Abstract
Neutrophils play a pivotal role in the innate immune response. The small cytokine CXCL8 (also known as interleukin-8 or IL-8) is known to be one of the most potent chemoattractant molecules which, among several other functions, is responsible for guiding neutrophils through the tissue matrix until they reach sites of injury. Unlike mice and rats that lack a CXCL8 homologue, zebrafish has two distinct CXCL8 homologues: Cxcl8-l1 and Cxcl8-l2. Cxcl8-l1 is known to be up-regulated under inflammatory conditions caused by bacterial or chemical insult but until now, the role of Cxcl8s in neutrophil recruitment has not been studied. Here, we show that both Cxcl8 genes are up-regulated in response to an acute inflammatory stimulus, and that both are crucial for normal neutrophil recruitment to the wound and normal resolution of inflammation. Additionally, we have analyzed neutrophil migratory behavior through tissues to the site of injury in vivo, using open-access phagocyte tracking software, PhagoSight. Surprisingly, we observed that in the absence of these chemokines, the speed of the neutrophils migrating to the wound was significantly increased in comparison to control neutrophils, although the directionality was not affected. Our analysis suggests that zebrafish may possess a sub-population of neutrophils whose recruitment to inflamed areas occurs independently of Cxcl8 chemokines. Moreover, we report that Cxcl8-l2 signaled through Cxcr2 for inducing neutrophil recruitment. Our study, therefore, confirms the zebrafish as an excellent in vivo model to shed light on the roles of CXCL8 in neutrophil biology.
Keywords: Inflammation, Neutrophils, Zebrafish, CXCL8, Wounding, Recruitment
Introduction
Neutrophils are known to be one of the first lines of defense against invading microbes, playing a pivotal role in antimicrobial host defense by recognizing microorganisms through various receptor systems. In order to fulfill this function, they are the first leukocytes to be recruited towards areas of inflammation (1). Neutrophil recruitment towards areas of inflammation is considered to be the result of the concerted action of several chemoattractants including chemokines (2, 3). The first chemokine to be discovered more than 20 years ago was CXCL8 (4) and nowadays stands as the prototypical member of the family of CXC chemokines. CXCL8 is considered as one of the most potent neutrophil chemoattractants in inflammation (5) and which binds to two different chemokine receptors on leukocytes: the G protein-coupled receptors CXCR1 and CXCR2 (6). By using different mechanisms of activation, CXCL8 binding activates these receptors and thus induces specific intracellular signaling cascades that result in rapid neutrophil recruitment (3, 7-9). As neutrophils are initial players in acute inflammation, CXCL8 and other chemokines are consistently among the first signals to be expressed and released by the various cell types involved in inflammation (2, 3).
The in vivo study of the role of CXCL8 in neutrophil biology and, more precisely, in neutrophil recruitment in inflammation has been hampered by the lack of true CXCL8 homologues in most widely used animal models, such as mice and rats (10, 11). As discussed below, this may now have been circumvented by the identification of CXCL8 homologues in another model organism, the zebrafish. Remarkably, the zebrafish immune system resembles that of mammals. In particular, zebrafish development offers a window of opportunity for the study of innate immunity during initial larval stages independently of the adaptive component, which is only active after 4 weeks post fertilization (12, 13). In addition, the optical transparency of zebrafish and the development of fluorescent cell-specific transgenic lines have enabled the in vivo study of leukocyte biology (14, 15). Zebrafish neutrophils have now been extensively studied in inflammation (16-23), infection (24-27) and tumor progression (28-30).
Importantly, the zebrafish has been shown to express chemokines from the various families, including CXC chemokines (31, 32). Recently, two Cxcl8 lineages were identified in teleosts. In the carp, members of both lineages were shown to be differentially expressed during early phases of inflammation (33, 34). Although lacking the ELR motif, teleost Cxcl8s possess high homology with human CXCL8 (33, 34). Despite the description of both lineages in zebrafish, so far it has only been reported that the cxcl8-l1 gene is induced in inflammation (35). To date, neither the expression of cxcl8-l2 in this context nor the involvement of these chemokines in neutrophil recruitment has been addressed in the zebrafish.
The objective of this study was to understand in vivo the role of zebrafish Cxcl8s on neutrophil behavior and function in the inflammation elicited by tissue injury. Firstly, our analysis indicated that both cxcl8-l1 and cxcl8-l2 were up-regulated in wound inflammation. Importantly, we observed that recruitment of neutrophils towards the wound was significantly reduced in the absence of these Cxcl8s. By analyzing in vivo neutrophil migration and behavior with new open source tracking algorithms, PhagoSight, we have unexpectedly observed that in the absence of these chemokines, the velocity of the neutrophils migrating to the wound was significantly increased in comparison to normal controls. Furthermore, we have found that Cxcr2 mediated neutrophil recruitment to wounds and that Cxcl8-l2 signaled through this receptor to promote neutrophil recruitment in vivo. Overall, these observations led us to propose that zebrafish Cxcl8 chemokines are both required for efficient neutrophil recruitment in inflammation. Our data further support the idea that the zebrafish may possess, at least at the larval stage, a sub-population of neutrophils whose recruitment to inflamed areas occurs independently of Cxcl8 chemokines.
Materials and Methods
Characterization of zebrafish CXCL8
A search for cxcl8 was performed at the Ensembl (http://www.ensembl.org/index.html) zebrafish database (zv8 and zv9) in order to check for the localization of CXCL8s in the zebrafish genome. Further genomic DNA analysis was performed in order to obtain accurate exon/intron sequences. Protein sequence alignments of zebrafish Cxcl8-l1 (XP_001342606) and Cxcl8-l2 (HF674400) with human CXCL8 (NP_000575) were generated using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/).
Zebrafish Husbandry
All experiments with live animals were performed using protocols approved by the European Union Council Guidelines (86/609/EU) and the Bioethical Committee of the University of Murcia (approval number #333/2008). Zebrafish fertilized eggs were obtained from natural spawning of wild-type (obtained from the Zebrafish International Resource Center), and the Tg(mpx:gfp)i114 (22) line held at our facilities following standard husbandry practices. Animals were maintained in a 12 hr light/dark cycle at 28.5°C.
Morpholino knockdown
The following splice blocking morpholino-modified antisense oligonucleotides (morpholinos (MO), Gene Tools) were injected into 1-cell-stage fertilized eggs (2-6 ng/egg): MO cxcl8-l1 E1/I1 (4 ng/egg), MO cxcl8-l1 E2/I2 (6 ng/egg), MO cxcl8-l2 E1/I1 (4 ng/egg), MO cxcl8-l1 I2/E3 (2 ng/egg) (see Table 1).
Table 1.
