Abstract
Cutaneous metastasis is considered as an advanced presentation of colorectal cancer and mostly managed with palliative measures. We present a case report where such a case was radically treated with good result and review such reports published in literature. This shows a distinct subgroup that could benefit from such radical approach.
Background
A radical approach to the management of advanced disease by identification of clear subgroup which could benefit from such approach.
Introduction
Skin metastasis occurs in up to 10% of all internal malignancies with 3% of colorectal cancer (CRC) presenting with them. Such metastasis is generally considered terminal with a projected survival counted in months and management directed to palliation. Significant improvements achieved in 5-year survival of CRCs and expanding array of chemotherapy has, however, resulted in offering radical management for those with colorectal lung and liver metastasis. Extrapolation of such an approach to cutaneous metastasis has not been reported, possibly due to its rarity of occurrence and lack of quality evidence.
We report a case with isolated cutaneous metastasis managed radically with review of published case reports with an aim to identify patient or tumour characteristics which might benefit from a radical approach to its management.
Methodology
To supplement the case report, an extensive search strategy using MEDLINE from 1950 to April 2012 and the PubMed interface portal was conducted with the following keyword terminology [MeSH] ‘skin metastasis’, ‘rectal AND OR colon’, AND/OR ‘colorectal’ AND ‘cancer’ OR ‘carcinoma’. The related citations option in PubMed was used to expand the search and we included all languages. Articles available in full reporting on cutaneous metastasis in isolation or as review of case reports were included and non-English articles translated by online Google Translate tool. A total of 50 publications were returned of which 24 case reports with 33 cases were selected for review (table 1). We excluded those on Sister Mary Joseph's nodule and with inadequate information for analysis. Statistical analysis was undertaken using Graphpad Prism and EndNote to reference citations.
Table 1.
| Author | Number of cases reported | Months since primary tumour | Site of skin metastasis |
|---|---|---|---|
| Alexandrescu et al1 | 2 | 60 and 36 | Previous abdominal scar |
| Placer et al2 | 1 | 26 | Perineum |
| Wright et al3 | 1 | Synchronous | Previous abdominal scar |
| Saladzinskas et al4 | 1 | 54 | Lip |
| McWeeney et al5 | 1 | 11 | Scrotal skin |
| Fyrmpas et al6 | 1 | 36 | Chin |
| Teo et al7 | 1 | Synchronous | Lower body |
| Moonda and Fatteh8 | 1 | 12 | Lip |
| Kurihara and Watanabe9 | 1 | 12 | Lower body |
| Sarid et al10 | 1 | 16 | Abdominal wall |
| Iwase et al11 | 1 | Synchronous | Previous abdominal scar |
| Hobdy et al12 | 1 | 14 | Chin |
| Tan et al13 | 2 | 24 | Back |
| Gazoni et al14 | 6 | Synchronous | Perineum and extremities |
| Proffer et al15 | 1 | 24 | Inner thigh |
| Melis et al16 | 1 | Synchronous | Perineum |
| Gallagher et al17 | 1 | 60 | Subungual |
| Attili et al18 | 1 | Synchronous | Upper lip |
| De Friend et al19 | 1 | 7 | Perineum |
| Kilickap et al20 | 1 | 14 | Chest and axilla |
| Ayadi et al21 | 1 | 5 | Scalp |
| Tranchart et al22 | 1 | 14 | Perineum |
| Shahidi-Dadras and Rahimi23 | 1 | 60 | Face |
| Gupta and Singh24 | 1 | Synchronous | Previous abdominal scar |
Case report
A 61-year-old woman underwent a curative low anterior resection for adenocarcinoma in 2004. Two years later, she developed an isolated right lung metastasis, confirmed by CT scans, positron emission tomography (PET) and aspiration cytology with no evidence of a new primary or colonic recurrence. She subsequently underwent a right thoracotomy and radical metastectomy, which confirmed the colorectal nature of the lesion. Surveillance was continued with CT scans, colonoscopy and carcinoembryonic antigen (CEA) levels.
