Abstract
A significant proportion of castration-resistant prostate cancers (CRPC) remain driven by ligand activation of the androgen receptor. Although the testes are the primary source of testosterone, testosterone can also be produced from peripheral conversion of adrenal sex hormone precursors dehydroepiandrosterone (DHEA) and androstenedione (AD) in the prostate and other tissues. CYP17A1 catalyzes two essential reactions in the production of DHEA and androstenedione: the hydroxylation (hydroxylase activity) and the subsequent cleavage of the C17-20 side-chain (lyase activity). Potent and selective inhibition of CYP17A1 by abiraterone depletes residual non-gonadal androgens and is an effective treatment for CRPC. Elucidation of the mechanisms that underlie resistance to abiraterone will inform on the development of novel therapeutic strategies post abiraterone. Preclinical evidence that androgen biosynthesis in prostate cancer cells does not necessarily follow a single dominant pathway and residual androgens or alternative ligands (including administered glucocorticoids) can reactivate androgen receptor signaling supports co-targeting of more than one enzyme involved in steroidogenesis and combining a CYP17A1 inhibitor with an anti-androgen. Furthermore, given the drawbacks of 17α-hydroxylase inhibition, there is considerable interest in developing new CYP17A1 inhibitors that more specifically inhibit lyase activity and are therefore less likely to require glucocorticoid co-administration.
Keywords: abiraterone acetate, 5α-androstanedione, backdoor pathway, prostate cancer
BACKGROUND
For the past 70 years, gonadal androgen depletion by medical or surgical castration has been the standard of care for men with metastatic prostate cancer 1. Despite significant initial responses, patients invariably relapse and several studies suggest intra-tumoral androgens (most commonly testosterone) in castration-resistant prostate cancer (CRPC) tumors are restored to equivalent levels found in non-castrate prostates 2-4. Intra-tumoral testosterone and/or dihydrotestosterone (DHT) in castrate men could be generated from conversion of circulating adrenal androgens 4,5 or could be synthesized de novo from cholesterol 6. The latter has been suggested in a number of preclinical models but remains unproven in patients. High doses of ketoconazole, which inhibits many cytochrome P450 enzymes, have been used for over a decade to inhibit androgen biosynthesis and induce tumor responses in CRPC. The high doses of ketoconazole required to inhibit cytochrome P450c17 (17α-hydroxylase/17,20-lyase, CYP17A1), however, are associated with significant toxicity in up to 30% of patients. Moreover, CYP17A1 inhibition with ketoconazole is incomplete, and a rise in adrenal androgens has been reported at disease progression 7. The development of abiraterone as a specific and irreversible inhibitor of CYP17A1 offered a less toxic and more effective option. Abiraterone acetate is now approved in combination with prednisone for the treatment of CRPC, based on demonstration of an improvement in survival when administered with prednisone to docetaxel-treated and chemotherapy-naïve patients 8,9. Abiraterone acetate and prednisone also significantly delay pain progression and skeletal-related events and improve quality of life and pain control 10. These data have unequivocally confirmed that directly targeting androgen biosynthesis is a valid therapeutic option for prostate cancer. This review will discuss the challenges of inhibiting CYP17A1 and other enzymes involved in steroid synthesis and review strategies that are being evaluated to further improve results achieved to date with abiraterone.
