1Abstract
The Human Leukocyte Antigen HLA-B27(B27) is strongly associated with the spondyloarthritides. B27 can be expressed at the cell surface of antigen presenting cells (APC) as both classical β2m-associated B27 and as B27 free heavy chain forms (FHC) including disulphide-bonded heavy chain homodimers (termed B272). B27 FHC forms but not classical B27 bind to KIR3DL2. HLA-A3 which is not associated with spondyloarthritis (SpA) is also a ligand for KIR3DL2. Here we show that B272 and B27 FHC bind more strongly to KIR3DL2 than other HLA-class I, including HLA-A3. B272 tetramers bound KIR3DL2 transfected cells more strongly than HLA-A3. KIR3DL2Fc bound to HLA-B27-transfected cells more strongly than to cells transfected with other HLA-class I. KIR3DL2Fc pulled down multimeric, dimeric and monomeric free heavy chains from HLA-B27 expressing cell lines. Binding to B272 and B27 FHC stimulated greater KIR3DL2 phosphorylation than HLA-A3. B272 and B27 FHC stimulated KIR3DL2CD3ε–transduced T cell IL-2 production to a greater extent than control HLA-class I. KIR3DL2 binding to B27 inhibited NK IFNγ secretion and promoted greater survival of KIR3DL2+CD4 T and NK cells than binding to other HLA-class I. KIR3DL2+ T cells from B27+SpA patients proliferated more in response to antigen presented by syngeneic APC than the same T cell subset from healthy and disease controls. Our results suggest that expansion of KIR3DL2-expressing leukocytes observed in B27+ SpA may be explained by the stronger interaction of KIR3DL2 with B27 FHC.
Introduction
HLA-B27 (B27) is strongly associated with a group of inflammatory arthritic disorders collectively known as the spondyloarthritides (SpA) (1). Several hypotheses have been proposed to explain B27 involvement. These include activation of cross-reactive autoimmune T cells by “arthritogenic peptides” and stimulation of proinflammatory cytokine production by induction of ER stress resulting from B27 misfolding during assembly (2-4).
We have shown that B27 can be expressed on the surface of patient and B27 transgenic rodent leukocytes as B27 free heavy chain forms (FHC) including cysteine-67 dependent disulphide bonded heavy chain homodimers (termed B272)(5-7). HLA-class I molecules bind members of the Killer cell Immunoglobulin-like Receptor family (KIR)(8). B272 binds to different but overlapping groups of immune receptors compared with classical β2-microglobulin-associated B27(5, 9). We have proposed that differences in the strength of binding and specificity of immune receptors binding to B27 FHC forms and classical HLA-class I could lead to altered immune regulation and promote inflammation in spondyloarthritis (SpA)(10).
Killer cell immunoglobulin-like receptors are expressed by Natural Killer, NK T cells and minor subsets of CD4 and CD8 T cells. KIRs are highly polymorphic and bind to HLA-class I in an allele-specific fashion(11). For example the cognate KIR for classical HLA-B27 is KIR3DL1 which also binds to B272 (5). KIR can be distinguished by the presence or lack of a long cytoplasmic tail incorporating regulatory ITIM motifs. These regulatory motifs are phosphorylated upon ligation by class I at immunological synapses. Subsequently, KIR ligation modulates cytokine production and promotes immune cell survival by upregulating the expression of anti-apoptotic genes and downregulating expression of pro-apoptotic genes such as FasL(12). B272 but not β2m-associated B27 binds to KIR3DL2 which has also been shown to bind to β2m-associated HLA-A3 and A11 (13, 14). KIR3DL1 and 2 binding to classical β2-microglobulin-associated HLA-class I is dependent on the sequence of peptide bound to the class I molecule (14, 15). By contrast B27 dimers bind to KIR3DL2 in a peptide-independent fashion (16).
Increased proportions of KIR3DL2-expressing NK and CD4 T cells are present in the blood and peripheral joint synovial fluid of patients with spondyloarthritis (17, 18). Moreover, KIR3DL2+Th17 account for the majority of IL17-producting CD4 T cells in SpA patients compared with controls (18). Since KIR3DL2-ligation by B272 enhances the survival of NK and CD4 T cells, we have proposed that KIR3DL2-B272 interactions promote the survival of proinflammatory leukocytes in SpA (17, 18).
By contrast with HLA-B27, HLA-A3 is not strongly associated with spondyloarthritis. We hypothesised that differences between the strength of binding of B272 and B27 free heavy chains and HLA-A3 to KIR3DL2 could explain the differential disease association of these different class I molecules.
We predicted that stronger interactions of B27 FHC with KIR3DL2 compared to HLA-A3 and other ligands would result in stronger effects on downstream functions modulated by KIR ligation.
Here we compare the strength of interaction of B272 and B27 free heavy chains and HLA-A3 and other HLA-class I with KIR3DL2. We compare KIR3DL2 binding to HLA-B27 and other HLA-class I using KIR3DL2 reporter cells and class I tetramer and KIR3DL2Fc staining of transfected cells. We also study the effect of KIR3DL2 ligation by HLA-B27 and other ligands on receptor phosphorylation, cell proliferation and survival and cytokine production. We show that cell surface B27 free heavy chains (FHC; which include B272) are ligands for KIR3DL2. KIR3DL2 bind more strongly to B27 FHC than other characterised ligands. KIR3DL2 binding to B27 FHC inhibits IFNγ production and promotes the survival of leukocytes to a greater extent than binding to other HLA-class I including HLA-A3.
Materials and Methods
Recombinant protein expression
Recombinant class I proteins were expressed as inclusion bodies, refolded as either heterotrimeric or homodimer complexes and purified by gel exclusion chromatography as previously described (5). Heterotrimers of HLA-class I comprise β2m, peptide class I heavy chains. HLA-A3 and HLA-B27 heterotrimer and homodimer tetramers were made as previously described(16).
FACS staining with tetramers and tetramer competition experiments
Tetrameric complexes were used to stain Baf3 cells transduced with KIR3DL1 and KIR3DL2 as previously described (16). For tetramer competition experiments cells were first stained with a saturating concentration of phycoerythrin-labelled tetramer before competition at room temperature with a two-fold excess of tetramer without fluorescent label. Subsequently FACS staining with fluorescent conjugated tetramer was assessed at different times after adding unlabelled tetramer.
KIR3DL2Fc expression and purification, FACS staining and pull-downs
A KIR3DL2Fc lentiviral expression cassette was constructed by overlapping PCR to generate cDNA encoding the D0, D1 and D2 domains of KIR3DL2*0101 fused to the Fc portion of human IgG1 cloned in PHR-SIN. Lentivirus were used to transduce 293T cells and soluble KIR3DL2Fc purified from supernatants on protein A sepharose.
200,000 LBL.721.221 (221) cells transfected with HLA-B27 or control HLA were stained for FACS analysis with 5μg of KIR3DL2 or control Death Receptor 5 DR5 Fc protein, washed and stained with phycoerythrin-conjugated goat anti-human immunoglobulins (Biolegend).
2 × 107 parental 221 cells or transfected cells were stained with 50μg KIR3DL2Fc or control DR5Fc. Cells were washed twice in ice-cold PBS and lysed in lysis buffer (1%NP-40, 20mM Tris, 150mM NaCl, iodoacetamide 0.5mM, 1mM EDTA with peptidase inhibitors; Roche UK Ltd) and bound proteins were precipitated with protein G Dynal beads (Dynal UK Ltd) washed 6 times with lysis buffer and resolved by non-reducing/reducing SDS-PAGE as previously described. Resolved lysates were western blotted with the anti-HLA-class I heavy chain MAb HC10.