In December 2009, the patient reported a sizeable increase of a scalp lesion, for which she had a dermatological assessment. Clinical examination and excision biopsy confirmed a 15×12×5 mm scalp nodule at the vertex of the scalp. Histopathology confirmed it to be a metastatic adenocarcinoma of colorectal origin positive for CK20, CK7 and negative for thyroid transcription factor 1 (figure 1). A second primary, recurrence and other sites of metastasis were ruled out by CT scans of chest and abdomen, colonoscopy and a normal CEA level. Radiotherapy (30 Gy in 10 fractions) was also performed on the scalp as an adjuvant to surgical excision biopsy as decided at the multidisciplinary team meeting.
Figure 1.
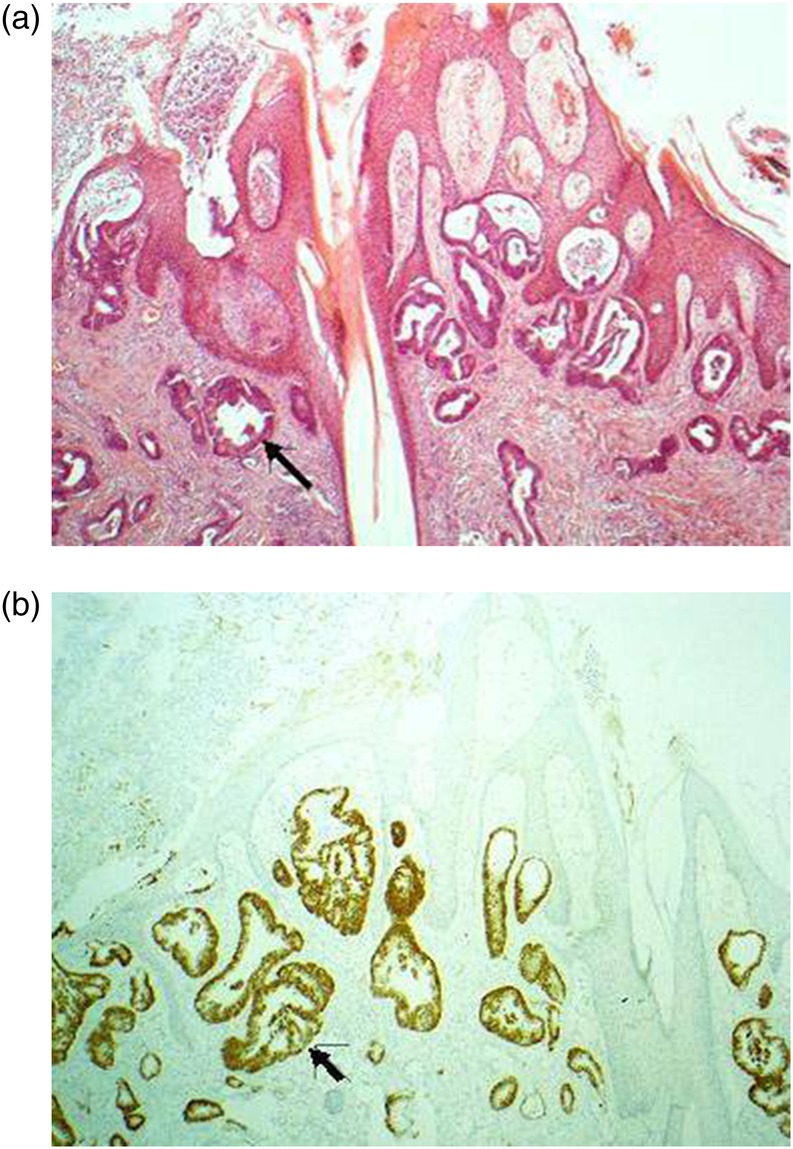
Metastatic colorectal adenocarcinoma involving skin, denoted by an arrow to an involved sweat gland. (A) H&E section and (B) same section with CDX2 immunostain (tumour positive).
In January 2011, she developed a right neck swelling, which was confirmed on CT and MRI scans as cervical lymph node at levels II–III of up to 10 mm in size. Fine needle aspiration cytology confirmed metastatic adenocarcinoma. PET scan confirmed metastasis of the neck nodes, again with no evidence of metastasis or recurrence in the rest of the body. A right selective neck dissection yielded 40 lymph nodes of which 19 had metastatic adenocarcinoma of colorectal origin. However, 4 months later a recurrent small nodule was noted at the lateral margin of the scalp lesion excision site, which was excised widely. Six years after the resection of primary tumour, this patient remains under follow-up with the combined colorectal, maxillofacial, dermatology and oncology team, and leads an active life.