Androgen biosynthesis pathways
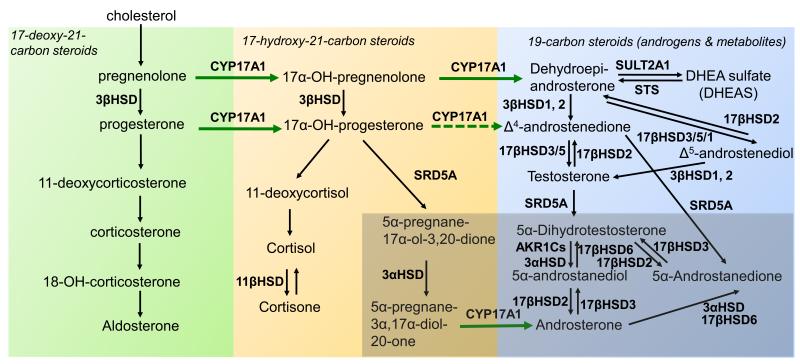
Steroidogenesis entails processes by which cholesterol is converted to biologically active steroid hormones. Steroidogenesis begins with the irreversible cleavage of a 6-carbon group from cholesterol, producing pregnenelone, by cytochrome P450scc (side chain cleavage enzyme, CYP11A1). A small repertoire of cytochrome P450 and non-P450 enzymes then convert pregnenelone to other 21-carbon steroids (including progestins, glucocorticoids, and mineralocorticoids), 19-carbon steroids (androgens) and 18-carbon steroids (estrogens) 11. The transformations catalyzed by the P450s, 5α-reductases, and 3β-hydroxysteroid dehydrogenase-Δ5/Δ4-isomerases (3βHSDs) are all irreversible reactions, giving rise to the general pathways of steroidogenesis (Figure). In contrast, the 3α-, 11β-, and 17β-HSD reactions at the terminal stages of the pathways are reversible pseudoequilibria, with each isoenzyme strongly favoring either steroid oxidation or reduction in intact cells. In human beings, each steroidogenic P450 derives from one gene yielding one isoform, whereas all other enyzmes exist as two or more isoenzymes, each with a unique cognate gene expressed in a tissue-specific fashion. Consequently, steroidogenesis generally follows a canonical pathway up to a point, but the final steps vary amongst tissues and cells, particularly in cancer cells, where genetic changes are frequent and ectopic expression of various genes is typical. CYP17A1 is the key enzyme for the synthesis of 19-carbon sex steroid precursors from 21-carbon pregnanes. CYP17A1 catalyzes both the 17α-hydroxylation (hydroxyl addition to pregnenolone and progesterone) and the subsequent 17,20-lyase cleavage (side-chain cleavage from 17-hydroxyprogesterone and 17-hydroxypregnenolone). The latter activity requires the presence of adequate amounts of cytochrome b5 12. Exploiting the requirement of the 17,20-lyase but not 17α-hydroxylase reaction for cytochrome b5 could allow development of therapeutics that specifically inhibit the former reaction. As cytochrome b5 is involved in a multitude of other essential processes, this approach will be challenging but could be possible since the critical residues of b5 for stimulating 17,20-lyase activity are E48 and E49 and these are not required for enhancing the activities of CYP2E1 or CYP2C19 13,14. In addition to its two primary activities, human CYP17A1 also 16α-hydroxylates progesterone during 25% of turnovers and cleaves pregnenolone and allopregnanolone directly to their Δ16, 19-carbon homologs in the presence of b5.
Figure. Androgen biosynthesis pathways.
The basic pathways are demarcated with respect to CYP17A1 and SRD5A activities. The 17-deoxy, 21-carbon steroids upstream of CYP17A1 are shown at left in green box, including 11-deoxycorticosterone (DOC). The 17-hydroxy, 21-carbon steroids are in center in peach box, and 19-carbon steroids are at right in blue box, generated via 17-hydroxylase and 17,20-lyase reactions of CYP17A1, respectively. The 5-reduced steroids are highlighted with darker background at lower right. Reversible interconversions catalyzed by HSDs are shown with double arrows at terminal steps. CYP17A1, cytochrome P450c17; 3β-HSD, 3-beta-hydroxysteroid dehydrogenase/isomerase; 11 α -HSD, 11-beta-hydroxysteroid dehydrogenase; 17β-HSD, 17-beta-hydroxysteroid dehydrogenase; 3α-HSD, 3-alpha-hydroxysteroid dehydrogenase; SRD5A, steroid 5α reductase; DHEA, Dehydroepiandrosterone; DHEA-S, Dehydroepiandrosterone sulfate; STS, steroid sulfatase; SULT2A1, sulfotransferase 2A1
Although small amounts of androstenedione (AD), testosterone, and other 19-carbon steroid metabolites can be directly produced by the adrenal glands, most Δ4-androgens in the castrated male are produced in peripheral tissues, where 3βHSD converts DHEA to AD and Δ 5-androstenediol to testosterone, respectively (Figure). In the testis, 17βHSD3 efficiently converts AD to testosterone and DHEA to Δ5-androstenediol, respectively, but in the adrenal and peripheral tissues, the much slower type 5 (17βHSD5) enzyme catalyzes these conversions, and 17βHSD1 also reduces DHEA to Δ5-androstenediol. Testosterone is then irreversibly 5α-reduced to the higher affinity ligand DHT by steroid 5α-reductase (SRD5A) isoenzymes (this is referred to as the canonical or “conventional” pathway for DHT synthesis). DHT is inactivated in part by 3-keto-reduction to 5α-androstane-3α,17β-diol through a single step, catalyzed by the AKR1C isoenzymes 1-4 (reductive 3αHSDs; mainly AKR1C2) and to 3α-androsterone through two steps (Figure). DHT, 5α-androstane-3α,17β-diol and 3α-androsterone can all be inactivated by the enzymes UDP glycosyltransferase 2, B15 (UGT2B15) or UGT2B17 15. The sulfotransferase SULT2A1 rapidly sulfonates the majority of DHEA synthesized in the adrenal gland, and most of the adrenal androgenic product therefore circulates as DHEA-sulfate (DHEA-S).