Analysis of KIR3DL2 phosphorylation
Transduced T cells were starved overnight in DMEM medium without supplements (D0). 5×106 jurkat cells transduced with C-terminal haemagglutinin-tagged KIR3DL2 were stimulated with 10ng/ml staphylococcal enterotoxin E (SEE) and 5×106 parental or transfected 221 cells in DMEM without supplements for 20 minutes prior to staining with anti-KIR3DL2 MAb for 10 minutes on ice (Clone#1, Innate Pharma, Marseille, France). After washing in ice-cold Tris buffered saline, cells were lysed in lysis buffer with phosphatase inhibitors (Thermo Scientific UK Ltd). KIR3DL2 was subsequently immunoprecipitated by tumbling with anti-mouse immunoglobulin Dynal beads (Dynal UK). Immunoprecipitates were washed and resolved by reducing SDS-PAGE. Resolved lysates were western blotted with a mix of anti-phospho tyrosine MAbs (PY20, InVitrogen; and P-Tyr 100; Cell Signalling) and were subsequently reprobed with anti-HA MAb (HA-7; Sigma).
KIR3DL2CD3ε reporter cell assay
A KIR3DL2CD3ε PHR-SIN lentiviral expression cassette was generated by overlapping PCR with primers designed to amplify the extracellular and transmembrane portions of KIR3DL2*0101 and the cytoplasmic region of CD3ε. KIR2DL3CD3ε PHR-SIN has been previously described ((19). Subsequently, KIR3DL2CD3ε and KIR2DL3CD3ε lentivirus were made as described previously for LILRB2 (20).
All experiments for cytokine assay were set up in RPM1640 medium (Sigma) supplemented with 10% FCS and antibiotics (R10, (16)). 200,000 transduced T cells were incubated with 200,000 221B27 or parental 221 cells, EBV-immortalised B cell lines or MACs-sorted (Mitenyi Ltd) CD14+ monocytes for 24 hours in with/without HC10, W632, ME1 or isotype control IgG1/G2a MAbs (MOPC 123 and MG1-45; Biolegend) at 50 μg/ml. Subsequently, supernatants were harvested for IL-2 ELISA according to the manufacturer’s instructions. (EBiosciences Ltd UK).
The tetramer and KIR3DL2 constructs used in this study are summarized in a schematic in figure 1S.
Generation of T and NK cell lines and clones. Coculture of T or NK cell lines with HLA-B27-expressing antigen presenting cells (APCs)
T and NK cell lines and clones were generated from FACS-sorted CD4 T cells from B27+SpA patients and maintained as described previously.(17, 18). LBL.721.220 and LBL.721.221 parental B lymphocyte-derived cell lines (abbreviated to 220 or 221) transfected with HLA-A3,-B7,-B8, -B27, -B27C67S, -B35, B44 and HLA-B27 together with human tapasin have been described previously (21, 22). CD4 T or NK cell lines were labelled with CFSE according to the manufacturer’s instructions (Invitrogen). T and NK cell cocultures with 221 APCs were set up as previously described for 220 cells (17, 23). 1×106 T or NK cells were cocultured with 0.5×106 221 cells. On day 5 cells were stained for KIR3DL2 expression with the DX31 MAb, Annexin V APC (BD Biosciences) and pacific blue Live Dead stain (InVitrogen) in Annexin V buffer according to the manufacturer’s instructions (BD Biosciences). Alternatively in CFSE proliferation experiments dead cells stained with live dead were excluded before FACS analysis. Total viable numbers of KIR3DL2+ CD4 T cells were enumerated with fluocount beads (Beckman Coulter).
Supernatants for IFNγ ELISA (eBioscience) were harvested from NK cells after .24 hour stimulation in R10 medium.
Results
B27 dimer tetramers bind more strongly to KIR3DL2 than HLA-A3
HLA-class I molecules are expressed as heterotrimeric complexes with heavy chain, β2m and peptide. In addition to β2m-associated heterotrimer HLA-B27 is also expressed as β2m-free heavy chain dimers (termed B272). We first compared staining of KIR3DL2-transfected Baf3 cells with HLA-A3 heterotrimers and B272 tetramers. B272 tetramer consistently stained KIR3DL2 transfectants more strongly than HLA-A3 heterotrimer tetramers (Figure 1A and B). B272 tetramers did not stain parental Baf3 cells (Figure 1A). B272 tetramers may have an enhanced avidity for KIR3DL2 because each dimer tetramer incorporates eight molecules of HLA-B27 compared with HLA-A3 which incorporates 4 molecules. Because of this we stained KIR3DL2-expressing cells with non-tetramerised recombinant B27 dimer, HLA-A3 or control HLA-class I protein. Subsequently bound protein was detected by staining with extravidin PE. Recombinant B27 dimer protein stained KIR3DL2 transfectants more strongly than HLA-A3 and other HLA-class I proteins (Figure 1C).
Figure 1. B272 protein and tetramers bind to KIR3DL2-transfected cells more strongly than HLA-A3.
A. Representative FACS stain of KIR3DL2 transfected Baf3 cells with saturating concentrations of B27 dimer and HLA-A3 tetramers. Geometric MFIs for staining were 206 and 52 for B27 dimer and HLA-A3 tetramer staining respectively. Representative of one of four independent experiments. B. Titration of phycoerythrin (PE)-labelled B272 and HLA-A3 tetramer in FACS staining of KIR3DL2 transfected Baf3 cells. Representative stain from one of four independent experiments. C. FACs staining of KIR3DL2 Baf3 cells with B27 dimer (B272) HLA-A3, HLA-B8 and HLA-B27 protein. Representative staining of one of three independent experiments. D. B272 tetramers compete for binding to KIR3DL2 more strongly than HLA-A3. Left hand top panel. B272 tetramer competition with PE-labelled HLA-A3 tetramer bound to KIR3DL2 Baf3 cells. Staining with HLA-A3 tetramer at 0, 30 and 60 minutes after addition of HLA-A3 or B272 tetramer. Left hand bottom panel. HLA-A3 tetramer competition with PE-labelled B272 tetramer bound to KIR3DL2 Baf3 cells. Staining with B272 tetramer at 0, 30 and 60 minutes after addition of HLA-A3 or B272 tetramer. Results are presented as the mean reduction in the geometric MFI of tetramer staining from three independent experiments ± 1SD.
Next we studied the ability of B27 dimer and HLA-A3 heterotrimer to compete for binding to KIR3DL2. We stained KIR3DL2-expressing cell lines with fluorescent-conjugated HLA-A3 heterotrimer or B27 dimer tetramers and then measured remaining bound tetramer at 30 and 60 minutes after adding unlabelled tetramer. B272 tetramers effectively competed HLA-A3 heterotrimer tetramer binding to KIR3DL2 transfectants whereas HLA-A3 tetramers had little effect on B272 binding (Figure 1D upper and lower panels). B27 dimer tetramers competed for bound B27 dimer tetramer weakly. HLA-A3 tetramers also competed for bound HLA-A3 tetramer weakly (Figure 1D). B272 tetramer staining of KIR3DL2-expressing cell lines was strongly inhibited by heavy chain MAb which recognises B27 dimers compared to HLA-A3 tetramer staining which was only weakly inhibited (HC10 or HCA2; figure 1S). KIR3DL2 staining by both tetramers was inhibited by anti-KIR3DL2 MAb (DX31) (Figure 1S).