Morpholinos used in this study.
| Morpholino’s name | MO Sequence | Target | [Mo] ng/egg |
|
|---|---|---|---|---|
| cxcl8-l1 | MO cxcl8-l1 E1/I1 | 5′-GGTTTTGCATGTTCACTTACCTTCA-3′ | E1/I1 | 4 |
| cxcl8-l1 | MOcxcl8-l1 E2/I2 | 5′-TTAGTTTGAAAACACATGATCTC-3′ | E2/I2 | 6 |
| cxcl8-l2 | MO cxcl8-l2 E1/I1 | 5′-TTAGTATCTGCTTACCCTCATTGGC-3′ | E1/I1 | 4 |
| cxcl8-l2 | MO cxcl8-l2 I2/E3 | 5′-GGCGCTGTTGAAAACAGATGTAAAA-3′ | I2/E3 | 2 |
For assessment of morphant efficacy, total RNA was prepared from 3 days post-fertilization (dpf) whole-larvae using TRIzol reagent and purified with PureLink RNA MiniKit (Invitrogen), following the manufacturer’s instructions and treated with DNase I, amplification grade (1 U/μg RNA; Invitrogen). The SuperScript III RNase H− reverse transcriptase (Invitrogen) was used to synthesize first-strand cDNA with oligo(dT)18 primer from 1 μg of total RNA at 50°C for 50 min. To confirm MO efficiency, semi-quantitative PCR was performed using specific primers for each cxcl8 gene (see Table 2). After gel electrophoresis, bands of amplified products were extracted and sequenced.
Table 2.
Primers used to analyze gene expression in this study.
| Gene | Acession number |
Name | Nucleotide sequence | Use |
|---|---|---|---|---|
| bactin2 | AF025305 | F | 5′-GTGCCCATCTACGAGGGTTA-3′ | PCR |
| R | 5′-TCTCAGCTGTGGTGGTGAAG-3′ | |||
| F | 5′-CCAGCTGAACTGAGCTCCTC-3′ | |||
| cxcl8-l1 | XM_001342570 | R | 5′-GGAGATCTGTCTGGACCCCT-3′ | PCR/qPCR |
| F1 | 5′-GTCGCTGCATTGAAACAGAA-3′ | |||
| R1 | 5′-CTTAACCCATGGAGCAGAGG-3′ | |||
| F1 | 5′-GCTGGATCACACTGCAGAAA-3′ | |||
| cxcl8-l2 | HF674400 | R1 | 5′-TCAAGGAAAAGTTTGCAGCA-3′ | PCR/qPCR |
| F3 | 5′-CCACACACACTCCACACACA-3′ | |||
| R3 | 5′-CCACTGAATTGTCCTTTCATCA-3′ | |||
| il1b | NM_212844 | F5 | 5′-GGCTGTGTGTTTGGGAATCT-3′ | qPCR |
| R5 | 5′-TGATAAACCAACCGGGACA-3′ | |||
| ptgs2b | NM_001025504 | F1 | 5′-TGGATCTTTCCTGGTGAAGG-3′ | qPCR |
| R1 | 5′-GAAGCTCAGGGGTAGTGCAG-3′ | |||
| rps11 | NM_213377 | F | 5′-ACAGAAATGCCCCTTCACTG-3′ | qPCR |
| R | 5′-GCCTCTTCTCAAAACGGTTG-3′ |
In the subsequent experiments employing the above mentioned MOs, standard control MOs (MO StdC) purchased from GeneTools were used in parallel to control for the specificity of the identified MO-mediated effects.
Zebrafish tail tissue sample collection and gene expression analysis
At 3 dpf, larvae were anesthetized in embryo medium with 0.16 mg/ml tricaine (ethyl 3-aminobenzoate, Sigma Aldrich) and complete transection of the tailfin tip was performed with a disposable sterile scalpel. Larvae were recovered in embryo medium at 28.5°C. At the time-points indicated, larvae were anesthetized again with 0.16 mg/ml tricaine and, using a sterile scalpel, the body portion between the cloaca and the wounded tail tip was excised from 80 larvae at each time-point. Tail tissue samples were then pooled and frozen in liquid nitrogen. Total RNA was extracted from cell pellets with TRIzol reagent (Invitrogen) and purified with RNAquous Micro Kit, total RNA purification system (Ambion), following the manufacturer’s instructions and first-strand cDNA synthesized as above. Real-time PCR was performed with an ABIPrism 7500 instrument (Applied Biosystems) using SYBR-Green (AppliedBiosystems). Reaction mixtures were incubated for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 1 min at 60°C, and finally 15 s at 95°C, 1 min 60°C, and 15 s at 95°C. For each mRNA, gene expression was normalized against the expression of ribosomal protein S11 (rps11) in each sample. The primers used are shown in Table 2. In all cases, PCR was performed with triplicate samples and repeated at least twice. Statistical analysis was performed using two-way ANOVA with Bonferroni post-test in Graph Prism5 software.
Tail fin wounding
At 3 dpf, larvae were anesthetized in embryo medium with 0.16 mg/ml tricaine. Then, complete transection of the tailfin tip was performed with a disposable sterile scalpel and fish were mounted in 1% (w/v) agarose low melting point (Sigma-Aldrich) dissolved in embryo medium supplemented with 0.16 mg/ml tricaine. The success of transection was immediately confirmed a fluorescence stereo microscope MZ16FA (Leica) equipped with green fluorescent filters. After solidification, embryo medium with 0.16 mg/ml tricaine solution was added in order to keep embryos hydrated during experiments. Thereafter, images were captured at the selected times while animals were kept in their agar matrixes with added medium at 28.5°C.
SB225002 pharmacological treatment
For CXCR2 inhibition assays, we choose to use a bath immersion method. Briefly, larvae were pre-incubated 1 hour at 28°C in presence or absence of the selective non-peptide inhibitor SB225002 (Tocris) at a final concentration of 5 μM diluted in embryo medium supplemented with 1% DMSO. In tail fin wounding experiments, during recovery larvae were kept in embryo medium supplemented with 1% DMSO in presence or absence of SB225002, until imaging at 6 hours post wounding (hpw).
Production of recombinant Cxcl8-l2
Recombinant zebrafish Cxcl8-l2 was produced in Escherichia coli. Briefly, the open reading frame of cxcl8-l2 encoding the mature protein without the signal peptide (residues 27-118) was synthesized, cloned in vector E3, produced as an N-terminal 6xHis fusion protein in E. coli, obtained from inclusion bodies with 6 M guanidine hydrochloride and purified by Ni-HiTrap column (GenScript).
Otic injection
At 3 dpf larvae were pre-incubated in presence or absence of SB225002 (Tocris) as described before, anesthetized in embryo medium with 0.16 mg/ml tricaine and mounted in 1% of agarose low melting point. Injection in the otic vesicle of 1 nl of PBS, Cxcl8-l2 recombinant protein at 30 μM or leukotriene B4 (LTB4) at 30 nM was performed. Embryo medium with 0.16 mg/ml tricaine solution supplemented with 1% DMSO and when required with 5 μM SB225002 was added on the top, in order to keep embryos hydrated during the experiments. Images were taken after 1 hour post-injection (hpi).