The inter-conversion of the 5α-reduced androgens occurs through reversible HSD reactions with the possibility of “back conversion” of inactive terminal products to DHT. 5α-reduction of upstream steroids, as opposed to 5α-reduction of testosterone, leads to DHT synthesis that bypasses testosterone through at least two pathways. In the 5α-androstanedione pathway, AD is converted by SRD5A1 to 5α-androstanedione, which is then converted into DHT by 17βHSD(s) 16 (Figure). The alternative or “backdoor” pathway to DHT synthesis occurs when progesterone and 17-hydroxyprogesterone (17OHP) accumulate and SRD5A enzymes are present. In this pathway, 17OHP is 5α- and 3α -reduced prior to the 17,20-lyase reaction of CYP17A1, yielding the 5α-reduced androgen androsterone (Figure). If 17βHSD activity is also present, 3α-androsterone is converted to 5α-androstane-3α,17β-diol via oxidative 3α-HSD activity, possibly catalyzed by 17βHSD6 17. This pathway yields DHT without DHEA, AD and testosterone as intermediates and occurs naturally in tammar wallaby pouch young testes, in the neonatal testes of several rodent species, and in certain types of congenital adrenal hyperplasia 18,19.
CLINICAL-TRANSLATIONAL ADVANCES
Metabolic Consequences of Therapeutic Inhibition of CYP17A1
Abiraterone was rationally designed in the early 1990s using pregnenolone as a backbone to bind the active site of CYP17A1 and inhibit its activity 20. This inhibition occurs secondary to formation of an essentially irreversible coordination complex between the heme iron—that is required in the CYP17A1 active site for enzymatic activity—and the azole nitrogen atom of abiraterone, plus hydrogen bonding interactions between the abiraterone 3β-OH and conserved polar residues in CYP17A1 21,22. The structural similarities between the latter interactions and ligands binding to steroid receptors could explain abiraterone’s (and other CYP17A1 inhibitors’) weak (relatively) antagonism of the androgen receptor (AR) 23. The specificity of abiraterone for inhibition of 17,20-lyase versus 17α-hydroxylase is low (1.4-fold, IC50=2.9 nM compared with 4 nM) 21 and treatment with abiraterone acetate therefore blocks both activities. When used as a single-agent, abiraterone acetate suppresses cortisol and causes a rise in ACTH with a consequent increase in 11-deoxycorticosterone (DOC) and corticosterone, mimicking the effects observed in families with congenital inactivating CYP17A1 mutations 24. When administered to noncastrate men, abiraterone acetate (a maximum of 750mg was evaluated) suppresses testosterone, but a subsequent LH surge overcomes inhibition of gonadal testosterone synthesis 25. Significantly higher doses than the currently approved 1000mg would be required to suppress androgens if abiraterone acetate was administered to noncastrate men, probably without any obvious sparing of the side-effects associated with pharmacologic castration with gonadotropin-releasing hormone agonists (GnRHa). Importantly, when administered with GnRHa, significant suppression of circulating DHEA, DHEA-S, AD, testosterone and estradiol is achieved with no obvious rise at disease progression 26-28. Evaluation of the latter has however been limited by the sensitivity of assays used.
CYP17A1 inhibition with single-agent abiraterone acetate is not associated with adrenocortical insufficiency, because a compensatory increase in ACTH leads to very high levels (30-40 fold rise) of the weak glucocorticoid corticosterone that maintains the glucocorticoid requirements of patients. However, raised levels of corticosterone precursors that have mineralocorticoid properties, particularly DOC, lead to a syndrome of mineralocorticoid excess, characterized by hypokalemia, hypertension and fluid retention 26,29,30. In order to effectively prevent or treat ACTH-induced side-effects of mineralocorticoid excess, two different strategies could be adopted: 1) the administration of exogenous glucocorticoids to prevent a compensatory ACTH rise, 2) the administration of mineralocorticoid receptor antagonists (MRA) that inhibit the peripheral effects of raised mineralocorticoids.
