Abstract
Cytokinesis is asymmetric along the apical–basal axis of epithelial cells, positioning the midbody near the apical domain. However, little is known about the mechanism and purpose of this asymmetry. We use live imaging of Drosophila follicle cell division to show that asymmetric cytokinesis does not result from intrinsic polarization of the main contractile ring components. We show that adherens junctions (AJs) maintain close contact with the apical side of the contractile ring during cytokinesis. Asymmetric distribution of AJ components within follicle cells and in the otherwise unpolarized S2 cells is sufficient to recruit the midbody, revealing that asymmetric cytokinesis is determined by apical AJs in the epithelia. We further show that ectopic midbody localization induces epithelial invaginations, shifting the position of the apical interface between daughter cells relative to the AB axis of the tissue. Thus, apical midbody localization is essential to maintain epithelial tissue architecture during proliferation.
Keywords: cytokinesis, midbody, adherens junction, cell polarity, epithelium
INTRODUCTION
Cytokinesis physically separates daughter cells at the end of division. It is driven by a contractile actomyosin ring, which deforms the cortex and generates a cleavage furrow at the cell equator [1]. Cleavage ingression forms a small intercellular bridge or midbody ring (MR) that associates with the midbody consisting of bundles of microtubules and cytokinesis proteins [2]. Although in most cells the contractile ring (CR) cuts symmetrically across the division plane, asymmetric constriction is a prevalent phenomenon [3]. Epithelial cells do not maintain circumferential symmetry of the CR during cytokinesis. Instead, it constricts from the basal to the apical side, thereby positioning the midbody at the apical cortex [4–6].
The midbody participates in abscission, the final step of cell division [1]. Additional roles have been described within the epithelial tissue, where the asymmetry of midbody localization can couple cell division to cell polarization and differentiation [7, 8]. The mechanism that determines midbody positioning in epithelial cells remains unknown. One possibility is that it relies on an intrinsic asymmetry of the structural components of the CR, such as observed in the first zygotic division of the Caenorhabditis elegans embryo [9]. However, the asymmetry of epithelial cytokinesis must be studied in light of their apical–basal (AB) polarity and the mechanical interactions established within the tissue [10, 11]. Adherens junctions (AJ) are key to these interactions by connecting adjacent epithelial cells via homophilic contacts between the extracellular domain of Cadherins [12]. Their position near the apical domain and maintenance through cell division [13] raise the question whether cleavage ingression faces a polarized effect from AJ-mediated cell adhesion along the epithelial AB axis.
Here we use clonal analysis and live imaging of the Drosophila follicular epithelium to address how asymmetric cytokinesis is determined and the significance of polarized midbody localization in epithelial tissue.
RESULTS AND DISCUSSION
Asymmetric cytokinesis in follicle cells
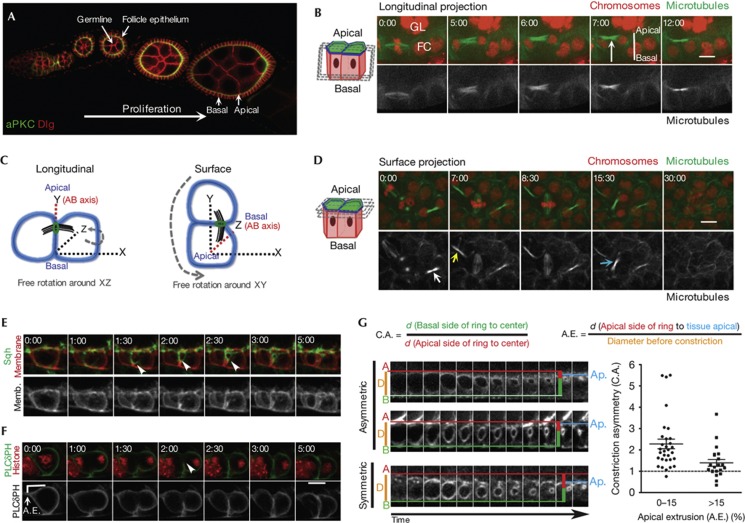
The follicular epithelium encloses the germline within each egg chamber of the ovariole, presenting an apical surface that contacts the germline and an opposite basal side (Fig 1A). The ovariole can be cultured ex vivo and division of follicle cells is observed until stage 6 of oogenesis. Imaging of follicle cell division shows that the midbody, marked by its associated microtubules, is always positioned at the apical side of the cortex (Fig 1B,C). This can be visualized using midbody markers, such as the kinesin Pavarotti, or markers of the midbody ring, such as Spaghetti squash (Sqh, Drosophila myosin II regulatory light chain (MRLC)) and Anillin [2] (supplementary Fig S1 online). Imaging along the apical surface of the egg chamber revealed that the midbody is also often positioned off the centre of the axis of division, closer to one of the neighbouring cells (Fig 1C,D, surface). This is consistent with a previous study showing that ring canals, small intercellular bridges that connect sibling follicle cells, are often formed at three-cell corners upon cell division [14].