Image acquisition and processing
For each experiment 3 dpf morphant and control larvae were imaged in three independent experiments. Images were taken from wounded or control larvae mounted as described above. Different methods of image acquisition were used according to the requirements of each experiment. Briefly, for total neutrophil counts and number of neutrophils at site of injury/injection, images were taken using a Leica MZ16F fluorescence stereo microscope. Time lapse images from wounded tail fins were acquired using a Zeiss 5 Live confocal line-scanning microscope with a NA1/20x water immersion objective in z-stack mode, every 3 minutes until 6 hpw, and assembled into time lapse movies. For neutrophil PhagoSight analysis, time lapse images were taken using a Zeiss Axiovert200 fully motorized, inverted, wide field fluorescence microscope using an NA 0.8/20x objective, in z-stack mode, every 2 minutes until 6 hpw. All data were processed using Image J (http://rsb.info.nih.gov/ij/).
Neutrophil response analysis- PhagoSight
The time-lapse movies from morphant and control larvae were processed using PhagoSight (http://www.phagosight.org.uk), an open-source neutrophil tracking software developed in MATLAB. This software allowed us to generate quantitative measurements with which we were able to perform an unbiased comparison of neutrophil migratory behavior between cxcl8 morphants and control larvae and between resting and inflammatory conditions. This was done by analyzing either neutrophil speed or velocity oriented/lateral with respect to the wound as well as distinguishing the movement before the neutrophil reached the wound (herein referred to as “in translation”) and after it reached the wound (herein referred to as “in exploration”). Among the many parameters compared, the following were analyzed: the meandering index, the number of neutrophils that leave the wound, the total number of tracks generated by the neutrophils, the number of tracks that enter the wound, the number of tracks that enter the wound and leave, and the time-point at which the neutrophils enter the wound.
Tail fin injury resolution assay
To address whether inflammation resolved normally in cxcl8-l1 morphants, 3 dpf morphant and control larvae were wounded as previously described and recovered at 28°C in embryo medium until live imaging acquisition. Larvae were then mounted in 0.5% (w/v) low melting point agarose in embryo medium with 0.16 mg/ml tricaine. Tailfin images were taken using a Zeiss Axiovert200 fully motorized, inverted, wide field fluorescence microscope, using an NA 0.8/20x objective, in a z-stack mode at 6, 24, and 48 hpw. Larvae were allowed to recover between acquisitions at 28°C in embryo medium. Images were processed with Image J and neutrophil counts were performed at the indicated time-points.
Statistical analysis
All error bars indicates standard error of the mean (SEM). For gene expression experiments data are shown as mean ± SEM of three separate experiments. One-way ANOVA with Bonferroni post-test was used for Figures 1, 2D, 3-5 and 7E, Supplemental Figure 1E, 4, 5A; two-tailed Mann-Whitney test was used for Figure 7B, and Two-way ANOVA with Bonferroni post-test for Figure 2B, 3 (il1b and ptgs2b) and 6B, Supplemental Figure 1D.
Figure 1.
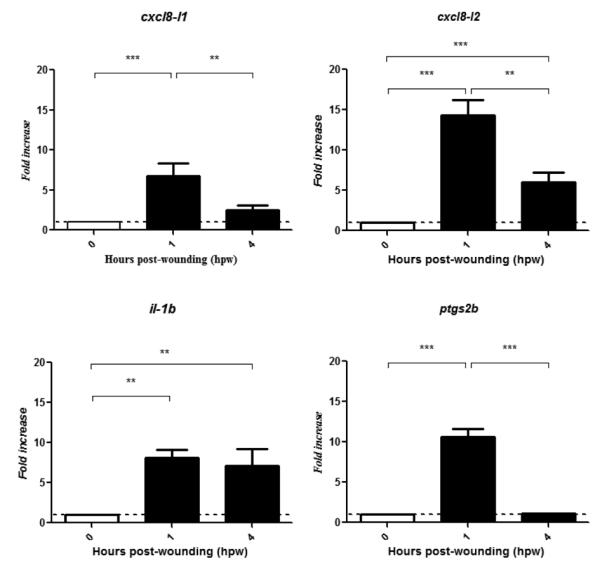
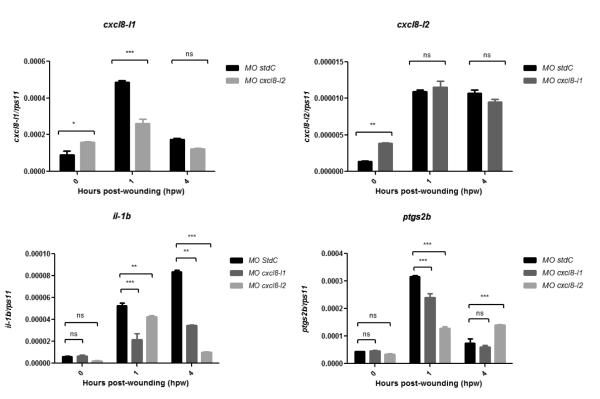
cxcl8-l1 and cxcl8-l2 mRNA levels are up-regulated in wounded zebrafish tail fin tissue. Tail fins from 3 dpf zebrafish larvae were wounded and mRNA levels of indicated genes were determined by qPCR in tail fin tissue at 0, 1 and 4 hpw (80 tail fins per time-point). Gene expression was normalized against rps11 and expressed as fold change compared with transcript expression levels of 3 dpf tail fin tissue from unwounded larvae (0 hpw). Each bar represents the mean ± SEM of triplicated samples. P values were calculated using one-way ANOVA and Bonferroni multiple comparison test, *P<0.05, **P<0.01, and ***P<0.001.
Figure 2.
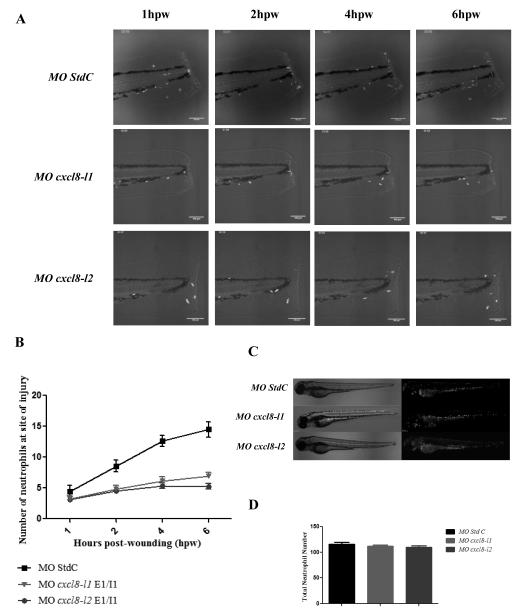
cxcl8 morphants display a reduced zebrafish neutrophil recruitment in acute inflammation. Tail fins of Tg(mpx:gfp)i114 previously microinjected with standard control morpholino (MO StdC), MO cxcl8-l1 and MO cxcl8-l2 were transected at 3 dpf. (A) Representative maximum intensity projections of 6 hours confocal time-lapse microscopy of 3 dpf Tg(mpx:gfp)i114 control and morphant larvae, acquired at 3 min intervals. This sequence is shown in Supplemental Movie 1. Scale bar=100 μm (B) Counts of fluorescent neutrophils at the wound were made at 1, 2, 4 and 6 hpw. Data are presented as means ± SEM (n=30 performed as 3 independent experiments). P values were calculated using two-way ANOVA and Bonferroni multiple comparison test. At 1 hpw no significant differences in neutrophil number were observed for both morphants in comparison to control larvae (P>0.05). For MO cxcl8-l1 significant decreases were observed at 2 hpw (P<0.01) and from 4 to 6 hpw (P<0.001). As for MO cxcl8-l2, we have observed a significant decrease from 2 to 6 hpw (P<0.001) (C) DIC and GFP wide field fluorescence microscope micrographs from 3 dpf control and morphant larvae. (D) Total neutrophil counts in whole-larvae for each condition. Each bar represents means ± SEM (n=20 performed as 2 independent experiments). P values were calculated using one-way ANOVA and Bonferroni multiple comparison test (P>0.05, no significant differences were observed).