Results
Literature review and analysis of 33 case reports showed two distinct clinical entities (see table 1). The first subgroup demonstrates advanced CRC disease at the time of presentation with multiple solid visceral and cutaneous metastases (synchronous lesions). The second subgroup developed cutaneous metastases during the period of disease surveillance after the primary tumour was treated with curative intent.
Synchronous presentation of cutaneous metastases in primary CRC
Twelve of the 33 cases had cutaneous metastases at the time of presentation with the primary CRC. The mean age of this group was 65 years (range 35–80 years), with male-to-female ratio of 2 : 1. Rectal cancer was primary in 75% of the cases (9 of 12). Histology was reported as poor or undifferentiated in 75% (9 of 12) of cases.
At the time of primary CRC diagnosis, more than 65% had additional solid visceral metastases, involving either liver, lungs or both. In patients with no detected visceral metastasis at diagnosis, skin metastasis was exclusively found on the abdominal wall or perineum (table 2).
Table 2.
| Site of metastasis | Synchronous presentation (%) | Late skin metastasis (%) |
|---|---|---|
| Perineum/external genitalia | 50 | 23 |
| Abdominal wall | 25 | 14 |
| Scalp and face | 8 | 33 |
| Extremities and others | 17 | 30 |
Definitive surgery with curative intent for the primary tumour was considered for 25% (3 of 12), while palliative diversion in the form of defunctioning colostomy or stent insertion performed in over 50%. The mean survival of these cases was 5.8 months (range 1–18 months).
Cutaneous metastasis detected during surveillance following curative treatment of CRC
This group of 21 patients had primary resection of their tumour with curative intent and were mostly rectal lesions in men (66%), with a mean age of 61 years (range 29–77 years). They did not have any evidence of metastasis at the time of resection. The tumours were moderate or poorly differentiated. Few cases of signet ring differentiation showed infiltration to muscularis mucosa or beyond in more than 70% (T3 or T4) and lymph node involvement in 50% of cases.
The mean time to presentation with cutaneous metastasis following the curative CRC treatment was 28 months (range 5–60 months). Postoperative chemotherapy did not make any difference to the time of presentation with skin metastases from resection (28 vs 29 months with and without adjuvant chemotherapy, respectively).
Solid visceral distant metastasis was detected in 66% (14 of 21), either at the same time or before detection of cutaneous metastatic lesions. Lungs were the commonest site of visceral metastasis (40%) with other sites including ovarian (Krükenberg's tumour), distant lymph nodes, bone and muscle. There was associated local recurrence of primary tumour in 4 of 21 (19%) cases. Of the eight case reports where CEA was mentioned, four had shown an elevation with visceral metastases, while the other four had normal range levels despite cutaneous secondary metastases.
Cutaneous metastases were treated by excision biopsy in 80% of cases (17 of 21) the rest had only incision biopsy. Added radiotherapy, chemotherapy or a combination was used in 30% of cases. The mean survival of patients with such delayed presentation of skin metastasis was 15.2 months (range 4–60 months).
Correlation between different groups revealed no survival advantage for those who had adjuvant chemotherapy as part of management of primary cancer, delayed presentation following resection of primary and addition of chemotherapy or radiation to management of skin metastasis over excision. However, comparison of survival curves of groups with cutaneous metastases at the time of diagnosis (median survival 4.5 months) with those who developed cutaneous metastases during the period of surveillance (median survival 13 months) showed a significant survival advantage with log rank test (figure 2, p<0.0185).
Figure 2.
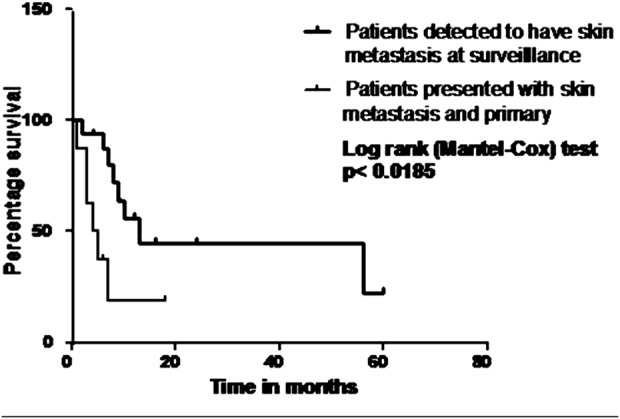
Comparison of survival in patients presenting with skin metastasis versus those who develop while in surveillance after curative resection of primary tumour.
