Btn2a2-Fc co-ligation (Figure 3 C).
Btn2a2 ligation inhibits PI3K and Akt signaling
We next studied the influence of Btn2a2-Fc on the PI3K/Akt pathway. This pathway is activated by co-stimulatory molecules, such as CD28, ICOS, and LFA-1. In addition, it can be activated by signaling from the TCR/CD3 complex. Signaling from CD28 activates the regulatory subunit of PI3K, p85, by tyrosine phosphorylation.
Stimulation with anti-CD3 and anti-CD28 induced phosphorylation of p85 at 5 min (Fig. 4A). Conversely, phosphorylation of p85 was not detectable in the presence of Btn2a2-Fc. An increase in phosphorylation of Akt at Ser478 and Tyr308 was detected 10 min after stimulation with anti-CD3, anti-CD28 and Fc (Figure 4B). A weaker increase in Akt phosphorylation was observed in the presence of Btn2a2-Fc, even after 30 min. These results indicate that Btn2a2 co-ligation inhibits anti-CD3 and anti-CD28-induced activation of the PI3K/Akt pathway.
Figure 4. Btn2a2 binding interferes with activation of the PI3K/Akt pathway in anti-CD3 and anti-CD28-activated T cells.
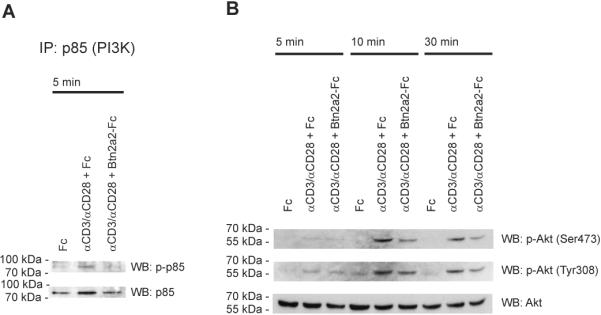
CD3+ T cells stimulated with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 and 10 μg/ml fusion protein as indicated. (A) Samples were immunoprecipitated with anti-p85 antibody, treated as in 3, and phosphorylation analysed using a p-p85 antibody. p85 antibody (total-p85) controlled loading. (B) Blotted proteins were analysed for phosphorylation of Akt using antibodies (p-Akt (Ser473) or p-Akt (Tyr308)). Antibody to total Akt (Akt) - sample loading. Data representative of 3 experiments.
Influence of anti-CD28 on T cells inhibited by Btn2a2
The extent of T cell activation depends on the balance of positive and negative co-regulatory stimuli. Increasing doses of anti-CD28 overcame the inhibition of T cell proliferation by PD-L1-Fc (30). This effect, of CD28 signaling on anti-CD3-activated CD3+ primary T cells co-inhibited with PD-L1-Fc, was reproduced by us. To compare T cell co-inhibition exerted by Btn2a2-Fc with the inhibition by PD-L1-Fc, CD3+ T cells were stimulated with anti-CD3 and anti-CD28 along with Btn2a2-Fc. As the surface expression levels and affinities of receptors for Btn2a2 and PD-L1 may differ, we titrated the concentration of anti-CD28. Stimulated samples were analysed by flow cytometry for expression of the T cell activation markers CD25 and CD69, and DNA content, 3d after initial stimulation (Figure 5 A). The right hand panels of the Figure indicate the relative inhibition values. The percentage of cells progressing through the cell cycle, expressing CD25, or CD69, increased in line with increasing anti-CD28 concentrations in presence of Btn2a2-Fc as well as the control. Hence, inhibition by Btn2a2 on anti-CD3-induced T cell activation was not overcome by anti-CD28 co-stimulation.
Figure 5. Btn2a2-mediated inhibition of anti-CD3-induced T cell activation cannot be overcome by anti-CD28 co-stimulation.