Figure 1.
Asymmetric cytokinesis is conserved in the follicle epithelium. (A) Longitudinal section of an ovariole stained apically for aPKC and laterally for Dlg. (B,D) Time-lapse images of follicle cells expressing Jupiter-GFP and His-RFP. (B) Longitudinal projection shows that the midbody localizes apically during cytokinesis (arrow), (D) whereas projections along the apical surface shows dividing cells that display off-centred positioning of the midbody (arrows). (C) Diagrams representing the planar division of follicle cells and the position of the midbody in cells observed at different planes of the egg chamber. (E,F) Time-lapse sequences of cells expressing plasma membrane markers (E) Myristoylated-Tomato and (F) PLCδ1(PH)-Cerulean, (E) Sqh-GFP to label the CR and (F) His-RFP. Asymmetric furrowing occurs (white arrows) from the basal to the apical side. See also supplementary Movie S1 online. (G) Quantification of ring constriction asymmetry along the AB axis. CA and AE were measured as exemplified. Average±s.e.m. is shown for cells with low (n=31) and high (n=21) extrusion. Time-lapse sequences of Sqh-GFP-expressing cells representative of different degrees of CA and AE are shown. Scale bar, 5 μm. AE, apical extrusion; aPKC, atypical protein kinase C; CA, constriction asymmetry; FC, follicle cell; GFP, green fluorescent protein; GL, germline; RFP, red fluorescent protein.
To address the correlation between midbody positioning and the constriction of the actomyosin ring, we imaged Sqh–GFP and fluorescent plasma membrane probes to follow cleavage furrow ingression (Fig 1E,F; supplementary Movie S1 online). Furrow ingression occurs from the basal to the apical side of the cell (Fig 1E,F). We also observed that dividing follicle cells are often apically extruded from the monolayer (Fig 1F), positioning its geometrical centre closer to the apical plane of the tissue. We quantified constriction asymmetry as the ratio of the distances measured from the basal and from the apical side of the CR before constriction to the centre of the constricted ring (diameter<0.5 μm; Fig 1G). The mean constriction asymmetry is 1.95, indicating a clear bias of basal to apical constriction. Cells with low apical extrusion have higher constriction asymmetry (2.28) than cells with significant extrusion (1.39), indicating that asymmetric cytokinetic constriction positions the midbody close to the apical plane of the epithelium regardless of the degree of extrusion.
Midbody positioning is not due to intrinsic ring asymmetry
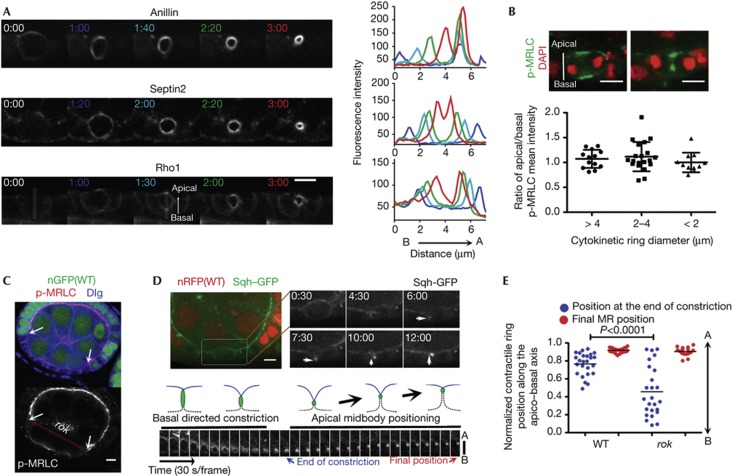
Asymmetric constriction in the C. elegans embryo requires localized Anillin and septins, which induce higher accumulation of actin and myosin on the side that constricts further [9]. Despite the apical enrichment of Sqh during interphase in follicle cells, a basal accumulation of Sqh was not evident during constriction (Fig 1E,G). Thus, we investigated if intrinsic asymmetric distribution of other CR components could underlie asymmetric constriction. Follicle cells display symmetric distribution of Septin2 during constriction, whereas Peanuts (human Septin7) and Anillin are nearly symmetric, showing slight apical enrichment (Fig 2A; supplementary Fig S2A online and supplementary Movie S1 online), opposite to what is expected if their accumulation mediates basal to apical constriction. Moreover, we observed that although pnut mutant cells often fail cytokinesis, the midbody is normally positioned whenever mutant cells reach midbody formation (supplementary Fig S2B,C online). RhoA (Rho1 in Drosophila) participates in the cascade that mediates the activating phosphorylation of MRLC, necessary to assemble the bipolar myosin filaments that drive actomyosin constriction [1]. Rho1 distribution is symmetric along the ring AB axis during constriction (Fig 2A). Also, using an antibody that recognizes the activated form of MRLC/Sqh, we show that there is no asymmetry of the actomyosin activation state from the initial deformation of the plasma membrane to the final stages of constriction (Fig 2B). Taken together, these results indicate that in the follicle epithelium, asymmetric constriction does not result from asymmetry of septins, anillin or myosin activation in the CR.