Discussion
Cutaneous metastasis is a rare manifestation of advanced CRC. Mostly the tumour burden is so significant with signs of progression of metastatic disease at other visceral sites that management will be palliative. Being a rare presentation, published case reports are the only source of information which tend to highlight the morphology of the disease and little on management option. In most of the oncology literature, they are bunched together as part of extrahepatic, extrapulmonary metastasis. Thus a review of published case reports is the best possible evidence obtainable in this rare presentation.
This review was able to define a subgroup of patients who could be considered for radical approach of management. They had apparently curative resection of their primary, and mostly had treatment for metastasis, as in the case presented, and diagnosed to have cutaneous metastasis at routine follow-up or from symptoms. From the review of case reports, more than 60% of cases detected at follow-up, have active metastasis at the time of finding cutaneous metastasis. The index case in the report was offered radical procedure as it was isolated with no detectable visceral or recurrent disease. It is predictable that the number of patients with such isolated metastasis will be very less.
The median survival as a group in those diagnosed at follow-up is better than those who present with primary and cutaneous metastasis (4.5 vs 13 months, p<0.0). The difference in survival could be due to earlier diagnosis and lower tumour burden at diagnosis in the latter. More than 65% of cases presenting with synchronous primary and cutaneous metastasis have visceral metastasis as well at diagnosis reflecting their advanced disease.
The case reports were extracted from different languages and countries, which reflected the heterogeneity in protocol for investigation, including that for PET and CEA. The same applies to treatment modalities and hence no firm conclusions could be drawn.
Some broad general principles, however, could be drawn from this review. Presence of visceral metastasis or recurrent disease, especially in rectal cancer should warn the clinician and patient regarding possibility of such metastasis. Early diagnosis will be the key element in its effective management and the American Society of Clinical Oncology (ASCO) guidance already recommends physical examination to rule out cutaneous deposits in CRCs. Patient education to report any changes in skin is crucial in early detection and should be included in information leaflets and guidance for surveillance. Such cutaneous metastasis should trigger a search for any visceral metastasis, the best modality available being PET scan, which is also the only other way of detecting other skin lesions unless clinically obvious. In the light of the current review, for isolated cutaneous metastasis following consideration of previous metastatic disease and patient’s choice, a radical approach might be considered as it can provide a median survival advantage of nearly 10 months. In the presented case, the patient benefitted with a disease-free survival of 12 months.
Conclusion
Cutaneous metastasis is often accompanied by visceral metastasis at presentation reflecting advanced disease. However, in cases where the cutaneous deposit is isolated, as in visceral metastasis, there is a role for radical management. This case review showed benefit in median survival for those cutaneous metastasis detected at follow-up following apparent curative resection. Although rare, the morbidity related to such metastasis can be reduced by early detection, which requires awareness on the part of the patient and the physician.
Learning points.
The need for multidisciplinary team led individualised patient care in such advanced malignancy.
Importance of surveillance in early detection and management of uncommon sites of metastasis.
Patient education in early recognition of skin metastasis.