Figure 3.
Expression of inflammatory genes in cxcl8 morphants. Tail fins from 3 dpf zebrafish control and morphants larvae were wounded and mRNA levels of the indicated genes were determined by qPCR in tail fin tissue at 0, 1 and 4 hpw. Gene expression was normalized against rps11. Each bar represents the mean ± SEM of triplicated samples. P values were calculated using one-way ANOVA and Bonferroni multiple comparison test, *P<0.05, **P<0.01, and ***P<0.001.
Figure 5.
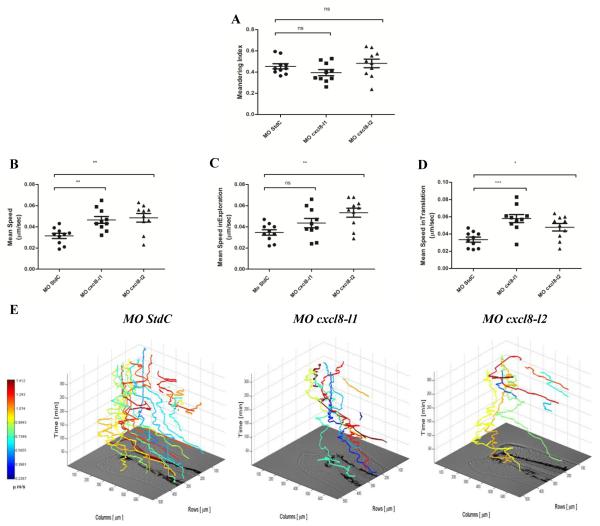
PhagoSight analysis reveals that cxcl8s morphants have an increased zebrafish neutrophil recruitment velocity in acute inflammation. Tailfins of Tg(mpx:gfp)i114 previously microinjected with MO StdC, MO cxcl8-l1 and MO cxcl8-l2 were transected at 3 dpf and time-lapse movies were made in wide field fluorescence microscope (MO StdC=270 tracks, MO cxcl8-l1=147 tracks and MO cxcl8-l2=128 tracks from n=10 larvae as 3 independent experiments) and further analyzed by PhagoSight. Distinct parameters were determined to study neutrophil migratory behavior such as: (A) Meandering Index; (B) Mean Speed; (C) Mean Speed inTranslation; (D) Mean Speed inExploration. Longer horizontal bars represent the mean values and shorter horizontal bars represent SEM. (E) 3D-Photomicrograph of a typical tracking experiment, with lines indicating the path of neutrophil movement over 6-hour time lapse during the recruitment phase of inflammation and colors indicating neutrophil velocity in μm/s. P values were calculated using one-way ANOVA and Bonferroni multiple comparison test, *P<0.05, **P<0.01, and ***P<0.001.
Figure 7.
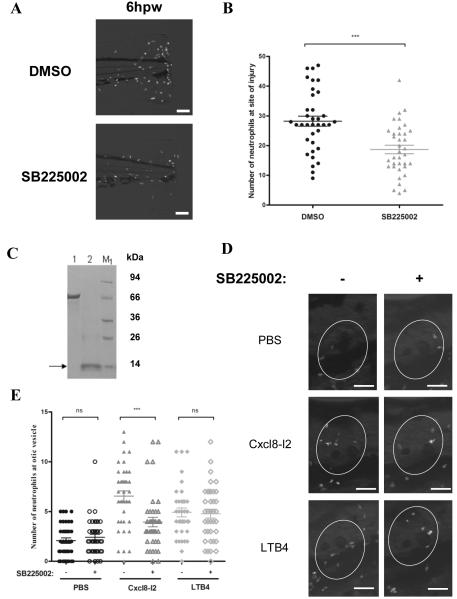
Pharmacological inhibition of Cxcr2 impaired neutrophil recruitment. (A) Representative wide field fluorescence microscope micrographs of 3 dpf Tg(mpx:gfp)i114 control and morphant larvae, pre-treated or not with 5 μM SB225002 followed by tail fin wounding. Scale bar=100 μm. (B) Counts of fluorescent neutrophils at the wound were made at 6 hpw. Data are presented as means ± SEM (n=25 performed as 3 independent experiments). P values were calculated using two-tailed Mann-Whitney test, ** P<0.01. (C) SDS-Page analysis of Cxcl8-l2 recombinant protein. Lane 1: BSA (2μg), lane 2: Cxcl8-l2 (2μg). (D) Representative wide field fluorescence microscope micrographs of 3 dpf Tg(mpx:gfp)i114 pre-treated with 5 μM SB225002 followed by otic vesicle injection of PBS, 30 μM Cxcl8-l2 and /or 30 nM LTB4. Images were taken at 1 hpi. Scale bar=100μm. (E) Counts of fluorescent neutrophils recruited to the ear (encircled) were made 1 hpi. Data indicate means ± SEM (n=32 performed as 3 independent experiments). P values were calculated using one-way ANOVA and Bonferroni multiple comparison, ***P<0.001.
Figure 4.
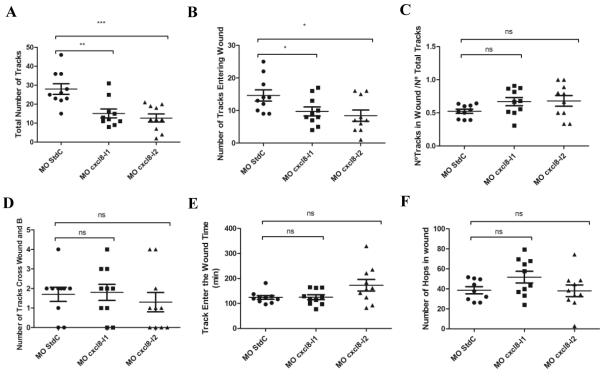
PhagoSight analysis confirms that CXCL8s morphants display a reduced zebrafish neutrophil recruitment in acute inflammation. Tailfins of Tg(mpx:gfp)i114 previously microinjected with MO StdC, MO cxcl8-l1 and MO cxcl8-l2 were transected at 3 dpf and time-lapse movies were performed under a wide field fluorescence microscope (MO StdC=270 tracks, MO cxcl8-l1=147 tracks and MO cxcl8-l2=128 tracks from n=10 larvae as 3 independent experiments) and further analyzed by PhagoSight. Distinct parameters were determined to study neutrophil migratory behavior namely: (A) Total Number of Tracks; (B) Number of Tracks Entering the Wound; (C) Ratio of Number of Tracks in Wound/Total Number of Tracks; (D) Number of Tracks Cross the Wound and Back; (E) Tracks Enter the Wound time; (F) Number of Hops in Wound. Longer horizontal bars represent the means and shorter horizontal bars represent SEM. P values were calculated using one-way ANOVA and Bonferroni multiple comparison test, *P<0.05, **P<0.01, and ***P<0.001.