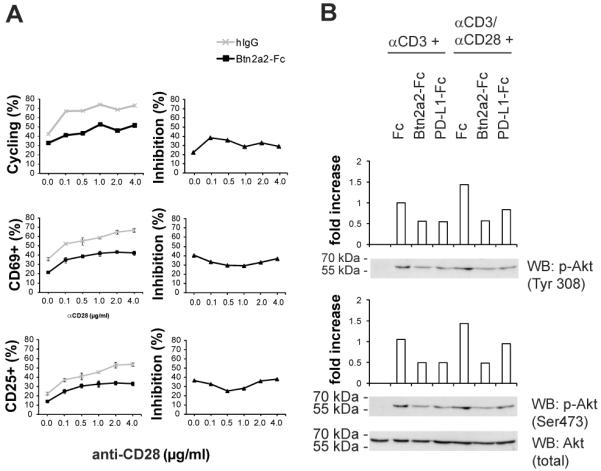
(A) CD3+ T cells activated for 3 d with 1 μg/ml anti-CD3, either 10 μg/ml Btn2a2-Fc or hIgG, and anti-CD28. Hoechst 33342 was used for DNA cell cycle analysis, and CD69 and CD25 antibodies for T cell activation (left). Relative inhibition (right) was calculated by dividing % cycling cells stimulated with anti-CD3 and Btn2a2-Fc by % cycling cells with anti-CD3 and hIgG. The quotient was multiplied by 100% for the % ratio. 3 replicates per sample with data from 3 experiments. (B) T cells stimulated for 30 min with 1 μg/ml anti-CD3 and 10 μg/ml fusion protein or 1 μg/ml anti-CD3, 5 μg/ml anti-CD28, and 10 μg/ml fusion protein. Fusion proteins: Fc backbone alone or the Btn2a2-Fc or PD-L1-Fc. Antibody for total Akt (Akt)was used for equal loading. Intensities measured were relative to stimulation with anti-CD3 and Fc. Data from 3 experiments.
To compare inhibition of Akt activation by Btn2a2, with PD-L1, CD3+ T cells were stimulated with anti-CD3, with or without anti-CD28. Btn2a2-Fc, or PD-L1-Fc, reduced activation of Akt in T cells stimulated with anti-CD3 (Figure 5 B). Stronger induction of phosphorylation of Akt was observed in control cells stimulated with anti-CD3 and anti-CD28. In the presence of Btn2a2-Fc, the levels of phosphorylation of Akt at Tyr308 and Ser473 were similar to the level in cells treated with anti-CD3 and Fc control, without anti-CD28 (Figure 5 B). In the presence of PD-L1-Fc, samples stimulated with anti-CD3 and anti-CD28 exhibited more Akt phosphorylation than the sample treated with anti-CD3 and PD-L1-Fc and more than those treated with anti-CD3, anti-CD28 and Btn2a2-Fc. These data suggest that Btn2a2 is more effective than PD-L1 at inhibiting anti-CD3 and anti-CD28-induced PI3K/Akt signaling.
Induction of Foxp3+ expression in naïve T cells by Btn2a2
Down-regulation of MAP kinase cascade signaling has been implicated in the development of regulatory T cells (31-33). In addition, inhibition of signalling via the PI3K/Akt pathway has been reported to control expression of the transcription factor Foxp3 (34; 35). We studied whether Btn2a2 engagement induced expression of Foxp3 in naïve T lymphocytes. T cells from Foxp3-GFP transgenic animals were negatively enriched and sorted for CD4+CD25−Foxp3−GFP−CD62Lhigh cells. The sorted naïve T cells were activated for 3d with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 plus 10 μg/ml of Fc fusion protein.
As shown in Figure 6 A, Btn2a2 co-inhibition induced de-novo expression of Foxp3 in naïve T cells stimulated for 3 days with anti-CD3 and anti-CD28 (5.59% vs. 1.29% in the Fc control). After 7 day of stimulation, 15.8% CD4+CD25+Foxp3+ T-cells were detected in the sample stimulated in presence of Btn2a2-Fc versus 1.15% in the Fc control stimulation.
Figure 6. Btn2a2-Fc binding induces de-novo expression of Foxp3 in naive primary T cells.