KIR3DL2 binds more strongly to cell surface B27 free heavy chains(FHC) than other HLA-class I
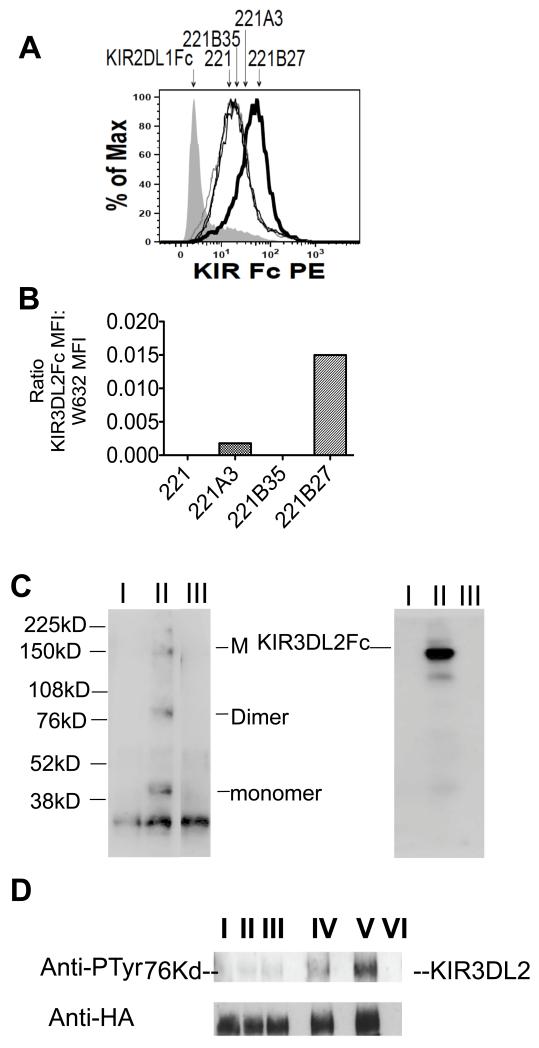
We next compared binding of KIR3DL2Fc fusion protein to HLA-B27 and other HLA-class I molecules. KIR3DL2 Fc consistently stained HLA-B27 transfected-LBL.721.221 cells (hereafter referred to as 221B27 cells) more strongly than parental 221 cells or 221A3 and 221B35 cells (Figure 2A and 2B). Figure 2B shows KIR3DL2Fc staining of transfected cells expressed as a ratio with the MFI for W632 staining after subtracting the MFIs for background KIR3DL2 staining of parental 221 cells. 221B35 cells did not stain with KIR3DL2Fc significantly above background staining of parental 221 cells. We hypothesised that the increased staining of B27-transfected cells with KIR3DL2Fc could result from KIR3DL2 binding to B27 free heavy chain forms expressed by these cells (22). Thus we stained 221B27 with KIR3DL2Fc and analysed bound protein precipitated with protein G from cell lysates by SDS PAGE and western blot with HC10 MAb. Bands corresponding to B27 dimers, monomers and multimers were detected by western blot (Figure 2B). No bands were detected when western blots were reprobed with the anti-β2m antibody BBM-1 (results not shown).
Figure 2. KIR3DL2Fc binds B272 more strongly than other HLA class I.
A. FACS staining of non-transfected LBL.721.221 (221) cells and 221 cells transfected with HLA-B27, HLA-A3 and HLA-B35 with KIR3DL2Fc. 221B27 cells stained with control DR5Fc fusion proteins is also shown. Geometric MFIs for staining of 221, 221B27, 221A3 and 221B35 were 18, 46, 22 and 17 respectively. Representative FACS stain from one of three independent experiments. B. Ratio of KIR3DL2Fc MFI:W632 MFI for staining of 221 transfectants. Ratios were calculated after subtracting the MFI for KIR3DL2Fc staining of parental 221 cells. Ratios were calculated from the mean MFIs from three independent experiments. C. Non-reducing SDS-PAGE gel and western blot with HC10 of precipitated proteins from 221B27 cells with control DR5Fc, lane I, and with KIR3DL2Fc from 221B27, lane II, and 221B35 transfected cells, lane III (left hand panel). Positions of multimeric, M, B27 dimer, B272, and monomeric B27 heavy chains, B27, are indicated. The right panel shows the same blot reprobed with HRP-conjugated anti-human Igs. The position of KIR3DL2Fc is indicated. Representative western blots from one of three independent experiments. D. Upper panel. Phosphotyrosine western blots of immunoprecipitates of KIR3DL2-transduced Jurkat T cells (lane I), or T cells stimulated with superantigen and parental 221 cells (lane II) or HLA-B35 (lane III), -A3 (lane IV), or -B27 transfectants (lane V). Lane VI: 221B27 cells alone. Lower panel. Western blot reprobed with anti-HA. Representative blots from one of three independent experiments.
We reasoned that stronger binding of B27 FHC would stimulate greater KIR3DL2 ITIM phosphorylation compared to other class I ligands. Thus we compared KIR3DL2 tyrosine phosphorylation in superantigen-activated KIR3DL2 expressing Jurkat cells with transfected 221 cells. HLA-B27 ligation consistently stimulated greater phosphorylation of KIR3DL2 compared to HLA-A3, HLA-B35 or parental 221 cells (Figure 2C).
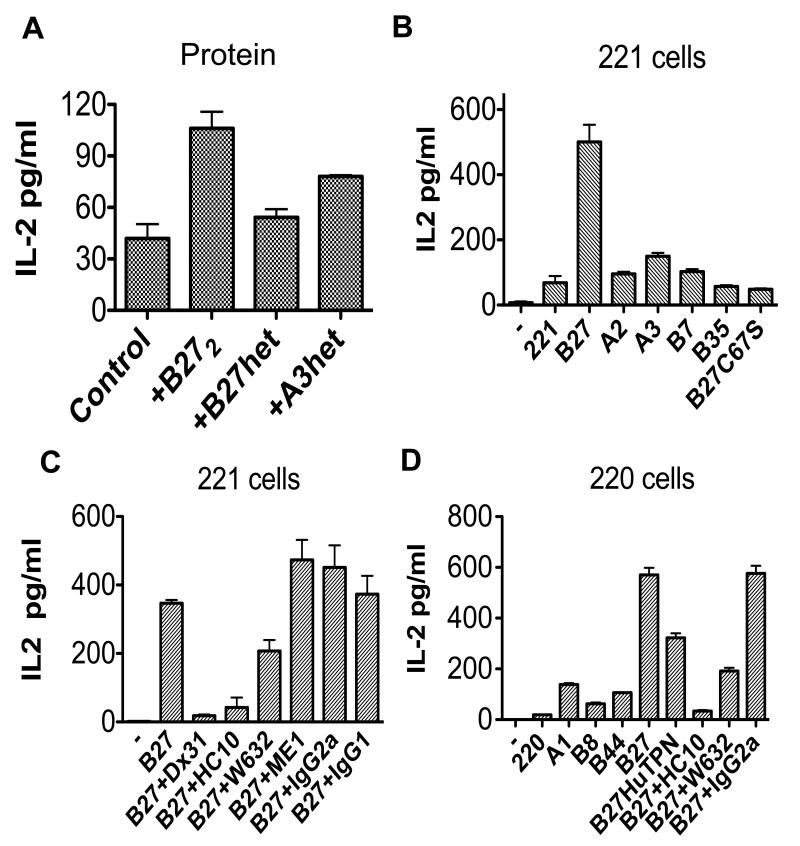
We then determined whether the stronger binding of B27 dimers to KIR3DL2 in vitro could be translated into differences in functional interactions with this receptor. We transduced Jurkat T cells with KIR3DL2CD3ε and measured functional interactions with plate-immobilised recombinant B27 dimers and HLA-class I heterotrimers by measuring IL-2 production by ELISA. B27 dimers but not control HLA-B27 heterotrimers consistently stimulated IL-2 production above background levels in the presence of PMA and anti-CD28 (Figure 3A). Equivalent quantities of immobilised B27 dimer consistently stimulated greater production of IL-2 than HLA-A3 heterotrimers.