Figure 6.
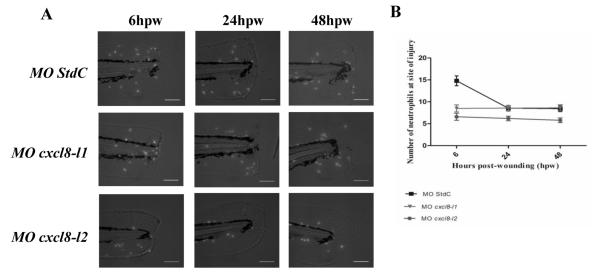
Resolution of the cellular component of inflammation is decreased in both cxcl8 morphant larvae. (A) Representative maximum intensity projections from wide field fluorescence microscope micrographs of 3 dpf Tg(mpx:gfp)i114 control and morphants larvae tail fins at 6, 24 and 48 hpw. Scale bar=100μm. (B) Counts of fluorescent neutrophils at the wound were made at 6, 24 and 48 hpw. Data is shown as mean ± SEM (n=40 performed as 3 independent experiments). P values were calculated using two-way ANOVA and Bonferroni multiple comparison test. For both CXCL8 morphants, significant differences were observed at 6 hpw in comparison to control larvae (P<0.001) but not at 24 or 48 hpw (P>0.05).
Results
Expression of both cxcl8 genes is up-regulated in wounded zebrafish tailfin tissue
In the carp, it has been shown that members of Cxcl8 lineages, namely Cxcl8-l1 and the Cxcl8-l2 play a role in acute inflammation. Consistently, the mRNA levels of both carp Cxcl8 genes are up-regulated under these conditions (33). In view of this, we first addressed the expression of both zebrafish cxcl8 at different time-points in 3 dpf Tg(mpx:gfp)i114 larvae under acute inflammatory conditions. This expression analysis was performed by qPCR by using the primers presented in Table 2. Here, we made use of the tail fin transection model, a validated model to investigate acute inflammation (16-23). We found that the mRNA levels of cxcl8-l1 and cxcl8-l2 were significantly increased in injured tail fin tissue and peaked at 1 hpw (Figure 1). il1b and ptgs2b (cox2b) mRNA levels were used as positive controls of inflammation in qPCR experiments (Figure 1).
Genetic inhibition of both cxcl8 genes attenuate zebrafish neutrophil recruitment in acute inflammation
Next, we decided to knockdown both cxcl8 by using splice-blocking morpholinos. In order to design these morpholinos, we had to know the complete genomic sequence for both chemokines. While cxcl8-l1 was already properly annotated in zv8 database, for cxcl8-l2 first we needed to use an EST sequence (EH557944) to design primers in order to perform RT-PCR analysis and sequencing. Next, after confirming the full coding sequence, we performed a BLAST search of the zebrafish genome from Ensembl using the zv9 database and we were able to identify the full cxcl8-l2 sequence on chromosome 7, position 8658505:8665964, opposite strand, this gene is now annotated at Gene EMBL with the accession number HF674400 (http://www.ebi.ac.uk/ena/). Two different splice-blocking morpholinos were designed and tested for each gene, in order to generate viable cxcl8-l1 and cxcl8-l2 morphants. Thus, we used MO cxcl8-l1 E1/I1 (referred to as MO cxcl8-l1) and MO cxcl8-l1 E2/I2 to generate cxcl8-l1 morphants and MO cxcl8-l2 E1/I1 (referred to as MO cxcl8-l2) and MO cxcl8-l2 I2/E3 for cxcl8-l2 morphants (Supplemental Figure 1A-B). At 3 dpf mpx:GFP morphant larvae were injured and neutrophil recruitment was quantified at different time-points from 1 to 6 hpw. Our results show that cxcl8-l1 and cxcl8-l2 knockdown significantly attenuated neutrophil recruitment to injured zebrafish tail fins (Figure 2A-B, Supplemental Movie 1-3 and Supplemental Figure 1C-D). Furthermore, none of the morpholinos used to knockdown cxcl8-l1 or cxcl8-l2 affected the total neutrophil number at 3 dpf in whole larvae (Figure 2C-D and Supplemental Figure 1E-F). Although all the four morpholinos reduced neutrophil recruitment, we decided to use MO cxcl8-l1 and MO cxcl8-l2 for further studies, since they had higher efficiencies in CXCL8 knockdown and thus affected neutrophil recruitment more significantly than the other two.
cxcl8-l2 knockdown impairs cxl8-l1 induction after wounding
Although both genes were important for neutrophil recruitment in zebrafish, MO cxcl8-l2 caused a larger inhibition of the neutrophil response. Considering this, we further asked whether knockdown of a given cxcl8 could impact on the expression of the other. By qPCR analysis, we observed that both morphants expressed higher basal levels of the other cxcl8 gene (Figure 3). Upon wounding, the cxcl8-l2 mRNA levels were not affected in the cxcl8-l1 morphant. In contrast, cxcl8-l1 transcript levels were significantly reduced in cxcl8-l2 morphants when compared to control conditions. Moreover, in both cxcl8 morphants, we observed that the mRNA levels of two inflammation control markers, namely il1b and ptgs2b, were also significantly reduced (Figure 3).
Neutrophil migratory behavior is affected in the absence of either Cxcl8
Our main objective was to study in vivo the function of Cxcl8s on the migratory behavior of neutrophils in acute inflammation. For such purpose, time-lapse images were acquired every 2 minutes from uninjured or previously injured 3 dpf larvae tail fins. Imaging was performed simultaneously for control conditions and for both cxcl8 morphants. To inspect neutrophil migratory behavior in detail, the acquired data sets were further analyzed using PhagoSight, software developed in Matlab (http://www.phagosight.org.uk/). PhagoSight provides an array of measurements which capture neutrophil behavioral traits, the most important of which are presented in Figures 4-5, Supplemental Figure 2, and Supplemental Movies 4-6.
In wounded larvae, we first confirmed that in both cxcl8 morphants, a significant lower number of neutrophils were being mobilized and recruited to the wound in the first 6 hpw (Figure 4A-C). Moreover, neutrophils in cxcl8-l2 morphants were observed to enter the wound at significantly later time-points than in control and cxcl8-l1 morphants (Figure 4E). The ratio between the total number of tracks and the number of tracks in the wound (Figure 4C), the number of tracks that crossed the wound and back (Figure 4D) and the number of neutrophil time-points in the wound (Figure 4F) were not significantly affected.
Interestingly, the knockdown of either cxcl8-l1 or cxcl8-l2 did not affect the meandering index of the neutrophil movement in the tail fin tissue under acute inflammatory conditions (Figure 5A). Unexpectedly, the speed of neutrophils in both cxcl8 morphants was significantly higher comparing with the control condition (Figure 5B). Consistently, the mean speed from neutrophils moving towards the wound (referred here as “in translation”) was significantly increased in both cxcl8 morphants (Figure 5C). Furthermore, the mean neutrophil speed was also significantly reduced for neutrophils moving within the wound (referred here as “in exploration”) in the absence of Cxcl8-l2 chemokine (Figure 5D). Both lateral and oriented velocities (Supplemental Figure 2A-F) were significantly increased in both cxcl8 morphants, indicating that the overall neutrophil speed was increased and not just the speed towards the wound.