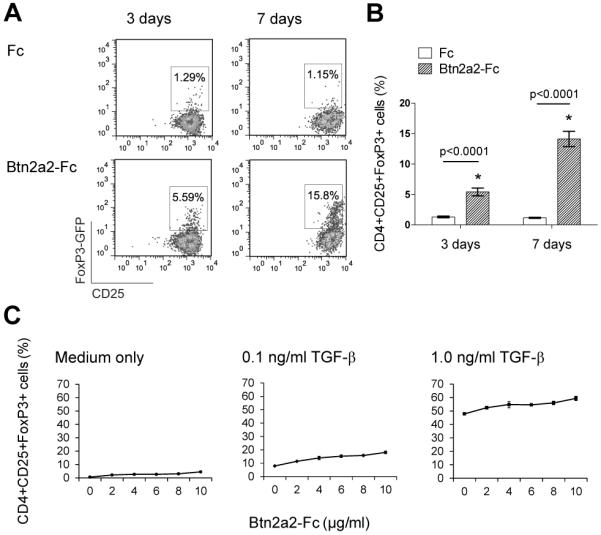
Naive T cells (CD4+ Foxp3−, CD25−, CD62Lhigh) were sorted from enriched CD4+ T cells, harvested from spleen and lymph nodes of NOD Foxp3-GFP mice. (A) Cells were activated for 3 d or 7 d with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 and 10 μg/ml fusion protein and analysed for Foxp3-GFP expression. (B) Statistical analysis using an ANOVA test. Significance (p < 0.05). (C) Cells activated for 3 d with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 and 10 μg/ml fusion protein as indicated in medium only, or in medium supplemented with 0.1 ng/ml, or 1.0 ng/ml TGF–β. Cells shown in (A) and (B) were gated on lymphocytes, single cells, CD4+ cells. Cells in (C) also gated on CD25+. Data from > 3 independent experiments. Three independent samples analyzed per data set (B) and (C). Data shown in (A) is representative of 4 replicates.
Titration of Btn2a2-Fc fusion protein indicated that the induction of Foxp3 in naïve CD4+ T cells was dose-dependent (Figure 6 C). We investigated induction of Foxp3 expression by Btn2a2-Fc in relation to induction by exogenous TGF-β. A strong induction of Foxp3+ expression was observed when T cells were stimulated with anti-CD3, anti-CD28 and control Fc protein, in presence of TGF-β. The induction of Foxp3 by TGF-β was further increased by Btn2a2-Fc, in a dose-dependent way (Figure 6 C).
Assessment of cell culture supernatants harvested at day 4 after activation with anti-CD3, anti-CD28, and Btn2a2-Fc or Fc, or 1 ng/ml TGF-β using a biological assay suggested that Btn2a2-Fc did not induce expression of TGF-β (Figure 7 A). However, a contribution of TGF-β induced by Btn2a2 cannot be ruled out entirely due to the detection limit of the assay.
Figure 7. Btn2a2-Fc induced CD4+CD25+Foxp3+ cells inhibit T cell proliferation and do not secrete TGF-β or IFN-γ.
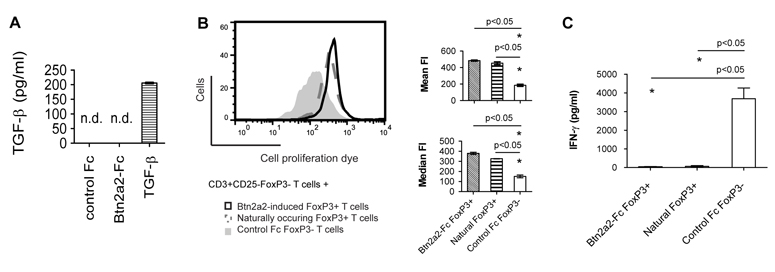
(A) CD4+CD25− CD62Lhigh Foxp3− cells from NOD Foxp3-GFP transgenic mice were activated for 4 d using 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 and 10 μg/ml Btn2a2-Fc or Fc fusion protein, or 1 ng/ml TGF-β. 4 days after initial stimulation, TGF-β was assayed in supernatants. (B) After 4 d, cells were sorted and CD4+CD25+Foxp3+ (“Btn2a2 Foxp3+”) or CD4+CD25+Foxp3− (“Control Foxp3−”) T cells were incubated 1:1 with freshly harvested and sorted CD3+CD25−Foxp3− from the same mouse strain. CD3+CD25−Foxp3− T cells were stained with 1 μM eFluor. Mixed cells were activated with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 and 10 μg/ml control Fc fusion protein. Positive control: CD4+CD25+Foxp3+ (Natural Foxp3+) from freshly harvested spleen and lymph nodes. After 4 d, proliferation of CD8+ T cells analyzed by FACS. Left: representative FACS plot; Right: summary MFI data (C) Concentration of IFN-γ was measured by ELISA. Experiments were repeated x3 and 3 independent samples were analysed. Significance (p < 0.05) with unpaired t-test (*). n.d. not detected.