Footnotes
Contributors: RK and PB contributed to analysis of the data, drafting and revising the manuscript, and approved the final version of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Alexandrescu DT, Vaillant J, Yahr LJ, et al. Unusually large colon cancer cutaneous and subcutaneous metastases occurring in resection scars. Dermatol Online J 2005;2013:22. [PubMed] [Google Scholar]
- 2.Placer C, Elosegui JL, Irureta I, et al. Perineal cutaneous metastases from adenocarcinoma after surgery for colorectal cancer. Cir Esp 2007;2013:41–3 [DOI] [PubMed] [Google Scholar]
- 3.Wright PK, Jha MK, Barrett PD, et al. Colonic adenocarcinoma presenting as a cutaneous metastasis in an old operative scar. J Postgrad Med 2003;2013:157–8 [PubMed] [Google Scholar]
- 4.Saladzinskas Z, Tamelis A, Paskauskas S, et al. Facial skin metastasis of colorectal cancer: a case report. Cases J 2010;2013:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McWeeney DM, Martin ST, Ryan RS, et al. Scrotal metastases from colorectal carcinoma: a case report. Cases J 2009;2013:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fyrmpas G, Barbetakis N, Efstathiou A, et al. Cutaneous metastasis to the face from colon adenocarcinoma. Case report. Int Semin Surg Oncol 2006;2013:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teo EY, Wang YS, Mancer K. Extensive cutaneous metastases from colonic adenocarcinoma. Ann Acad Med Singapore 2009;2013:1107–8 [PubMed] [Google Scholar]
- 8.Moonda A, Fatteh S. Metastatic colorectal carcinoma: an unusual presentation. J Cutan Pathol 2009;2013:64–6 [DOI] [PubMed] [Google Scholar]
- 9.Kurihara A, Watanabe M. Massive cutaneous metastases in the lower part of the body of a patient with rectal cancer. Dig Surg 2009;2013:288. [DOI] [PubMed] [Google Scholar]
- 10.Sarid D, Wigler N, Gutkin Z, et al. Cutaneous and subcutaneous metastases of rectal cancer. Int J Clin Oncol 2004;2013:202–5 [DOI] [PubMed] [Google Scholar]
- 11.Iwase K, Takenaka H, Oshima S, et al. The solitary cutaneous metastasis of asymptomatic colon cancer to an operative scar. Surg Today 1993;2013:164–6 [DOI] [PubMed] [Google Scholar]
- 12.Hobdy EM, Ciesielski TE, Kummar S. Unusual sites of colorectal cancer metastasis. Clin Colorectal Cancer 2003;2013:54–7 [DOI] [PubMed] [Google Scholar]
- 13.Tan KY, Ho KS, Lai JH, et al. Cutaneous and subcutaneous metastases of adenocarcinoma of the colon and rectum. Ann Acad Med Singapore 2006;2013:585–7 [PubMed] [Google Scholar]
- 14.Gazoni LM, Hedrick TL, Smith PW, et al. Cutaneous metastases in patients with rectal cancer: a report of six cases. Am Surg 2008;2013:138–40 [PubMed] [Google Scholar]
- 15.Proffer LH, Czarnik KL, Sartori CR. Colon carcinoma cutis: a case report. Cutis 1999;2013:301–2 [PubMed] [Google Scholar]
- 16.Melis M, Scintu F, Marongiu L, et al. Inflammatory cutaneous metastasis from rectal adenocarcinoma: report of a case. Dis Colon Rectum 2002;2013:562–3 [DOI] [PubMed] [Google Scholar]
- 17.Gallagher B, Yousef G, Bishop L. Subungual metastasis from a rectal primary: case report and review of the literature. Dermatol Surg 2006;2013:592–5 [DOI] [PubMed] [Google Scholar]
- 18.Attili VS, Rama Chandra C, Dadhich HK, et al. Unusual metastasis in colorectal cancer. Indian J Cancer 2006;2013:93–5 [DOI] [PubMed] [Google Scholar]
- 19.De Friend DJ, Kramer E, Prescott R, et al. Cutaneous perianal recurrence of cancer after anterior resection using the EEA stapling device. Ann R Coll Surg Engl 1992;2013:142–3 [PMC free article] [PubMed] [Google Scholar]
- 20.Kilickap S, Aksoy S, Dincer M, et al. Cutaneous metastases of signet cell carcinoma of the rectum without accompanying visceral involvement. South Med J 2006;2013:1137–9 [DOI] [PubMed] [Google Scholar]
- 21.Ayadi L, Zribi J, Mziou TJ, et al. Scalp metastasis from small cell carcinoma of the rectum: an unusual case. Tunis Med 2009;2013:354–5 [PubMed] [Google Scholar]
- 22.Tranchart H, Benoist S, Penna C, et al. Cutaneous perianal recurrence on the site of Lone Star Retractor after J-pouch coloanal anastomosis for rectal cancer: report of two cases. Dis Colon Rectum 2008;2013:1850–2 [DOI] [PubMed] [Google Scholar]
- 23.Shahidi-Dadras M, Rahimi H. Facial metastasis from colon cancer. Arch Iran Med 2011;2013:64–5 [PubMed] [Google Scholar]
- 24.Gupta SS, Singh O. Carcinoma colon presenting as cutaneous metastasis to an old operative scar of hysterectomy. J Cancer Res Ther 2010;2013:316–17 [DOI] [PubMed] [Google Scholar]