Prednisone (prednisolone in the UK) 5mg bid was used in the regulatory Phase III studies of abiraterone, primarily because most CRPC patients are already receiving this glucocorticoid during taxane treatment. Prednisone is a glucocorticoid prodrug that is converted by 11β-HSD in the liver into the active form, prednisolone. Prednisolone is four times a more potent glucocorticoid than cortisol, and 5-7.5 mg daily is used to manage adrenal insufficiency 31. However, in the COU-301 and COU-302 Phase III studies up to 40% of patients treated with prednisone and placebo suffered side-effects associated with mineralocorticoid excess 8,9 suggesting prednisone (or prednisolone) or their metabolites could activate the mineralocorticoid receptor. Moreover, as anticipated side-effects of mineralocorticoid excess were significantly more common in the abiraterone-prednisone arm in both studies. The half life of prednisone is three hours and the biological effect of 5mg lasts up to twelve hours 32 although this can be variable due to prednisone’s interconversion with prednisolone. Once daily prednisone dosing is being used in several clinical studies in early prostate cancer and breast cancer (for example NCT01751451, NCT01381874, NCT00268476) but there is a hypothetical increased risk of more mineralocorticoid excess due to a compensatory nocturnal rise in ACTH, Modified-release formulations or higher doses could be considered for once daily dosing if these studies report significant mineralocorticoid excess. Overall, hypokalemia is usually corrected with oral potassium supplementation, and fewer than 2% of patients treated with prednisone 5mg BID and abiraterone require MRA or intervention to control these side-effects 8. Dexamethasone is a potent glucocorticoid, and a dose of 0.5 mg daily is usually used. In retrospective studies, dexamethasone was reported to have a significant response rate (equivalent or potentially superior) to prednisone as monotherapy for CRPC 33. Dexamethasone has no mineralocorticoid activity and a long duration of action: this could make it the preferred combination glucocorticoid. Orthostatic hypotension has been rarely reported (~2/100 patients) after addition of dexamethasone to patients on single-agent abiraterone, presumably due to dexamethasone’s absence of mineralocorticoid properties coupled with a rapid decline in raised mineralocorticoids 28,29. Hydrocortisone (cortisol) could be administered at a daily dose of 10–12 mg/m2/daily in divided doses (corresponding to a total daily dose of 15-25 mg). However, due to its short duration of action even thrice daily dosing is unlikely to completely suppress ACTH without over-dosing.
Overall, it is challenging to suppress ACTH whilst not administering supra-physiological glucocorticoid doses and avoiding Cushingoid symptoms from long-term treatment. Moreover, it is possible that long-term use of exogenous glucocorticoids is detrimental (see final section). The alternative option is to use abiraterone acetate alone and treat patients who develop mineralocorticoid excess with a MRA. However, spironolactone, the first-generation, cheapest and most readily available (competitive) MRA binds to the AR as a mixed agonist/antagonist and could lead to disease progression 34,35. Eplerenone, a second-generation MRA was developed as a more selective MRA that does not bind wild-type AR but can activate AR mutations previously detected in prostate cancer 23. Also, significantly raised levels of steroids upstream of CYP17A1 in patients on single-agent abiraterone acetate could activate mutant AR, and very high levels of 17α-hydroxylase substrates could overcome CYP17A1 inhibition by abiraterone. In fact, when dexamethasone was added to single-agent abiraterone in patients with a rising PSA, a secondary PSA decline ≥50% was reported in 25% of patients regardless of prior treatment with the same dose and regimen of dexamethasone suggesting high levels of upstream steroids were causing resistance 29. Amiloride and triamterine, which are potassium-sparing diuretics and inhibitors of the epithelial sodium channel in the distal nephron, are useful agents for controlling the hypokalemia and hypertension of mineralocorticoid excess states and might be effective treatments for these complications of abiraterone therapy without activating AR.