Figure 3. B27 dimers and free heavy chains stimulate KIR3DL2CD3ε reporter T cells more strongly than control HLA class I.
A. IL-2 production by KIR3DL2CD3ε–transduced T cells stimulated with recombinant HLA-B27 dimer and -B27 or HLA-A3 heterotrimers. Results, presented as mean values from triplicate samples (pg/ml)±1SD. B IL-2 secretion by KIR3DL2CD3ε–transduced Jurkat T cells, stimulated with parental LBL.721.221 (221) cells and cells transfected with HLA-B27, HLA-B27C67S, HLA-B7, HLA-B35, HLA-A2 and HLA-A3. Results presented as mean values from 5 independent experiments (pg/ml) ± 1SEM. C. IL-2 production by KIR3DL2CD3ε–transduced T cells stimulated with 221B27 cells with HC10, W632, ME1 and IgG2a and IgG1 isotype control MAbs or the anti-KIR3DL2 MAb (DX31). Results presented as mean values from triplicate samples (pg/ml) ± 1SD are representative of 4 independent experiments. D. IL-2 production by KIR3DL2CD3ε transduced T cells stimulated with parental LBL.221.220 cells, 220B8, 220B27 cells with HC10, W632, ME1 and IgG2a and IgG1 isotype control MAbs and 220B27 cells transfected with human tapasin (HuTPN). Results presented as mean values from triplicate samples (pg/ml) ± 1SD are representative of 4 independent experiments.
Subsequently we studied whether cell surface HLA-B27 bound to KIR3DL2CD3ε-Jurkat T cells. 221B27 cells stimulated greater IL-2 secretion than 221A2, 221A3, 221B7, 221B35, 221B27C67S and parental 221 cells (Figure 3B). HLA-class I transfectants expressed similar quantities of class I, as assessed by FACS staining with W632 MAb (Figure 2S). Parental Jurkat T cells and KIR2DL3CD3ε-transduced jurkat T cells produced negligible quantities of IL-2 in response to these stimuli (Figure 3S). IL-2 production by KIR3DL2CD3ε-transduced Jurkat T stimulated with 221B27 cells was inhibited by KIR3DL2-specific MAb (DX31), by heavy chain (HC10) and W632 antibodies (Figure 3B). By contrast the B27-specific antibody ME1 had no significant effect (Figure 3B).
LBL.721.220 cells transfected with HLA-B27 (hereafter referred to as 220B27) also express cell surface B27 heavy chain dimers and multimers (22). These cells lack functional human tapasin and as a consequence form more unstable B27 heterotrimers on their cell surface. B27 dimers and multimers form from unstable cell surface B27 heterotrimers(22). Supertransfection of 220B27 cells with human tapasin (220B27 huTPN), which optimises peptide cargo and stabilises B27 heterotrimers reduces the level of cell surface B27 dimer expression (16). As a consequence 220B27huTPN cells express higher levels of ME1 and W632 reactive B27 and lower levels of HC10-reactive B27 FHC. As expected, 220B27 cells stimulated production of IL-2 by KIR3DL2CD3ε-transduced T cells to a greater extent than parental 220 cells or 220 cells transfected with control HLA-A1, -B8 or -B44 (Figure 3D). 220B27 cells transfected with human tapasin stimulated less IL-2 production by KIR3DL2CD3ε reporter cells compared to 220B27 transfectants (Figure 3D). As seen for the 221B27 cells, production of IL-2 by reporter cells was reduced by antibodies which inhibit KIR3DL2 interactions with B27 free heavy chains and dimers (HC10 and W632 respectively; Figure 3D).
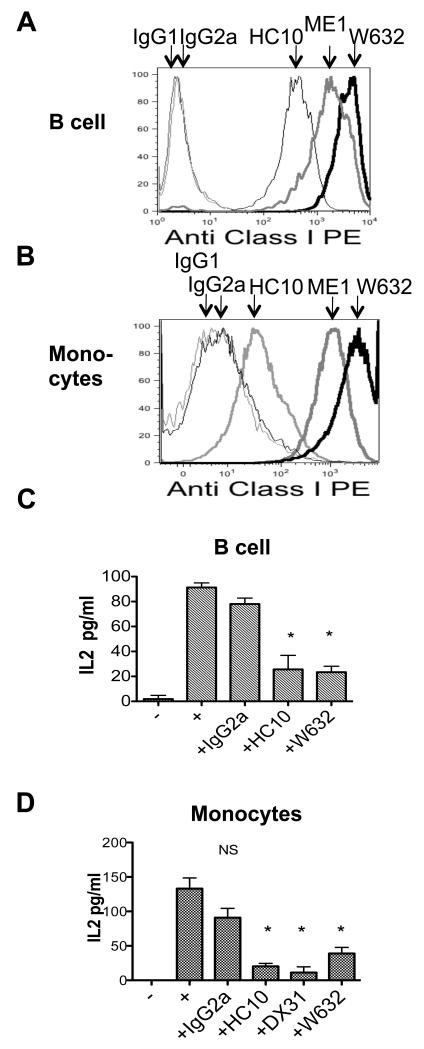
We have shown that B27 dimers and heavy chains are expressed by antigen presenting cells (APC) in B27+SpA patient peripheral blood and synovial fluid (5, 7). We sought to determine whether HLA-class I on the surface of APC from B27+SpA patients and B27+healthy controls could interact with KIR3DL2 on KIR3DL2CD3ε Jurkat T cells. The representative FACS stains in Figure 4A and B show the relative levels of expression of HC10 and W632-reactive forms of HLA-class I by EBV transformed B cells and purified monocytes from B27+individuals. B cell lines and monocyte lines from B27+healthy controls and B27+SpA patients both stimulated IL-2 production by KIR3DL2CD3ε-transduced T cells (Figure 4C and 4D). Reporter cell IL-2 production was inhibited by HC10 and W632 MAbs but not isotype control MAb (Figure 4A and 4B). B cell lines from healthy controls also weakly stimulated IL-2 production by KIR3DL2CD3ε-transduced T cells (data not shown).
Figure 4. B cell lines and monocytes from B27+SpA patients and B27+controls express HLA-class I ligands for KIR3DL2.
A Representative FACS stain with W632, HC10 and ME1 antibodies of an EBV transformed B cell line from a B27+SpA patient. Representative stain from one of four different B cell lines. Geometric MFIs for staining with HC10, ME1 and W632 were 406, 1443 and 3464 respectively. B Representative FACS stain with HC10 and ME1 antibodies of CD14+ monocytes from a B27+SpA patient. Representative stain from one of four monocyte samples. Geometric MFIs for staining with HC10, ME1 and W632 were 82, 1315 and 2724 respectively. C IL-2 production by KIR3DL2CD3ε reporter cells stimulated with primary B cell lines and D primary monocyte lines from a B27+ healthy control and a B27+ AS patient. IL-2 production by reporter cells is inhibited by anti-heavy chain (HC10), -KIR3DL2 and W632 monoclonals. Representative data from experiments with 4 different B cell lines and 3 monocyte lines. Results with B cell lines were repeated on 3 independent occasions. Results are expressed as mean values of IL-2 pg/ml +/− 1SD. * values P<0.05 by ANOVA.