Foxp3+ T cells induced by Btn2a2 ligation are functional regulatory T cells
In order to show that the Btn2a2-Fc induced CD4+CD25+Foxp3+ cells are functional, regulatory T cells, we sorted for CD4+CD25+Foxp3+ T cells (“Btn2a2-induced Foxp3+ T cells”) and incubated them with non-activated CD3+CD25−Foxp3− T cells, stained with cell proliferation dye, at a 1:1 ratio and stimulated with 1 μg/ml anti-CD3, 1 μg/ml anti-CD28 plus 10 μg/ml Fc fusion protein (Figure 7 B). For the negative control arm of the experiment, T cells were activated in presence of activated CD4+CD25+Foxp3− T cells (“Control Foxp3− T cells”) at a 1:1 ratio. As positive control non-activated T cells were activated in presence of naturally occurring CD4+CD25+Foxp3+ cells (“Natural Foxp3+”), harvested and sorted from spleen and lymph nodes of NOD Foxp3-GFP mice. After 4d, proliferation of CD8+ T cells in the samples was analysed. CD8+ T cells were chosen for analysis to exclude the possibility that proliferation of Btn2a2-induced CD4+ Foxp3+, CD4+ Foxp3− or naturally-occurring CD4+ T cells influenced analysis. As shown in Figure 7 B, Btn2a2-induced Foxp3+ T cells inhibited proliferation of CD8+ T cells with a similar efficiency as naturally-occurring Foxp3+ T cells from NOD mice. Secretion of IFN-γ was not detected in samples containing Btn2a2 Foxp3+ or naturally-occurring Foxp3+ T cells, but was detectable in the sample containing control Foxp3− T cells (Figure 7 C). These results show that CD4+ Foxp3+ T cells induced in presence of Btn2a2-Fc are functional regulatory T cells.
Discussion
Co-regulatory molecules expressed on APCs are involved in fine-tuning of T cell activation and differentiation. We found that TLR ligands such as LPS and Pam3CysK4 increased cell surface expression of Btn2a2 on APCs, consistent with a role for Btn2a2 in limiting T cell activation in inflammation.
Binding of Btn2a2 to anti-CD3 and anti-CD28-activated T cells reduced the number of proliferating cells and inhibited entry into the cell cycle. Inhibition of cell cycle entry has been noted for binding of Btnl1, PD-L1 and PD-L2 fusion proteins to T cells (16; 36). In T cells co-inhibited with Btn2a2-Fc, higher levels of the G0/G1 to S phase inhibitor p27kip1 were detected. High p27kip1 levels are considered as a hallmark of naïve, as well as of anergic, T cells (37). Activation of the PI3K/Akt pathway promotes degradation of p27kip1(28). The high level of p27kip1 in cells co-inhibited by Btn2a2 is consistent with inhibition of the PI3K/Akt pathway by Btn2a2.
Little has been reported on the signaling pathways influenced by binding of butyrophilin-like molecules to T cells. Anti-CD3-treated DO11.10 T cells co-stimulated with BTNL2-Fc exhibited reduced activation of the transcription factor NFAT and NF-κB (19). We show here that Btn2a2 inhibited anti-CD3 induced phosphorylation of CD3ε, Zap70 and Erk1 and Erk2. Our time course studies indicated that this was not simply a delayed activation.
Signals transmitted by the PI3K/Akt pathway are important for full activation of T cells. Engagement of the TCR/CD3 complex activates the PI3K/Akt pathway but stronger activation is achieved through concomitant signals from CD28 (38). In our experiments, tyrosine phosphorylation of p85, which took place 5 min after anti-CD3/CD28 stimulation of T cells, was negated by Btn2a2-Fc. Co-inhibition with Btn2a2-Fc inhibited activation of Akt, a downstream target of PI3K, 10 min after activation.
Inhibition of anti-CD3-induced T cell activation by Btn2a2-Fc was not overcome by anti-CD28-induced co-simulation, as was the case with PD-L1 (30). Similarly, inhibition of Akt phosphorylation by Btn2a2-Fc was not reversed by high concentrations of anti-CD28. These findings indicate that Btn2a2 has a unique role in regulation of T cells.