Novel CYP17A1 inhibitors in clinical development
Given the drawbacks of 17α-hydroxylase inhibition, there is considerable interest in developing new CYP17A1 inhibitors that more specifically inhibit 17,20-lyase and are therefore less likely to require glucocorticoid co-administration. Orteronel (TAK-700; Millennium Pharmaceuticals) inhibits 17,20-lyase activity 5.4 times more potently than 17α-hydroxylase activity 36. This relative specificity might however require a compromise between higher doses that achieve maximum profound inhibition of 17,20-lyase with the risk of a decrease in cortisol and consequent raised ACTH and mineralocorticoid excess versus lower doses that do not suppress cortisol. Orteronel is currently undergoing evaluation in Phase III studies in chemotherapy-naïve and chemotherapy-treated CRPC (NCT01193244; NCT01193257). Both studies utilize a dose of 400mg twice daily and combine orterenol with prednisone. Lower doses of orteronel (for example 300mg bid) that may not require concomitant exogenous glucocorticoids are also being evaluated. Another CYP17A1 inhibitor, galeterone (VN/124-1, TOK-001; Tokai Pharmaceuticals), is a combined inhibitor of CYP17A1, AR and SRD5A 37, which has recently completed phase I testing 38. VT-464 (Viamet Inc) has a 60-fold greater specificity for C17,20-lyase than 17α-hydroxylase. Treatment of monkeys with VT-464 did not cause a rise in steroids upstream of CYP17A1 in contrast to abiraterone 39. This agent is now in early clinical trials.
Reactivation of AR signaling in patients progressing on abiraterone – will combination therapy prove more effective?
The mechanisms that underlie resistance to abiraterone are unknown, and their elucidation will inform on the development of novel therapeutic strategies post abiraterone and biomarkers for selecting patients for treatment. The majority of patients progressing on abiraterone have a rise in PSA, suggesting reactivation of AR or other steroid signaling pathways that could increase PSA transcription 40. Several studies have shown that the AR can become promiscuously activated by very low levels of androgens (that could persist in patients treated with abiraterone acetate), other steroid metabolites and drugs that bind the AR 41-44. The latter could include abiraterone or co-administered glucocorticoids. AR mutations previously described in prostate cancer can be activated by cortisol and other glucocorticoids at levels significantly lower than are reported in patients 23,44. Studies are required to evaluate whether the development of or clonal selection of mutant AR-expressing clones occur on inhibitors of steroid biosynthesis as was described nearly two decades ago with first-generation anti-androgens 45. Moreover, glucocorticoid receptor signaling could activate AR regulated genes in the absence of ligand activation of AR signaling 46. These or other mechanisms of AR stimulation on abiraterone therapy may explain persistent or resumed AR signaling observed in circulating tumor cells that appear to portend a poorer prognosis 47.
Gene expression studies have identified alterations in the expression of multiple steroidogenic enzymes in CRPC tissue including increased levels of SRD5A1, 3βHSD, 17β-HSD5 (also called AKR1C3) and a new isoform of SRD5A (SRD5A3) and reduced expression of SRD5A2 4,48-50. Moreover, CYP17A1 and other steroidogenic enzymes have been shown to become upregulated as a consequence of abiraterone treatment in preclinical models 51,52. Although increased expression of steroidogenic enzymes does not necessarily equate with increased androgen production, these observations raise the possibility that resistance to abiraterone may be due in-part to mechanisms that maintain androgen synthesis. Translational studies to date have failed to identify a rise in circulating androgens on treatment 27,53 but measurement of intracellular androgens has been limited by the availability of CRPC tissue and the technical and analytical challenges of controlling for losses during extraction and processing, and definitively separating, detecting, and identifying particular steroids amongst highly related compounds. It is hypothesized that androgen biosynthesis in prostate cancer cells does not necessarily follow a single dominant pathway, particularly under therapy pressure 17. This model would support co-targeting of more than one enzyme involved in steroidogenesis. Nonetheless, both the conventional and alternative pathways of androgen biosynthesis are dependent on CYP17A1 17,20-lyase for production of androgens, and to date a CYP17A1-independent mechanism for androgen biosynthesis has not been identified. The role of non-CYP17A1 steroidogenesis inhibitors may therefore be limited to combinations with CYP17A1 inhibitors when 17,20-lyase blockade is incomplete, potentially reversing resistance or allowing the use of doses that do not concurrently suppress 17α-hydroxylase activity.