In Figure 5E, we present representative 3D tracking plots of wounded tail fins of 3 dpf larvae from control, cxcl8-l1 and cxcl8-l2 morphants. Tracks were plotted highlighting the faster ones. As mentioned above, a significantly lower number of neutrophils were recruited to the wounds at higher speeds in both cxcl8 morphants in comparison to normal conditions. For the unwounded conditions, no significant differences were observed for neutrophil migratory speed, directionality or number of neutrophils migrating to the tail (Supplemental Figure 2G-L). In addition, neutrophils migrated extravascularly, through the tissue matrix, in both wild type and cxcl8 morphants (Fig. 5E and Supplemental Movies 1-6).
Inflammation resolution in wounded tail fin tissue is affected by the absence of Cxcl8 chemokines
Having established that neutrophil recruitment to wound heavily depends on both Cxcl8s, we further addressed whether inflammation resolution is affected by the absence of these chemokines. For these experiments, we wounded 3 dpf control and morphant larvae at the tail fins and counted the number of neutrophils present at the wound at 6, 24 and 48 hpw. Control larvae had a significantly reduced number of neutrophils at 24 hpw when compared to 6 hpw (Figure 6), indicating a successful inflammation resolution. However, in both cxcl8 morphants, a reduction of the number of recruited neutrophils was not observed at either 24 or 48 hpw. Interestingly, cxcl8-l2, and to some extent cxcl8-l1, morphant larvae showed a faster healing and regenerative capacity than control larvae (data not shown).
Pharmacological inhibition of Cxcr2 impairs neutrophil recruitment to recombinant Cxcl8-l2
In order to address whether also in zebrafish Cxcl8s signal through Cxcr2 for inducing neutrophil recruitment, we designed two different in vivo recruitment assays using a human CXCR2 selective non-peptide inhibitor, SB225002 (36). The presence of SB225002 at 5 μM significantly reduced the number of neutrophils recruited to the wound upon tail fin injury (Figure 7A and B). Due to the fact that cxcl8-l2 was induced at higher levels than cxcl8-l1 in wounded tissue (Figure 1), recombinant Cxcl8-l2 protein was produced (Figure 7C). Consistently with its chemoattractant function, injection of the recombinant chemokine into the otic vesicle increased neutrophil recruitment to the ear in comparison to control conditions (Figure 7D and E). When recombinant protein injection was performed into the otic vesicle of larvae pre-treated with SB225002 so as to inhibit Cxcr2, the number of neutrophils recruited to the ear was reduced to a basal level (Figure 7D and E). In contrast, leukotriene B4 (LTB4)-induced neutrophil recruitment was unaffected by SB225002 (Figure 7D and E), confirming the specificity of this inhibitor.
Discussion
CXCL8 is an important chemokine that mediates neutrophil migration, accumulation and function at sites of inflammation (37, 38). Up to now, its in vivo study has been greatly hampered by the lack of true homologues in rats and mice (11). This difficulty can now be circumvented by using zebrafish models, as this teleost fish has been shown to express close CXCL8 homologues (33-35, 39). This is evidenced by the protein alignment (data not shown), which further allowed us to conclude that Cxcl8-l2 phylogenetically constitutes a closer homologue to human CXCL8 than Cxcl8-l1 (45.4% of identity between zebrafish Cxcl8-l2 and human CXCL8 versus 35.2% of identity between zebrafish Cxcl8-l1 and human CXCL8). As such, we aimed here to address in vivo the requirement of the zebrafish Cxcl8s for the recruitment and behavior of neutrophils in acute inflammation. As an inflammatory model, we used here the transection of the tail fin, which is currently one the best studied and most used models in zebrafish.
In this study, we hypothesized that zebrafish Cxcl8s play a role in neutrophil recruitment under acute inflammatory conditions. Consistently, we first demonstrated that the expression of both cxcl8 genes is rapidly up-regulated upon wounding. A similar increase in expression of cxcl8s has also been observed in response to infectious stimuli (data not shown). These results are in agreement with those published previously for Cxcl8-l1 (35) as well as for the carp Cxcl8s (33, 34) and strongly suggest that both zebrafish Cxcl8s play a role in acute inflammation.
Considering the literature published on the inflammatory function of CXCL8, we have further addressed the requirement of zebrafish Cxcl8 lineages for neutrophil recruitment to sites of inflammation. For such, neutrophil recruitment to wounded tail fins was assayed in the absence of these chemokines via the use of specific MOs that enabled the knockdown of their expression. Our analysis revealed that both chemokines are necessary for a normal neutrophil response, as in their absence, neutrophil recruitment to the wound was severely impaired. These findings are in agreement with the reduced neutrophil recruitment observed in inflammation in knockout mice for CXCR1, CXCR2 or CXCL1 (one of the mouse CXCL8 functional homologues) (40-44). Furthermore the mRNA levels of key pro-inflammatory mediators, such as IL-1β and PTGS2b, were affected in both morphants, strongly suggesting that in the absence of either Cxcl8 zebrafish acute inflammation is significantly attenuated. Similarly, infection of CXCL1-deficient mice with Klebsiella pneumonia has been shown to affect the expression of several chemokines and cytokines in the lungs in comparison with control animals (44). It is also important to point out that although Cxcl8-l2 deficiency impaired the induction of Cxcl8-l1 upon wounding, Cxcl8-l1 knockdown had no effect on Cxcl8-l2 induction. This crosstalk between the two zebrafish CXCL8 chemokines will require further investigation.
Having established the requirement of both Cxcl8s for neutrophil recruitment, we next investigated in detail by PhagoSight analysis how the absence of these chemokines affected this process. Inspection of the total numbers of neutrophil tracks and of tracks entering the wound allowed us first to confirm that neutrophil recruitment is significantly reduced in the absence of both Cxcl8s. Moreover, we also observed that the number of neutrophils that entered the wound and left until 6 hpw were similar in control and morphant larvae. These results suggest that the absence of both chemokines does not favor reverse migration that could perhaps function as a compensatory response to achieve resolution of inflammation. Additionally, our data reveal that in cxcl8 morphants, the meandering index of migrating neutrophils was not significantly affected whereas, surprisingly, the mean neutrophil migratory speed, as well as the lateral and oriented velocities, increased. Moreover, a sensitivity analysis has been performed to demonstrate that all these measurements are not sensitive to variation in the selected size of the wound region, or to variation in track length (data not shown). A similar analysis performed for unwounded larvae allowed us to exclude the possibility that these results reflected an altered migratory pattern typical of cxcl8 morphant neutrophils, regardless of any elicited inflammatory response. Altogether these experiments led us to conclude that while reducing neutrophil recruitment, the absence of both Cxcl8s increases the velocity of the neutrophils migrating to the wound area. As it is generally accepted that chemotactic cues guide leukocytes to sites of injury by increasing their velocity and directionality (usually measured by the meandering index) (45, 46), these findings are quite paradoxical. One possible scenario for explaining these controversial results would be that distinct neutrophil sub-populations may respond differentially to CXCL8 chemokines and other chemoattractants expressed locally at the site of inflammation and could thus display distinct migratory behaviors. Under normal inflammatory conditions, these neutrophil subpopulations could be mobilized via sensing distinct chemotactic cues to inflamed areas where they could perhaps exert different functions. In the absence of either Cxcl8 signal, only the Cxcl8-unresponsive neutrophils would then migrate towards the inflamed area, possibly with an increased velocity by responding to other chemoattractants, such as for example, LTB4 which induces neutrophil recruitment as demonstrated by us and others (21, 47). In agreement with a scenario of distinct functional populations, a recent study has proposed that zebrafish neutrophils can be functionally classified either as consumers or producers of H2O2 (48). The idea that neutrophils have distinct functional populations is taking shape (49, 50), and the acceptance of different functional subpopulations of neutrophils will require additional work. In this respect, a differential neutrophil response to CXCL8 may actually constitute a pivotal criterion to discriminate between the identified neutrophil populations.