Ligation of KIR3DL2 by HLA-B27 inhibits NK IFNγ production and promotes NK cell survival to a greater extent than binding to HLA-A3, HLA-B7 and HLA-B35
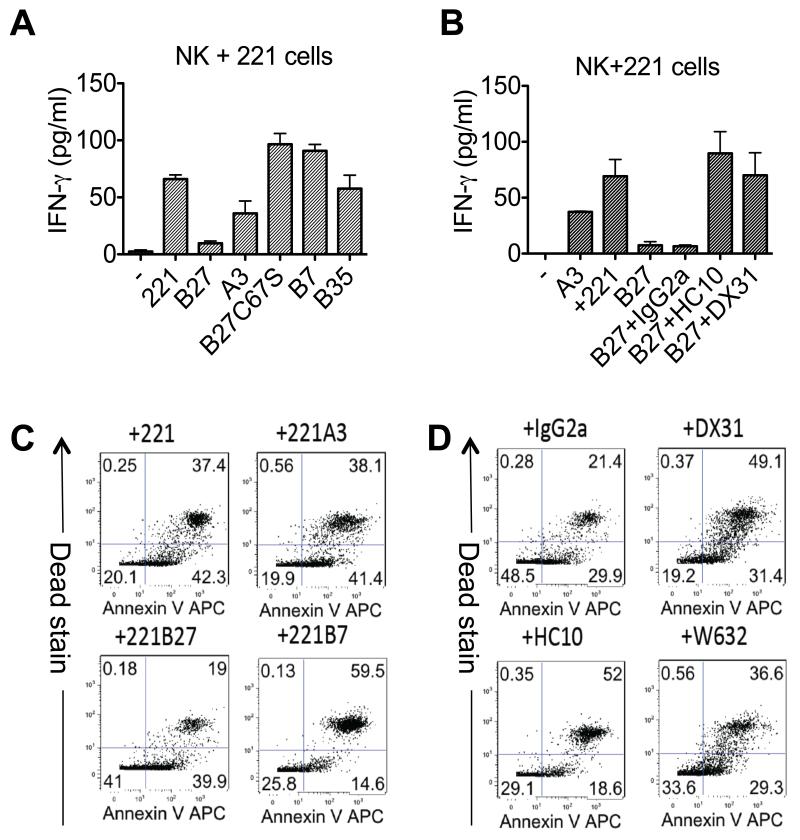
We next investigated the effect of KIR3DL2 binding to HLA-B27 on cytokine production and survival of primary NK cells. 221B27 cells inhibited the production of IFNγ by KIR3DL2-expressing NK cells to a greater extent than cells expressing control HLA-class I (Figure 5A). Inhibition of NK cytokine production by 221B27 cells was reduced by anti-KIR3DL2 and HC10 MAbs but not isotype control MAb (Figure 5B). Stimulation with 221B27 cells promoted the survival of KIR3DL2-expressing NK cells to a greater degree than parental 221 cells and cells expressing control HLA-class I (Figure 5C). The effect of 221B27 cells in promoting NK cell survival could be inhibited by anti-class I (HC10 and W632) and anti-KIR3DL2 (DX31) MAbs (Figure 5D).
Figure 5. KIR3DL2 ligation by HLA-B27 inhibits NK cell IFNγ and promotes NK cell survival.
A. IFNγ production by KIR3DL2-expressing NK cells stimulated with parental 221 cells and 221 cells transfected with HLA-B27, -A3,-B7, -B35 or -B27C67S. Representative data from triplicate samples from one of three independent experiments. B IFNγ production by KIR3DL2-expressing NK cells stimulated with parental 221B27 cells and anti-KIR3DL2 (DX31), anti HLA-class I heavy chain (HC10) or isotype control MAbs (IgG2a). Representative data from triplicate samples from one of three independent experiments. C. Annexin V and live-dead (Dead) staining of KIR3DL2+NK cells stimulated for 7 days with parental 221 cells or 221A3, -B27 or B7 cells. Representative data from one of three independent experiments. Lower left hand quadrants indicate percentages of viable NK cells remaining at the end of the assay. D. Annexin V and live-dead staining of KIR3DL2+NK cells stimulated for 7 days with 221B27 cells and anti-KIR3DL2 (DX31), anti-HLA-class I MAbs (HC10 and W632) or isotype control MAb.
Stimulation with HLA-B27 enhances the survival of KIR3DL2+CD4 T cells compared to stimulation with HLA-A3 and other HLA-class I. Increased expansion of antigen-stimulated KIR3DL2+T cells in B27+SpA patients
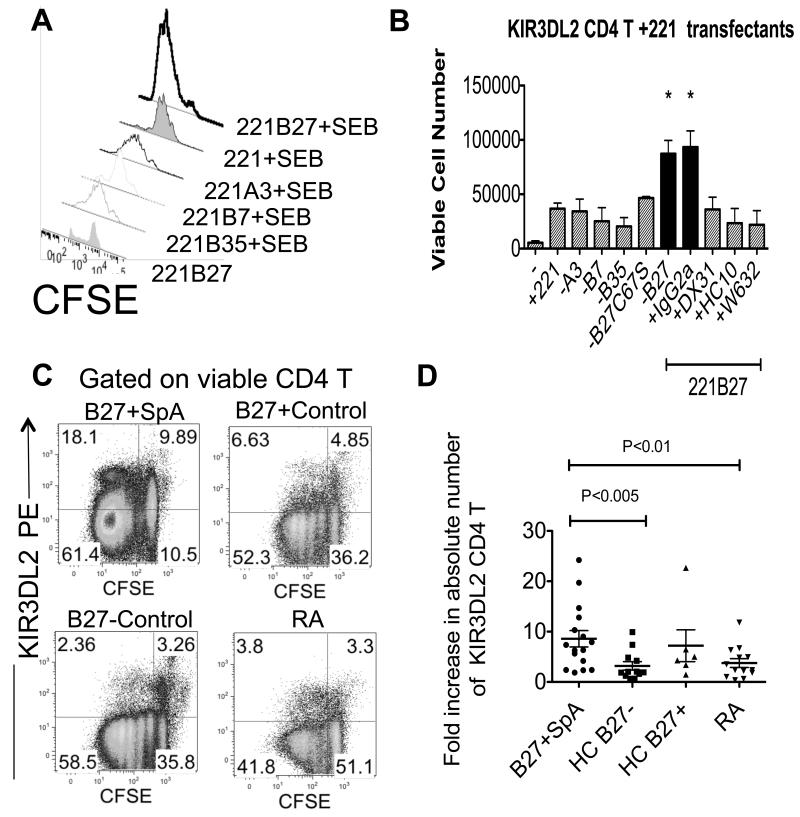
We next studied proliferation and survival of primary KIR3DL2+ CD4 T cells, stimulated with 221B27 cells by CFSE dilution. 221B27 cells promoted the survival of SEB-activated KIR3DL2+CD4 T cells to a greater degree than parental 221 and 221A3, (figure 6A). Superantigen-driven proliferation and survival of KIR3DL2+CD4 T cells with 221B27 transfected cells was inhibited with anti-KIR3DL2 (DX31), anti class I heavy chain (HC10) and W632 MAbs but not isotype control IgG2a MAb (Figure 6B).
Figure 6. Enhanced survival of KIR3DL2-expressing T cells stimulated with 221B27 cells. Increased expansion of antigen stimulated KIR3DL2+ CD4 T cells in B27+SpA patients.