Significant pathway diversity and redundancy pose a challenge to targeting steroidogenesis downstream of CYP17A1. SRD5A1 is highly expressed in prostate cancer and mediates the 5α-androstanedione pathway synthesis of DHT that appears preferentially activated in prostate cancer cell lines and freshly collected CRPC patient tissue 16,54-56. Although most studies to date suggest that testosterone concentrations are higher than DHT in CRPC, this could be explained by inefficient testosterone 5α-reduction by SRD5A1, leading to its accumulation. 4,16,57. Furthermore, studies of intratumoral androgens disproportionately sample the interstitial space and cellular cytoplasm and DHT concentrations are enriched in the cellular nucleus, which is where most of the AR protein resides in the presence of agonist 58. Combinations of a 5α-reductase inhibitor with 17,20-lyase inhibition would prevent 5α-reduction of 17OHP and could prevent the accumulation of “backdoor pathway” steroids upstream of CYP17A1 27. A phase II study is evaluating the addition of dutasteride to abiraterone acetate in metastatic CRPC and studying levels of testosterone and DHT at baseline and at progression (NCT01393730). Dutasteride is preferred to finasteride as the latter is relatively specific for the type 2 enzyme, whilst dutasteride inhibits all three isoforms 17. 17β-HSDs play an important role by catalyzing the reduction of 19-carbon-17-ketosteroids to their corresponding 17β-hydroxy forms, as well as the reverse reaction (oxidation). To date, 14 different 17β-HSD genes and cognate isoenzymes have been identified; consequently, specific inhibition of one isoform could be by-passed if other 17β-HSDs are also expressed. For example, inhibition of 17β-HSD3 would not prevent DHT synthesis due to SRD5A-mediated conversion of AD to 5α-androstandione, followed by 17β-HSD5-mediated conversion to DHT (Figure). Similarly, inhibition of 17βHSD5 could be by-passed by the 5α-androstanedione pathway. Inhibition of 3βHSD could effectively block the conventional pathway at the less active metabolite DHEA, but the backdoor pathway to DHT synthesis would not be suppressed and 3βHSD inhibitors developed to date demonstrate unfavorable properties such as AR agonism 59. Interestingly, abiraterone also inhibits 3βHSD1 and 3βHSD2 in vitro (albeit less potently than CYP17A1) and could therefore prevent conversion to active androgens of any DHEA that leaks through abiraterone’s block of 17,20-lyase 60. Steroid sulfatase hydrolyzes steroid sulfates, such as estrone sulfate and DHEA-S, to their unconjugated, biologically active forms estrone and DHEA. The first trial with a first-generation single-agent steroid sulfatase inhibitor (SXT64, 667 COUMATE) in postmenopausal women with locally advanced or metastatic breast cancer confirmed inhibition of sulfatase that was associated with reductions in serum Δ5-androstenediol, AD and testosterone 61. Second- and third-generation steroid sufatase inhibitors have now been developed 62. Inhibiting sulfatase activity in combination with abiraterone in men with prostate cancer could reduce the production of AR-activating androgens from DHEA-S, which would be particularly of relevance in select patients for whom DHEA-S rises on abiraterone treatment.
Increasing drug exposure of abiraterone could reverse resistance through more potent CYP17A1 (and 3ßHSD) inhibition and increasing AR antagonism. This strategy could potentially be achieved by exploiting increased absorption of abiraterone in the presence of a high fat meal. To date, anti-tumor activity data is not available from randomized trials of different abiraterone doses. The hypothesis that reactivation of AR signaling by residual low levels of androgens, reactivation of steroid biosynthesis or alternative ligands (including administered glucocorticoids) supports the combination of an anti-androgen with a CYP17A1 inhibitor. Moreover, this combination would prevent a rise in androgens that could occur on treatment with an anti-androgen: as testosterone and DHT have a higher affinity for the AR than enzalutamide and other anti-androgens developed to date, out-competing of the anti-androgen by natural ligand could reverse AR inhibition. A phase Ib/II safety evaluation of enzalutamide (MDV3100, Medivation) in combination with abiraterone acetate and prednisone in CRPC with bone metastates is currently ongoing (NCT01650194). Finally, inhibition of steroid biosynthesis merits evaluation in early-stage prostate cancer when greater efficacy and increased cure rates could be achieved – the STAMPEDE study (NCT00268476) is currently comparing abiraterone with androgen deprivation therapy (ADT) to ADT alone in high-risk M0 or newly diagnosed M1 patients.