Regulatory T cells are central mediators of immune tolerance by suppression of various effector T cells. They are characterized by the expression of the transcription factor Foxp3 (39; 40). Mutations of Foxp3 lead to fatal autoimmune pathologies (39; 41; 42). Naturally-occurring regulatory T cells (nTreg) develop in the thymus whereas induced regulatory T cells (iTreg) develop in the periphery (43). In the presence of TGF-β, peripheral naïve CD4+ T cells were induced to express Foxp3, thereby leading to the development of iTreg (inducible T reg) cells (44; 45). Inhibition of Erk1/2 or Akt signaling promotes induction of regulatory T cells. We found that Btn2a2 inhibits activation of Erk1/2 and is a potent inhibitor of Akt activation. We found a positive relationship between the concentration of Btn2a2-Fc and the frequency of CD4+CD25+Foxp3+ T cells induced in vitro. Btn2A2-induced CD4+CD25+Foxp3+ T cells inhibited proliferation of T cells and IFN-γ production.
A dose-dependent induction of Foxp3 expression by Btn2a2 was also observed in the presence of TGF-β. This cytokine is potent for induction and maintenance of Foxp3 expression but was apparently not required for expression of Foxp3 in the thymus (46). We detected no TGF-β in supernatants containing Btn2a2-induced Foxp3+ T cells, possibly indicating that Foxp3 expression is induced via the inhibition of TCR and PI3K/Akt signaling pathways, although some contribution of TGF-β cannot be ruled out at this stage. We have shown that Btn1a1 and Btn2a2 are expressed on epithelial cells in the thymus (12). Hence, butyrophilin molecules could play a role in induction of nTreg cells in the thymus. In addition, Btn2a2 might contribute to T cell tolerance in the periphery, involving encounters between TLR-activated professional APCs that induce iTreg cells. Taken together, our findings indicate that BTN-related molecules may be involved in the induction of both nTreg and iTreg cells.
By inducing expression of Foxp3, Btn2a2 might be involved in the suppression of Th17 cell differentiation, since Foxp3 represses expression of the IL-17 gene (40). This hypothesis is consistent with the finding that Btnl1, another butyrophilin-related molecule, reduced levels of Th17 cells in an autoimmune disease animal model (16). Reduction of Th17 cell levels could be due to repression of IL-17 gene expression by Foxp3 or by induction of T reg cells which suppress Th17 activation and proliferation. When expressed on enterocytes, Btnl1 inhibited production of the pro-inflammatory cytokine IL-6 (17). BtnL2 also inhibited IL-6 production (18). IL-6 inhibits T reg cell-mediated suppression (47), down-regulates TGF-β-induced Foxp3 expression (48), induces Th17 cells in presence of TGF-β (49), and re-programs T reg cells to express cytokines such as IL-17 and IFN-γ (50). BTN-related molecules may therefore be a therapeutic target, to be exploited for treatment of Th17-driven autoimmune diseases.
There is increasing evidence that butyrophilin family molecules regulate T cells (12; 16; 18; 19). With Btn2a2 this may be by direct inhibition of T cell activation, or indirectly, by inducing differentiation into Foxp3+ Treg cells. Inhibition of primary T cell activation by Btn2a2 engagement was profound and was not overcome by co-stimulation with anti-CD28. We traced the effect to inhibition of Akt activation, which was not subjugated by anti-CD28 co-stimulation, as it is by PD-L1 engagement. Due to the ability of BTN-related molecules to induce Foxp3 expression in T cells and to inhibit IL-6 production, they may be important regulators of the T reg – T effector balance, by shifting the equilibrium towards T cell tolerance.
Acknowledgements
We thank Helga Schneider and Nigel Miller for their kind help. Anti-mouse BTN2A2 was provided by Isobel Smith. We also thank Jenny Phillips, Sarah Gibbs, Steve Newland, Paola Zaccone, David Bending, and Oliver Burton as well as Jim Kaufman for critical comments.
J.U.A. was supported by the European Commission with a Marie Curie fellowship. J.T. and A.C. were supported by the MRC and the Wellcome Trust, with partial support (J.T.) from the NIHR Cambridge Biomedical Research Centre.
Footnotes
Conflict of interest disclosure
The authors have no conflicting financial interests.
Authorship contributions
J.U.A, A.C. und J.T designed research; J.U.A. performed research; J.U.A, A.C. and J.T. analyzed data; and J.U.A and J.T. wrote the paper.