A. Viable CFSE-labelled primary KIR3DL2+CD4 T cells after stimulation with parental 221 cells and 221B27,-B7,-B35 and −A3 and superantigen (SEB) or with 221B27 cells without superantigen. Geometric MFIs are 439 (221B27+SEB), 998(221+SEB), 851 (221A3+SEB), 1214 (221B7+SEB), 1935 (221B35+SEB) and 2876 (221B27). Representative FACS stain from one of five independent experiments. B. Numbers of viable CFSE-labelled primary KIR3DL2+CD4 T cells after 5 day stimulation with 221B27 cells without stimulus (−) or parental 221 cells, 221-A3, -B7, -B35, -B27C67S and -B27 cells with SEB. Numbers of viable CD4 T cells after 5 day stimulation with SEB and 221B27 cells with control (IgG2a), anti-HLA-class I(HC10 or W632) or anti-KIR3DL2 (DX31) MAbs are also shown. Mean data ±1SEM from three independent experiments. Values with an asterisk* indicate P<0.05 by ANOVA for T cells stimulated with SEB and 221B27 or SEB 221B27 and isotype MAb (IgG2a) compared to other stimuli. NS=not significant. C. Representative FACS stains of CFSE-labelled PBMC from an AS and RA patient and a healthy B27-control and healthy B27+ control showing proliferation of KIR3DL2+ and KIR3DL2-CD4 T cells following 5 day stimulation with superantigen (SEB). Representative FACS stains from 10 independent experiments. D. Fold increase in peripheral blood KIR3DL2 CD4 T cells from AS patients, B27− and B27+ healthy controls and RA patients following 5 day stimulation with SEB. Right hand panel. Mean fold increases in absolute KIR3DL2+CD4 T cell numbers with interquartile ranges for each of the groups studies are 8.6 and 9.3 (n=16) for B27+SpA, 3.2 and 3.4 for B27−HC (n=12), 7.2 and 9 for B27+HC (n=6), and 3.8 and 4.2 for RA patients (n=13).
We have previously shown increased proportions of CD4 T cells expressing KIR3DL2 in B27+SpA patients (17, 23). We hypothesised HLA-B27 on antigen presenting cells (APC) could promote the survival of antigen-stimulated KIR3DL2+ expressing leukocytes in ankylosing spondylitis.
Thus we compared the survival of CFSE-labelled KIR3DL2+CD4 T cells stimulated with superantigen presented by APCs in peripheral blood mononuclear cell samples from B27+ SpA patients and healthy B27+ and B27− controls and patients with rheumatoid arthritis (RA). PBMC were labelled with CFSE and stimulated with staphylococcal enterotoxin B (SEB) for 5 days. Viable proliferating CD4 T cells expressing KIR3DL2 or other KIR were determined by FACS staining.
We observed increased numbers of viable proliferating KIR3DL2 CD4 T cells from SpA patients after stimulation with superantigen compared to KIR3DL2 CD4 T cells from healthy and RA disease controls (Figure 6C). By contrast we did not see similar expansions of CD4 T cells expressing other KIR (Figure 4SA). Because of variability in the percentages of T cells expressing KIR and responding to superantigen between individuals we counted numbers of KIR3DL2+CD4 T cells before and after stimulation using fluocount beads. We then compared the fold increase in KIR3DL2+CD4 T cells between AS patients and controls when cells were stimulated with superantigen. Figure 6D shows that that there was a greater increase in numbers of KIR3DL2 CD4 T cells in patients compared to B27− healthy controls and RA disease controls. Expansion of KIR3DL2+ CD4 T cells in AS patients was not due to Vβ bias towards SEB-binding Vβ in this population in patients as FACS staining showed no enrichment for SEB-reactive Vβ in patients (Figure 4SB). We also observed a greater fold increase in the number of CD4-negative T cells between patients and controls (Figure 4SC). SEB-driven expansion of KIR3DL2+CD4 T cells in AS patients was inhibited by anti-KIR3DL2 MAb (DX31) and B27 FHC specific MAb (HD6) (Figure 4SD). By contrast B27 FHC specific MAb had little effect on expansion of KIR3DL2+CD4 T cells in B27− healthy controls (Figure 4SD).
Discussion
Here we show that KIR3DL2 binds more strongly to HLA-B27 free heavy chain species (FHC; which include B27 dimers) than to HLA-A3 and other HLA-class I. B27 dimer tetramers bound more strongly to KIR3DL2 and competed more effectively for binding to KIR3DL2 than HLA-A3 tetramers suggesting that B27 dimers are stronger ligands. The fact that non-tetramerised B27 dimer protein also bound more strongly to KIR3DL2 expressing cell lines than HLA-A3 suggests that this was not simply due to differences in tetramer stoichiometry. Moreover, B27 dimer tetramer binding to KIR3DL2 was strongly inhibited by the heavy chain specific monoclonal HC10. The HLA-A heavy chain MAbs HCA2 and HC10 MAb only slightly reduced HLA-A3 binding to KIR3DL2, suggesting that binding of B27 FHC and HLA-A3 to this receptor is distinct. HCA2 binds within and HC10 binds close to a region of HLA-class-1 which binds to the D0 domain of KIR3DL1 (24-26). KIR3DL1 has a related structure to KIR3DL2. KIR3DL2Fc stained 221B27 cells more strongly than HLA-A3 and control HLA-class I transfected cells.
We also observed greater phosphorylation of KIR3DL2 when T cells were stimulated with 221B27 cells compared with stimulation with parental 221, 221A3 and 221B35 cells. This increased phosphorylation of KIR3DL2 ITIMs by B27 also suggests stronger binding compared to other HLA-class I.
Recombinant B27 dimers stimulated greater production of IL-2 by KIR3DL2 reporter cells compared with HLA-A3 and -B27 heterotrimers. Moreover 221B27 cells stimulated greater production of IL-2 by KIR3DL2CDε reporter cells compared with 221A3, parental 221 cells and control HLA-class I transfected cells. 221A3 cells stimulated production of more IL-2 by KIR3DL2CDε-reporter cells compared to stimulation with 221 cells expressing control HLA-class I but not HLA-B27. These results suggest that peptide MHC (pMHC) complexes and possibly other forms of HLA-A3 are weaker ligands for KIR3DL2 than HLA-B27 heavy chain forms.
220B27 cells also stimulated greater production of IL-2 by KIR3DL2CD3ε-transduced T cells compared with cells expressing control HLA-class I. 220B27 cells lack functional tapasin which optimises peptides bound to HLA-class I. We have shown that B27 FHC form from β2m-associated HLA-B27 with suboptimal peptides (22). Thus 220B27 cells express increased levels of B27 dimers and other free heavy chain species because of the presence of more B27 pMHC complexes suboptimally loaded with peptide. Consistent with this hypothesis, 220B27 cells transfected with human tapasin stimulated less IL-2 production by reporter cells compared to 220B27 cells.
221B27 cells inhibited IFN-γ production by KIR3DL2 expressing NK cells to a greater extent than cells expressing other HLA-class I. 221B27 inhibition of IFNγ production was reduced by heavy chain and anti-KIR3DL2 antibodies suggesting that at least part of this effect was due to KIR3DL2 binding to B27 free heavy chain species. 221B27 cells also promoted the survival of KIR3DL2-expressing NK and T cells more strongly than HLA-A3 and other HLA-class I transfected cells. We have shown that KIR3DL2 expressing leukocytes are expanded in B27+ spondyloarthritis patients (17, 23). KIR ligation inhibits activation induced cell death of leukocytes(17, 27). The increased proportions of KIR3DL2+expressing leukocytes in SpA patients could thus be due to stronger KIR3DL2 binding to B27 promoting immune cell survival.