In order to restore the normal tissue function and homeostasis, it is crucial that an acute inflammatory process should be well resolved (51). Among other markers, inflammation resolution is characterized by a significant decrease in the number of neutrophils at the inflamed sites (17, 52-55). Taken the impact of both Cxcl8s in the recruitment of zebrafish neutrophils, we have also addressed whether and how resolution was affected in cxcl8-l1 and cxcl8-l2 morphants. As expected for a normal resolution, our results showed that at 24 hpw, control larvae presented a significantly reduced number of neutrophils comparing to 6 hpw. Such scenario was not observed for both morphants that were not able to reduce their neutrophil counts at the wound at 24 or even at 48 hpw. In fact, the number of neutrophils at wounds remained fairly constant in cxcl8 morphants from 6 to 48 hpw. These results, together with the impaired induction of the pro-inflammatory mediators IL-1β and PTGS2b in Cxcl8-deficient larvae after wounding, suggest that inflammation resolution was compromised in these larvae. In addition, these results also demonstrate that CXCL8 chemokines can modulate the expression of other inflammatory mediators such as factors with a pro-resolution function that might be enrolled in neutrophil apoptosis signaling pathways, in macrophage-mediated phagocytosis or even in neutrophil reverse migration, among other events critical for inflammation resolution (17, 23, 56, 57). As such, the unavailability of these chemokines during acute inflammation would necessarily affect the resolution of the process. Nevertheless, it is surprising that Cxcl8-deficient larvae showed faster healing than control larvae, despite being unable to resolve the inflammation. Although the mechanisms involved in this process deserve further investigations, our results are in agreement with a recent study that reported the absence of neutrophils to contribute to a faster and more successful repair of the tail fin (58).
In mammals CXCL8 binds to CXCR1 and CXCR2 with different affinities and thus activates different cascade pathways (3, 7-9). These receptors have been proposed to exert different functions in the neutrophil inflammatory response (7, 59). Due to its intrinsic higher affinity towards its ligand chemokines, CXCR2 is believed to play a more active role in neutrophil recruitment and degranulation (7, 36, 59). As for CXCR1, it is thought to be more important for regulating cytotoxic events such as, ROS formation and protease release, at the injury site, (7, 59, 60). As zebrafish also have CXCR1 and CXCR2 homologues (35), we made here use of a human selective non-peptide CXCR2 inhibitor, SB225002, to establish that (i) Cxcr2 plays a prominent role in neutrophil recruitment to wound and (ii) neutrophil recruitment mediated by Cxcl8-l2 mainly depends on Cxcr2 signaling.
In summary, we report that zebrafish Cxcl8-l1 and Cxcl8-l2 are both important in vivo for the recruitment of neutrophils in the wound-elicited acute inflammation. In particular, we have demonstrated that the presence of these chemokines is required for the maintenance of a normal neutrophil migratory behavior upon wounding. As evidenced in this report, there is still much work to be done in order to understand in detail how this small cytokine affects and modulates the neutrophil inflammatory response. In this respect, we believe that the zebrafish will undoubtedly stand as the optimal experimental animal model to pursue these issues and further to develop new therapeutic approaches strategically targeting CXCL8.
Supplementary Material
Acknowledgments
The authors would like to thank to Lara Carvalho, Dr. Jose Rino, Dr. António Temudo, Inma Fuentes and Pedro J. Martínez for their expert technical assistance, to Dr. Leonor Saúde and Dr. Susana Pascoal for their help, support and expertise.
This work was supported by Fundação para a Ciência e Tecnologia (FCT) via S. de Oliveira PhD Fellowship Grant (SFRH/BD/62674/2009), the Spanish Ministry of Science and Innovation (grants BIO2011-23400 and CSD2007-00002 to V.M.; and PhD fellowship to S.C., and Fundación Séneca-Murcia (grant 04538/GERM/06 to V.M.). SAR is funded by a UK Medical Research Council Senior Clinical Fellowship (G0701932) and an MRC Centre Grant (G0700091)
Abbreviations
- MO
Morpholino
- Tg
Transgenic
- dpf
days post-fertilization
- hpw
hours post-wounding
- hpi
hours post-injection
References
- 1.Nemeth T, Mocsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 3.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 4.Lindley I, Aschauer H, Seifert JM, Lam C, Brunowsky W, Kownatzki E, Thelen M, Peveri P, Dewald B, von Tscharner V, et al. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: biological equivalence between natural and recombinant neutrophil-activating factor. Proc Natl Acad Sci U S A. 1988;85:9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 6.Sarmiento J, Shumate C, Suetomi K, Ravindran A, Villegas L, Rajarathnam K, Navarro J. Diverging mechanisms of activation of chemokine receptors revealed by novel chemokine agonists. PLoS One. 2011;6:e27967. doi: 10.1371/journal.pone.0027967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183:3425–3432. doi: 10.4049/jimmunol.0900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 9.Raghuwanshi SK, Su Y, Singh V, Haynes K, Richmond A, Richardson RM. The Chemokine Receptors CXCR1 and CXCR2 Couple to Distinct G Protein-Coupled Receptor Kinases To Mediate and Regulate Leukocyte Functions. J Immunol. 2012 doi: 10.4049/jimmunol.1201114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, Frevert CW. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol. 2010;184:2677–2685. doi: 10.4049/jimmunol.0903274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284:L566–577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 12.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 13.Martin JS, Renshaw SA. Using in vivo zebrafish models to understand the biochemical basis of neutrophilic respiratory disease. Biochem Soc Trans. 2009;37:830–837. doi: 10.1042/BST0370830. [DOI] [PubMed] [Google Scholar]
- 14.Lieschke GJ, Trede NS. Fish immunology. Curr Biol. 2009;19:R678–682. doi: 10.1016/j.cub.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 15.Meeker ND, Trede NS. Immunology and zebrafish: spawning new models of human disease. Dev Comp Immunol. 2008;32:745–757. doi: 10.1016/j.dci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Mathias JR, Dodd ME, Walters KB, Rhodes J, Kanki JP, Look AT, Huttenlocher A. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J Cell Sci. 2007;120:3372–3383. doi: 10.1242/jcs.009159. [DOI] [PubMed] [Google Scholar]
- 17.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 18.Mathias JR, Walters KB, Huttenlocher A. Neutrophil motility in vivo using zebrafish. Methods Mol Biol. 2009;571:151–166. doi: 10.1007/978-1-60761-198-1_10. [DOI] [PubMed] [Google Scholar]