References
- [1].Heid HW, Winter S, Bruder G, Keenan TW, Jarasch ED. Butyrophilin, an apical plasma membrane-associated glycoprotein characteristic of lactating mammary glands of diverse species. Biochim Biophys Acta. 1983;728:228–238. doi: 10.1016/0005-2736(83)90476-5. [DOI] [PubMed] [Google Scholar]
- [2].Vernet C, Boretto J, Mattéi MG, Takahashi M, Jack LJ, Mather IH, Rouquier S, Pontarotti P. Evolutionary study of multigenic families mapping close to the human MHC class I region. J Mol Evol. 1993;37:600–612. doi: 10.1007/BF00182746. [DOI] [PubMed] [Google Scholar]
- [3].Rhodes DA, Stammers M, Malcherek G, Beck S, Trowsdale J. The cluster of BTN genes in the extended major histocompatibility complex. Genomics. 2001;71:351–362. doi: 10.1006/geno.2000.6406. [DOI] [PubMed] [Google Scholar]
- [4].Tazi-Ahnini R, Henry J, Offer C, Bouissou-Bouchouata C, Mather IH, Pontarotti P. Cloning, localization, and structure of new members of the butyrophilin gene family in the juxta-telomeric region of the major histocompatibility complex. Immunogenetics. 1997;47:55–63. doi: 10.1007/s002510050326. [DOI] [PubMed] [Google Scholar]
- [5].Stammers M, Rowen L, Rhodes D, Trowsdale J, Beck S. BTL-II: a polymorphic locus with homology to the butyrophilin gene family, located at the border of the major histocompatibility complex class II and class III regions in human and mouse. Immunogenetics. 2000;51:373–382. doi: 10.1007/s002510050633. [DOI] [PubMed] [Google Scholar]
- [6].Arnett HA, Escobar SS, Viney JL. Regulation of costimulation in the era of butyrophilins. Cytokine. 2009;46:370–375. doi: 10.1016/j.cyto.2009.03.009. [DOI] [PubMed] [Google Scholar]
- [7].Renard C, Hart E, Sehra H, Beasley H, Coggill P, Howe K, Harrow J, Gilbert J, Sims S, Rogers J, Ando A, Shigenari A, Shiina T, Inoko H, Chardon P, Beck S. The genomic sequence and analysis of the swine major histocompatibility complex. Genomics. 2006;88:96–110. doi: 10.1016/j.ygeno.2006.01.004. [DOI] [PubMed] [Google Scholar]
- [8].Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barbee SD, Woodward MJ, Turchinovich G, Mention J, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor MR, Peterson JA, Ceriani RL, Couto JR. Cloning and sequence analysis of human butyrophilin reveals a potential receptor function. Biochim Biophys Acta. 1996;1306:1–4. doi: 10.1016/0167-4781(19)60000-x. [DOI] [PubMed] [Google Scholar]
- [11].Jack LJ, Mather IH. Cloning and analysis of cDNA encoding bovine butyrophilin, an apical glycoprotein expressed in mammary tissue and secreted in association with the milk-fat globule membrane during lactation. J Biol Chem. 1990;265:14481–14486. [PubMed] [Google Scholar]
- [12].Smith IA, Knezevic BR, Ammann JU, Rhodes DA, Aw D, Palmer DB, Mather IH, Trowsdale J. BTN1A1, the mammary gland butyrophilin, and BTN2A2 are both inhibitors of T cell activation. J Immunol. 2010;184:3514–3525. doi: 10.4049/jimmunol.0900416. [DOI] [PubMed] [Google Scholar]
- [13].Malcherek G, Mayr L, Roda-Navarro P, Rhodes D, Miller N, Trowsdale J. The B7 homolog butyrophilin BTN2A1 is a novel ligand for DC-SIGN. J Immunol. 2007;179:3804–3811. doi: 10.4049/jimmunol.179.6.3804. [DOI] [PubMed] [Google Scholar]
- [14].Yamashiro H, Yoshizaki S, Tadaki T, Egawa K, Seo N. Stimulation of human butyrophilin 3 molecules results in negative regulation of cellular immunity. J Leukoc Biol. 2010;88:757–767. doi: 10.1189/jlb.0309156. [DOI] [PubMed] [Google Scholar]
- [15].Compte E, Pontarotti P, Collette Y, Lopez M, Olive D. Frontline: Characterization of BT3 molecules belonging to the B7 family expressed on immune cells. Eur J Immunol. 2004;34:2089–2099. doi: 10.1002/eji.200425227. [DOI] [PubMed] [Google Scholar]
- [16].Yamazaki T, Goya I, Graf D, Craig S, Martin-Orozco N, Dong C. A butyrophilin family member critically inhibits T cell activation. J Immunol. 2010;185:5907–5914. doi: 10.4049/jimmunol.1000835. [DOI] [PubMed] [Google Scholar]