While HLA-A3 binding to KIR3DL2 is dependent on the sequence of complexed peptide, B27 dimers bind to KIR3DL2 in a peptide-independent fashion (14, 16). This could also result in higher densities of B27 ligand at the surface of antigen presenting cells available for binding to KIR3DL2 compared to HLA-A3. Higher densities of ligand could also result in stronger interactions with KIR3DL2. HLA-A3-expressing individuals have NK cells with poor effector function suggesting that HLA-A3 may only interact weakly with KIR3DL2 in vivo in “licensing” NK cells (28). Our findings suggest that studying how HLA-B27 expression could “licence” better KIR3DL2+NK cell effector function merits further investigation.
We hypothesise that KIR3DL2-B27 interactions could promote the expansion of T cells and other leukocytes in vivo. In support of this superantigen-activated KIR3DL2+expressing T cells in peripheral blood mononuclear cell samples from SpA patients proliferated more than the same immune subset in control samples. We observed greater proliferation of both CD4+ and CD4-T cells expressing KIR3DL2 in SpA patients. Although we have previously shown enrichment for IL-23 receptor expression on KIR3DL2 expressing CD4 T cells we did not observe similar enrichment on CD4-T (18). This suggests that the increased expansion of KIR3DL2 T cells in these assays is not simply because of increased levels of IL-23 in SpA patients promoting the survival of these cells. We did not observe significantly increased proliferation of KIR3DL2+expressing T cells from B27+healthy controls compared to other control samples. We have previously reported intermediately increased proportions of KIR3DL2+CD4 T cells in healthy B27+ controls compared to B27-controls (18). The difference in proliferation of KIR3DL2 CD4 T cells between B27+SpA patients and B27+controls might be explained by higher levels of expression of B27 free heavy chain ligands for KIR3DL2 expressed by APC from B27+SpA. Indeed, in support of this, we and others have reported higher levels of expression of B27 and other free heavy chains on the surface of peripheral blood monocytes from B27+SpA patients (5, 7, 29, 30).
KIR3DL2CD3ε T cell interactions with B27 were inhibited by HC10, W632 and HD6 but not by ME1 antibodies (this study and (7)). W632 and HD6 antibodies inhibited KIR3DL2 binding more weakly than HC10 in these assays. HC10 was most effective at inhibiting KIR3DL2CD3ε T cell interactions suggesting that the different inhibitory antibodies recognise different B27 species which share the HC10 epitope. By contrast ME1 recognises a population of β2m-associated HLA-B27 molecules and did not inhibit KIR3DL2 interactions. W632 and ME1 recognise distinct epitopes of β2m-associated HLA-class I. W632 recognises a conformational-dependent epitope in the α2 and 3 domains of HLA-class I close to residues 86 and 121 of class I heavy chains (31). By contrast, ME1 binds to a conformational β2m-dependent epitope in the α1 domain of HLA-B27 which is critically dependent on interactions between residues 67-71(32). W632 has also been reported to recognise a population of B27 dimers on transfected cells and β2m-free HLA class 1 on transfected and Daudi cells but does not inhibit B27 dimer tetramer binding to KIR3DL2 (33, 34).
Our results suggest that B27 FHC ligands for KIR3DL2 are heterogeneous. KIR3DL2Fc pulled down monomeric, dimeric and multimeric heavy chain forms from B27-expressing cells. Dimerisation and multimerisation of B27 heavy chains could increase the binding avidity for KIR3DL2. Alternatively monomeric B27 heavy chains could bind more strongly to this receptor than other HLA-class I heavy chains.
Monocytes and B cells from B27+SpA patients and B27+healthy controls stimulated production of IL-2 by KIR3DL2CD3ε-transduced T cells which was inhibited by HC10 and W632 MAbs. Thus β2m-free class I heavy chains and other class I species on cells which express physiological levels of HLA-class I can interact with KIR3DL2. Our results with monocytes and immortalised B cell lines do not distinguish between B27 and other HLA-class I heavy chains binding to KIR3DL2. Indeed, interestingly, our observations suggest that KIR3DL2 can also weakly interact with other HLA-class I heavy chains in addition to HLA-B27 on transfected cells and EBV transformed B cell lines (our unpublished observations).
We propose that HLA-B27 forms higher levels of high avidity FHC ligands for KIR3DL2 under inflammatory conditions compared to other HLA-class I. In support of this, both we and other authors have shown increased expression of HLA-class I heavy chains and B27 FHC species on the surface of monocyte populations in B27+ SpA and PsA patients (5, 7, 29, 30, 35).
How could KIR3DL2 bind to HLA-class I heavy chains? One possibility is that KIR3DL2 binds to shared features of HLA-class I weakly in a similar way to KIR3DL1 (24, 36). Since the D0 domain of KIR3DL1 binds to a conserved region of all HLA-class I it is possible that the D0 domain of KIR3DL2 could bind to a similar region in HLA-B27. Disulphide-dependent dimerization and/or multimerisation of B27 heavy chains could increase the avidity of HLA-B27 for KIR3DL2.
How could these interactions be involved in arthritic disease? We propose that KIR3DL2 binding to B27 free heavy chain forms could promote the survival and differentiation of proinflammatory leukocytes in ankylosing spondylitis. One possibility is that KIR3DL2 ligation could divert the production of cytokines in inflammation from Th1 to Th17. Indeed KIR3DL2 ligation by B27 free heavy chains inhibits production of IFN-γ by NK cells and can promote survival of Th17 cells under some circumstances (16, 23).
Here we show that B27 free heavy chain species including B27 dimers bind more strongly to the NK receptor KIR3DL2 than conventional HLA-class I. Stronger binding of B27 FHC to KIR3DL2 compared to other HLA-class I could promote the survival and differentiation of inflammatory leukocytes in SpA.
Supplementary Material
Acknowledgements
We thank Alison Han, Rebecca Horsfall, Alistair Waugh and Kati diGleria for purification of antibodies and peptides used in this study.
Footnotes
Arthritis Research UK (SK, JS, OR), Oxford NIHR Biomedical Research Centre (AR, PB), and the Belmont Trust (PB). Consejo Nacional de Ciencia y Technologia (CONACyT) Mexico (IWB), HH Japan Society for the Promotion of Science.