- 19.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 23.Starnes TW, Huttenlocher A. Neutrophil reverse migration becomes transparent with zebrafish. Adv Hematol. 2012;2012:398640. doi: 10.1155/2012/398640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Q, Harvie EA, Huttenlocher A. Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell Microbiol. 2012;14:517–528. doi: 10.1111/j.1462-5822.2011.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benard EL, van der Sar AM, Ellett F, Lieschke GJ, Spaink HP, Meijer AH. Infection of zebrafish embryos with intracellular bacterial pathogens. J Vis Exp. 2012 doi: 10.3791/3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui C, Benard EL, Kanwal Z, Stockhammer OW, van der Vaart M, Zakrzewska A, Spaink HP, Meijer AH. Infectious disease modeling and innate immune function in zebrafish embryos. Methods Cell Biol. 2011;105:273–308. doi: 10.1016/B978-0-12-381320-6.00012-6. [DOI] [PubMed] [Google Scholar]
- 27.van der Vaart M, Spaink HP, Meijer AH. Pathogen Recognition and Activation of the Innate Immune Response in Zebrafish. Adv Hematol. 2012;2012:159807. doi: 10.1155/2012/159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cvejic A, Hall C, Bak-Maier M, Flores MV, Crosier P, Redd MJ, Martin P. Analysis of WASp function during the wound inflammatory response--live-imaging studies in zebrafish larvae. J Cell Sci. 2008;121:3196–3206. doi: 10.1242/jcs.032235. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Renshaw S, Martin P. Live Imaging of Tumor Initiation in Zebrafish Larvae Reveals a Trophic Role for Leukocyte-Derived PGE(2) Curr Biol. 2012;22:1253–1259. doi: 10.1016/j.cub.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomiyama H, Hieshima K, Osada N, Kato-Unoki Y, Otsuka-Ono K, Takegawa S, Izawa T, Yoshizawa A, Kikuchi Y, Tanase S, Miura R, Kusuda J, Nakao M, Yoshie O. Extensive expansion and diversification of the chemokine gene family in zebrafish: identification of a novel chemokine subfamily CX. BMC Genomics. 2008;9:222. doi: 10.1186/1471-2164-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alejo A, Tafalla C. Chemokines in teleost fish species. Dev Comp Immunol. 2011;35:1215–1222. doi: 10.1016/j.dci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 33.van der Aa LM, Chadzinska M, Tijhaar E, Boudinot P, Verburg-van Kemenade BM. CXCL8 chemokines in teleost fish: two lineages with distinct expression profiles during early phases of inflammation. PLoS One. 2010;5:e12384. doi: 10.1371/journal.pone.0012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Aa LM, Chadzinska M, Golbach LA, Ribeiro CM, Lidy Verburg-van Kemenade BM. Pro-inflammatory functions of carp CXCL8-like and CXCb chemokines. Dev Comp Immunol. 2012;36:741–750. doi: 10.1016/j.dci.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Oehlers SH, Flores MV, Hall CJ, O’Toole R, Swift S, Crosier KE, Crosier PS. Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev Comp Immunol. 2010;34:352–359. doi: 10.1016/j.dci.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 36.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 37.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 38.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1997;94:3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoll SJ, Bartsch S, Augustin HG, Kroll J. The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8. Circ Res. 2011;108:1367–1377. doi: 10.1161/CIRCRESAHA.111.244095. [DOI] [PubMed] [Google Scholar]
- 40.Hu N, Westra J, Rutgers A, Doornbos-Van der Meer B, Huitema MG, Stegeman CA, Abdulahad WH, Satchell SC, Mathieson PW, Heeringa P, Kallenberg CG. Decreased CXCR1 and CXCR2 expression on neutrophils in anti-neutrophil cytoplasmic autoantibody-associated vasculitides potentially increases neutrophil adhesion and impairs migration. Arthritis Res Ther. 2011;13:R201. doi: 10.1186/ar3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godaly G, Hang L, Frendeus B, Svanborg C. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J Immunol. 2000;165:5287–5294. doi: 10.4049/jimmunol.165.9.5287. [DOI] [PubMed] [Google Scholar]
- 42.Nagarkar DR, Wang Q, Shim J, Zhao Y, Tsai WC, Lukacs NW, Sajjan U, Hershenson MB. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol. 2009;183:6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batra S, Cai S, Balamayooran G, Jeyaseelan S. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188:3458–3468. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khandoga AG, Khandoga A, Reichel CA, Bihari P, Rehberg M, Krombach F. In vivo imaging and quantitative analysis of leukocyte directional migration and polarization in inflamed tissue. PLoS One. 2009;4:e4693. doi: 10.1371/journal.pone.0004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadirkamanathan V, Anderson SR, Billings SA, Zhang X, Holmes GR, Reyes-Aldasoro CC, Elks PM, Renshaw SA. The neutrophil’s eye-view: inference and visualisation of the chemoattractant field driving cell chemotaxis in vivo. PLoS One. 2012;7:e35182. doi: 10.1371/journal.pone.0035182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pase L, Nowell CJ, Lieschke GJ. In vivo real-time visualization of leukocytes and intracellular hydrogen peroxide levels during a zebrafish acute inflammation assay. Methods Enzymol. 2012;506:135–156. doi: 10.1016/B978-0-12-391856-7.00032-9. [DOI] [PubMed] [Google Scholar]
- 49.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 52.Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci (Lond) 1992;83:639–648. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- 53.Loynes CA, Martin JS, Robertson A, Trushell DM, Ingham PW, Whyte MK, Renshaw SA. Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J Leukoc Biol. 2010;87:203–212. doi: 10.1189/jlb.0409255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elks PM, van Eeden FJ, Dixon G, Wang X, Reyes-Aldasoro CC, Ingham PW, Whyte MK, Walmsley SR, Renshaw SA. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 55.Holmes GR, Dixon G, Anderson SR, Reyes-Aldasoro CC, Elks PM, Billings SA, Whyte MK, Kadirkamanathan V, Renshaw SA. Drift-Diffusion Analysis of Neutrophil Migration during Inflammation Resolution in a Zebrafish Model. Adv Hematol. 2012;2012:792163. doi: 10.1155/2012/792163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 57.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32:350–357. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL. Live imaging reveals differing roles of macrophages and neutrophils during Zebrafish tail fin regeneration. J Biol Chem. 2012 doi: 10.1074/jbc.M112.349126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–2594. [PubMed] [Google Scholar]
- 60.Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Chemokine antagonists that discriminate between interleukin-8 receptors. Selective blockers of CXCR2. J Biol Chem. 1997;272:16166–16169. doi: 10.1074/jbc.272.26.16166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.