Acknowledgments
Funding: R.F. and G.A are employees of the Section of Medicine that is supported by a Cancer Research UK programme grant and an Experimental Cancer Medical Centre (ECMC) grant from Cancer Research UK and the Department of Health (Ref: C51/A7401). R.F is funded by The Wellcome Trust (Clinical PhD Programme) G.A. is also supported by a Cancer Research UK Clinician Scientist Fellowship. The authors acknowledge NHS funding to the Royal Marsden NIHR Biomedical Research Centre. N.S. is supported by a Howard Hughes Medical Institute Physician-Scientist Early Career Award, the Prostate Cancer Foundation, an American Cancer Society Research Scholar Award, grant PC080193 from the U.S. Army Medical Research Command and 1R01CA172382 from the National Cancer Institute.
Footnotes
Disclosure: Abiraterone acetate was developed at The Institute of Cancer Research, which therefore has a commercial interest in the development of this agent. N.S. has received consulting fees from Janssen and Medivation. R.J.A. has received consulting fees from Cougar Biotechnology, now a unit of Johnson and Johnson. G.A. has received consulting fees and travel support from Janssen-Cilag, Veridex, Roche/Ventana, Astellas, Novartis and Millennium Pharmaceuticals, speakers fees from Janssen, Ipsen, Takeda and Sanofi-Aventis, and grant support from AstraZeneca and Genentech. G.A. is on The ICR rewards to inventors list of abiraterone acetate.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA: a cancer journal for clinicians. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer research. 2007;67:5033–41. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 3.Geller J, Albert J, Loza D, Geller S, Stoeltzing W, de la Vega D. DHT concentrations in human prostate cancer tissue. The Journal of clinical endocrinology and metabolism. 1978;46:440–4. doi: 10.1210/jcem-46-3-440. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer research. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clinical cancer research. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 6.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer research. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 7.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) Journal of clinical oncology. 2004;22:1025–33. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012;13:1210–7. doi: 10.1016/S1470-2045(12)70473-4. [DOI] [PubMed] [Google Scholar]
- 11.Sharifi N, Auchus RJ. Steroid biosynthesis and prostate cancer. Steroids. 2012;77:719–26. doi: 10.1016/j.steroids.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Lee-Robichaud P, Wright JN, Akhtar ME, Akhtar M. Modulation of the activity of human 17 alpha-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. The Biochemical journal. 1995;308:901–8. doi: 10.1042/bj3080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng HM, Auchus RJ. The Action of Cytochrome b(5) on CYP2E1 and CYP2C19 Activities Requires Anionic Residues D58 and D65. Biochemistry. 2013;52:210–2. doi: 10.1021/bi301384n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naffin-Olivos JL, Auchus RJ. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry. 2006;45:755–62. doi: 10.1021/bi051623y. [DOI] [PubMed] [Google Scholar]
- 15.Belanger A, Pelletier G, Labrie F, Barbier O, Chouinard S. Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends in endocrinology and metabolism. 2003;14:473–9. doi: 10.1016/j.tem.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13728–33. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohler JL, Titus MA, Bai S, Kennerley BJ, Lih FB, Tomer KB, et al. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer research. 2011;71:1486–96. doi: 10.1158/0008-5472.CAN-10-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends in endocrinology and metabolism. 2004;15:432–8. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Auchus RJ, Miller WL. Congenital adrenal hyperplasia--more dogma bites the dust. The Journal of clinical endocrinology and metabolism. 2012;97:772–5. doi: 10.1210/jc.2012-1080. [DOI] [PubMed] [Google Scholar]
- 20.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) The Journal of steroid biochemistry and molecular biology. 1994;50:267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 21.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. Journal of medicinal chemistry. 1995;38:2463–71. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 22.DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–9. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer research. 2012;72:2176–82. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinology and metabolism clinics of North America. 2001;30:101–19. doi: 10.1016/s0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. British journal of cancer. 2004;90:2317–25. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Journal of clinical oncology. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 27.Attard G, Reid AH, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. The Journal of clinical endocrinology and metabolism. 2012;97:507–16. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 28.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. Journal of clinical oncology. 2010;28:1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. Journal of clinical oncology. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. Journal of clinical oncology. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. A Summary of the Endocrine Society Clinical Practice Guidelines on Congenital Adrenal Hyperplasia due to Steroid 21-Hydroxylase Deficiency. International journal of pediatric endocrinology. 2010;2010:494173. doi: 10.1155/2010/494173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids: Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. The American journal of medicine. 1977;63:200–7. doi: 10.1016/0002-9343(77)90233-9. [DOI] [PubMed] [Google Scholar]