References
- 1.Brown MA, Pile KD, Kennedy LG, Calin A, Darke C, Bell J, Wordsworth BP, Cornelis F. HLA class I associations of ankylosing spondylitis in the white population in the United Kingdom. Ann Rheum Dis. 1996;55:268–270. doi: 10.1136/ard.55.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. HLA-B27 misfolding and spondyloarthropathies. Adv Exp Med Biol. 2009;649:217–234. doi: 10.1007/978-1-4419-0298-6_16. [DOI] [PubMed] [Google Scholar]
- 3.Fiorillo MT, Sorrentino R. T-cell responses against viral and self-epitopes and HLA-B27 subtypes differentially associated with ankylosing spondylitis. Adv Exp Med Biol. 2009;649:255–262. doi: 10.1007/978-1-4419-0298-6_19. [DOI] [PubMed] [Google Scholar]
- 4.Lopez de Castro JA. HLA-B27 and the pathogenesis of spondyloarthropathies. Immunol Lett. 2007;108:27–33. doi: 10.1016/j.imlet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, Bowness P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–2982. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- 6.Kollnberger S, Bird LA, Roddis M, Hacquard-Bouder C, Kubagawa H, Bodmer HC, Breban M, McMichael AJ, Bowness P. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J Immunol. 2004;173:1699–1710. doi: 10.4049/jimmunol.173.3.1699. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto J, Garnero P, van der Heijde D, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Yoshikawa H, Nishimoto N. A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod Rheumatol. 2009;19:273–282. doi: 10.1007/s10165-009-0170-4. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. Follow the leader: NK cell receptors for classical and nonclassical MHC class I. Cell. 1998;92:705–707. doi: 10.1016/s0092-8674(00)81398-7. [DOI] [PubMed] [Google Scholar]
- 9.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001;167:5543–5547. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 10.Kollnberger S, Bowness P. The role of B27 heavy chain dimer immune receptor interactions in spondyloarthritis. Adv Exp Med Biol. 2009;649:277–285. doi: 10.1007/978-1-4419-0298-6_21. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 12.Morita I, Nakagaki H, Taguchi A, Kato K, Murakami T, Tsuboi S, Hayashizaki J, Inagaki K, Noguchi T. Relationships between mandibular cortical bone measures and biochemical markers of bone turnover in elderly Japanese men and women. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;108:777–783. doi: 10.1016/j.tripleo.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Dohring C, Scheidegger D, Samaridis J, Cella M, Colonna M. A human killer inhibitory receptor specific for HLA-A1,2. J Immunol. 1996;156:3098–3101. [PubMed] [Google Scholar]
- 14.Hansasuta P, Dong T, Thananchai H, Weekes M, Willberg C, Aldemir H, Rowland-Jones S, Braud VM. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 15.Stewart-Jones GB, di Gleria K, Kollnberger S, McMichael AJ, Jones EY, Bowness P. Crystal structures and KIR3DL1 recognition of three immunodominant viral peptides complexed to HLA-B*2705. Eur J Immunol. 2005;35:341–351. doi: 10.1002/eji.200425724. [DOI] [PubMed] [Google Scholar]
- 16.Kollnberger S, Chan A, Sun MY, Chen LY, Wright C, di Gleria K, McMichael A, Bowness P. Interaction of HLA-B27 homodimers with KIR3DL1 and KIR3DL2, unlike HLA-B27 heterotrimers, is independent of the sequence of bound peptide. Eur J Immunol. 2007;37:1313–1322. doi: 10.1002/eji.200635997. [DOI] [PubMed] [Google Scholar]
- 17.Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum. 2005;52:3586–3595. doi: 10.1002/art.21395. [DOI] [PubMed] [Google Scholar]
- 18.Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, Cummings F, McMichael A, Kollnberger S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. Journal of immunology. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu XY, Wu XP, Xie H, Zhang H, Peng YQ, Yuan LQ, Su X, Luo XH, Liao EY. Age-related changes in biochemical markers of bone turnover and gonadotropin levels and their relationship among Chinese adult women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:275–285. doi: 10.1007/s00198-009-0943-9. [DOI] [PubMed] [Google Scholar]
- 20.Giles J, Shaw J, Piper C, Wong-Baeza I, McHugh K, Ridley A, Li D, Lenart I, Antoniou AN, DiGleria K, et al. HLA-B27 homodimers and free H chains are stronger ligands for leukocyte Ig-like receptor B2 than classical HLA class I. Journal of immunology. 2012;188:6184–6193. doi: 10.4049/jimmunol.1102711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peh CA, Burrows SR, Barnden M, Khanna R, Cresswell P, Moss DJ, McCluskey J. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8:531–542. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- 22.Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33:748–759. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- 23.Bowness P, Ridley A, Shaw J, Chan A, Wong Baeza I, Fleming M, Cummings F, McMichael A, Kollnberger S. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in Ankylosing Spondylitis. J Immunol. 2011;186:8. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Julian P, Vivian RCD, Richard Berry, Geraldine M. O’Connor, Hugh H. Reid, Travis Beddoe, Stephanie Gras, Philippa M. Saunders, M. AO, Jacqueline M. L. Widjaja, Christopher M. Harpur, Jie Lin, Sebastien M. Maloveste, David A. Price, B. APL, Daniel W. McVicar, Craig S. Clements, Andrew G. Brooks, Jamie Rossjohn. Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature. 2011;479:401–405. doi: 10.1038/nature10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer AL, Vittinghoff E, Ramachandran R, Mahmoudi N, Bauer DC. Laboratory reproducibility of biochemical markers of bone turnover in clinical practice. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21:439–445. doi: 10.1007/s00198-009-0974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer DC, Garnero P, Harrison SL, Cauley JA, Eastell R, Ensrud KE, Orwoll E. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2009;24:2032–2038. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ugolini S, Arpin C, Anfossi N, Walzer T, Cambiaggi A, Forster R, Lipp M, Toes RE, Melief CJ, Marvel J, et al. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat Immunol. 2001;2:430–435. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 28.Fauriat C, Andersson S, Bjorklund AT, Carlsten M, Schaffer M, Bjorkstrom NK, Baumann BC, Michaelsson J, Ljunggren HG, Malmberg KJ. Estimation of the size of the alloreactive NK cell repertoire: studies in individuals homozygous for the group A KIR haplotype. J Immunol. 2008;181:6010–6019. doi: 10.4049/jimmunol.181.9.6010. [DOI] [PubMed] [Google Scholar]
- 29.Tsai WC, Chen CJ, Yen JH, Ou TT, Tsai JJ, Liu CS, Liu HW. Free HLA class I heavy chain-carrying monocytes--a potential role in the pathogenesis of spondyloarthropathies. J Rheumatol. 2002;29:966–972. [PubMed] [Google Scholar]
- 30.Raine T, Brown D, Bowness P, Hill Gaston JS, Moffett A, Trowsdale J, Allen RL. Consistent patterns of expression of HLA class I free heavy chains in healthy individuals and raised expression in spondyloarthropathy patients point to physiological and pathological roles. Rheumatology (Oxford) 2006;45:1338–1344. doi: 10.1093/rheumatology/kel305. [DOI] [PubMed] [Google Scholar]
- 31.Tanabe M, Sekimata M, Ferrone S, Takiguchi M. Structural and functional analysis of monomorphic determinants recognized by monoclonal antibodies reacting with the HLA class I alpha 3 domain. Journal of immunology. 1992;148:3202–3209. [PubMed] [Google Scholar]
- 32.McCutcheon JA, Smith KD, Valenzuela A, Aalbers K, Lutz CT. HLA-B*0702 antibody epitopes are affected indirectly by distant antigen residues. Hum Immunol. 1993;36:69–75. doi: 10.1016/0198-8859(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 33.Martayan A, Sibilio L, Tremante E, Lo Monaco E, Mulder A, Fruci D, Cova A, Rivoltini L, Giacomini P. Class I HLA folding and antigen presentation in beta 2-microglobulin-defective Daudi cells. Journal of immunology. 2009;182:3609–3617. doi: 10.4049/jimmunol.0802316. [DOI] [PubMed] [Google Scholar]
- 34.Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–23468. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 35.Lan CE, T. WC, Wu CS, Lu CL, Yu HS. Psoriatic patients with arthropathy show significant expressionof free HLA class I heavy chains on circulating monocytes: apotential role in the pathogenesis of psoriatic arthritis. British Journal of Dermatology. 2004;151:24–31. doi: 10.1111/j.1365-2133.2004.05890.x. [DOI] [PubMed] [Google Scholar]
- 36.Fu L, Hazes B, Burshtyn DN. The first Ig domain of KIR3DL1 contacts MHC class I at a secondary site. J Immunol. 187:1816–1825. doi: 10.4049/jimmunol.1002125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.