- [17].Bas A, Swamy M, Abeler-Dörner L, Williams G, Pang DJ, Barbee SD, Hayday AC. Butyrophilin-like 1 encodes an enterocyte protein that selectively regulates functional interactions with T lymphocytes. Proc Natl Acad Sci U S A. 2011;108:4376–4381. doi: 10.1073/pnas.1010647108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arnett HA, Escobar SS, Gonzalez-Suarez E, Budelsky AL, Steffen LA, Boiani N, Zhang M, Siu G, Brewer AW, Viney JL. BTNL2, a butyrophilin/B7-like molecule, is a negative costimulatory molecule modulated in intestinal inflammation. J Immunol. 2007;178:1523–1533. doi: 10.4049/jimmunol.178.3.1523. [DOI] [PubMed] [Google Scholar]
- [19].Nguyen T, Liu XK, Zhang Y, Dong C. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol. 2006;176:7354–7360. doi: 10.4049/jimmunol.176.12.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Robenek H, Hofnagel O, Buers I, Lorkowski S, Schnoor M, Robenek MJ, Heid H, Troyer D, Severs NJ. Butyrophilin controls milk fat globule secretion. Proc Natl Acad Sci U S A. 2006;103:10385–10390. doi: 10.1073/pnas.0600795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Admyre C, Johansson SM, Qazi KR, Filén J, Lahesmaa R, Norman M, Neve EPA, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- [22].Harly C, Guillaume Y, Nedellec S, Peigné C, Mönkkönen H, Mönkkönen J, Li J, Kuball J, Adams EJ, Netzer S, Déchanet-Merville J, Léger A, Herrmann T, Breathnach R, Olive D, Bonneville M, Scotet E. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Palakodeti A, Sandstrom A, Sundaresan L, Harly C, Nedellec S, Olive D, Scotet E, Bonneville M, Adams EJ. The Molecular Basis for Modulation of Human Vγ9Vδ2 T Cell Responses by CD277/Butyrophilin-3 (BTN3A)-specific Antibodies. J Biol Chem. 2012;287:32780–32790. doi: 10.1074/jbc.M112.384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- [25].Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- [26].Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- [27].Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- [28].Appleman LJ, van Puijenbroek AAFL, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- [29].Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- [30].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luo X, Zhang Q, Liu V, Xia Z, Pothoven KL, Lee C. Cutting edge: TGF-beta-induced expression of Foxp3 in T cells is mediated through inactivation of ERK. J Immunol. 2008;180:2757–2761. doi: 10.4049/jimmunol.180.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adler HS, Kubsch S, Graulich E, Ludwig S, Knop J, Steinbrink K. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood. 2007;109:4351–4359. doi: 10.1182/blood-2006-09-047563. [DOI] [PubMed] [Google Scholar]
- [33].Huber S, Schrader J, Fritz G, Presser K, Schmitt S, Waisman A, Lüth S, Blessing M, Herkel J, Schramm C. P38 MAP kinase signaling is required for the conversion of CD4+CD25− T cells into iTreg. PLoS One. 2008;3:e3302. doi: 10.1371/journal.pone.0003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- [36].Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Y Iwai, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- [37].Lechner O, Lauber J, Franzke A, Sarukhan A, von Boehmer H, Buer J. Fingerprints of anergic T cells. Curr Biol. 2001;11:587–595. doi: 10.1016/s0960-9822(01)00160-9. [DOI] [PubMed] [Google Scholar]
- [38].Ward SG, Ley SC, MacPhee C, Cantrell DA. Regulation of D-3 phosphoinositides during T cell activation via the T cell antigen receptor/CD3 complex and CD2 antigens. Eur J Immunol. 1992;22:45–49. doi: 10.1002/eji.1830220108. [DOI] [PubMed] [Google Scholar]
- [39].Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- [40].Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- [41].Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of Foxp3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- [42].Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- [43].Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [44].Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- [48].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- [49].Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- [50].Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng X, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