- 33.Venkitaraman R, Thomas K, Huddart RA, Horwich A, Dearnaley DP, Parker CC. Efficacy of low-dose dexamethasone in castration-refractory prostate cancer. BJU Int. 2008;101:440–3. doi: 10.1111/j.1464-410X.2007.07261.x. [DOI] [PubMed] [Google Scholar]
- 34.Sundar S, Dickinson PD. Spironolactone, a possible selective androgen receptor modulator, should be used with caution in patients with metastatic carcinoma of the prostate. BMJ case reports. 2012;2012 doi: 10.1136/bcr.11.2011.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luthy IA, Begin DJ, Labrie F. Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture. Journal of steroid biochemistry. 1988;31:845–52. doi: 10.1016/0022-4731(88)90295-6. [DOI] [PubMed] [Google Scholar]
- 36.Yamaoka M, Hara T, Hitaka T, Kaku T, Takeuchi T, Takahashi J, et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. The Journal of steroid biochemistry and molecular biology. 2012;129:115–28. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Bruno RD, Vasaitis TS, Gediya LK, Purushottamachar P, Godbole AM, Ates-Alagoz Z, et al. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124-1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124-1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids. 2011;76:1268–79. doi: 10.1016/j.steroids.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery RB, Eisenberger MA, Rettig M, Chu F, Pili R, Stephenson J, et al. Phase I clinical trial of galeterone (TOK-001), a multifunctional antiandrogen and CYP17 inhibitor in castration resistant prostate cancer (CRPC) J Clin Oncol. 2012;30 (suppl; abstr 4665) [Google Scholar]
- 39.Eisner JR, Abbott DH, Bird IM, Rafferty SW, Moore WR, Schotzinger RJ. Assessment of Steroid Hormones Upstream of P450c17 (CYP17) in Chemically Castrate Male Rhesus Monkeys Following Treatment with the CYP17 Inhibitors VT-464 and Abiraterone Acetate (AA) Endocr Rev. Vol 33 (03_MeetingAbstracts): SAT-266 2012. [Google Scholar]
- 40.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–62. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer research. 1999;59:2511–5. [PubMed] [Google Scholar]
- 42.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochemical and biophysical research communications. 1990;173:534–40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 43.Chen G, Wang X, Zhang S, Lu Y, Sun Y, Zhang J, et al. Androgen receptor mutants detected in recurrent prostate cancer exhibit diverse functional characteristics. The Prostate. 2005;63:395–406. doi: 10.1002/pros.20191. [DOI] [PubMed] [Google Scholar]
- 44.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nature medicine. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 45.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 46.Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, Jänne OA. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2012 Dec 26; doi: 10.1158/0008-5472.CAN-12-2350. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer discovery. 2012;2:995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer research. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 49.Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, et al. Distinct Patterns of Dysregulated Expression of Enzymes Involved in Androgen Synthesis and Metabolism in Metastatic Prostate Cancer Tumors. Cancer research. 2012;72:6142–52. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, et al. Aldo-keto reductase (AKR) 1C3: role in prostate disease and the development of specific inhibitors. Molecular and cellular endocrinology. 2006;248:182–91. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71:6503–13. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clinical cancer research. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:637–43. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annual review of biochemistry. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 55.Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, et al. Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer science. 2008;99:81–6. doi: 10.1111/j.1349-7006.2007.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, et al. 5alpha-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. The Prostate. 2011;71:1033–46. doi: 10.1002/pros.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, 3rd, et al. The androgen axis in recurrent prostate cancer. Clinical cancer research. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 58.Bruchovsky N, Wilson JD. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. The Journal of biological chemistry. 1968;243:5953–60. [PubMed] [Google Scholar]
- 59.Sharifi N. New agents and strategies for the hormonal treatment of castration-resistant prostate cancer. Expert opinion on investigational drugs. 2010;19:837–46. doi: 10.1517/13543784.2010.494178. [DOI] [PubMed] [Google Scholar]
- 60.Li R, Evaul K, Sharma KK, Chang KH, Yoshimoto J, Liu J, et al. Abiraterone inhibits 3beta-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clinical cancer research. 2012;18:3571–9. doi: 10.1158/1078-0432.CCR-12-0908. [DOI] [PubMed] [Google Scholar]
- 61.Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clinical cancer research. 2006;12:1585–92. doi: 10.1158/1078-0432.CCR-05-1996. [DOI] [PubMed] [Google Scholar]
- 62.Purohit A, Foster PA. Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. The Journal of endocrinology. 2012;212:99–110. doi: 10.1530/JOE-11-0266. [DOI] [PubMed] [Google Scholar]