Figure 2.
Apical midbody positioning is independent of asymmetric ring structure and constriction. (A) Frames from time lapses showing the localization of the depicted markers during cytokinesis. Plots of mean pixel intensity along the AB axis measured for boxed regions defined for each colour-matching time point are shown. Time 0 was used to define the AB edges of the box used for quantification as exemplified. (B) Confocal projections of follicle cells stained with p-MRLC (green) and DAPI (red). Scatter dot plot of the apical to basal ratio of p-MRLC intensity is shown (average±s.d. for CR diameter: >4—1.07±0.18; 2–4—1.12±0.29; <2—1.00±0.20). (C,D) Mosaic egg chambers containing rok2 mutant clones marked by absence of nuclear GFP (C) or RFP (D). (C) Egg chambers are stained for p-MRLC and Dlg. (D) The actomyosin ring (marked with Sqh:GFP) constricts from the apical to the basal side (0:30–7:30) in rok mutant cells but is later positioned apically (7:30–12:00). CR constriction and midbody position in rok mutants are illustrated. Sequential projections are shown at the bottom. See also supplementary Movie S2 online. (E) Quantification of the normalized CR position along the AB axis (1 is defined as the apical cortex and 0 the position at the basal cortex) at the end of constriction versus final position in WT and rok2 mutant clones. n=25 for each genotype. (A–D) Scale bar, 5 μm. CR, contractile ring; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; MRLC, myosin II regulatory light chain; RFP, red fluorescent protein; WT, wild type.
Myosin/Sqh activity confers rigidity to the apical cortex of follicle cells, which is required to sustain compression forces from the germline [15]. As Rho kinase (Rok) regulates Sqh phosphorylation [16], we generated rok mutant clones to determine if midbody positioning was dependent on high myosin activity. rok mutant cells present lower levels of activated myosin (Fig 2C), which are sufficient to complete cytokinesis with a lower constriction rate than the wild type (supplementary Fig S3A,B online). In contrast to the wild type, the position of the CR at the end of constriction is unbiased along the AB axis in rok mutant cells (Fig 2D,E). Importantly, the midbody is always repositioned to the apical side when mutant cells regain their shape at the end of cell division (Fig 2D,E; supplementary Movie S2 online). Thus, the midbody is positioned in the apical domain independently of the initial direction of constriction.
AJs determine asymmetric cytokinesis
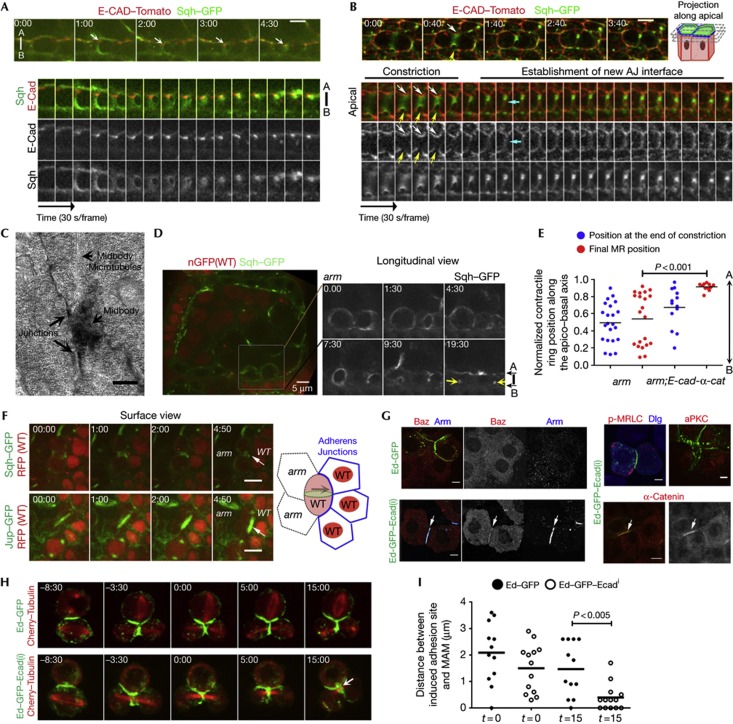
As rok mutants do not affect AB polarity (supplementary Fig S3C,D online, and Wang and Riechmann [15]), we considered if an apical cue could localize the midbody, and investigated the organization of apical polarity during follicle cell division. The key apical determinants aPKC and Par-6 [11] are depolarized during mitosis, and the apical enrichment of Par-6 and aPKC is only similar to interphase levels when the midbody is positioned apically (supplementary Fig S4 online). We then examined the distribution of AJ components during follicle cell division. In these cells, E-cadherin adhesion complexes are maintained apically during mitosis. During cytokinesis, we observed that the apical side of CR is in close contact with AJs (Fig 3A; supplementary Fig S5A online and supplementary Movie S3 online). In most follicle cells (74.3%, n=70), the CR remains coupled to the AJs of one adjacent cell, whereas it disengages from the opposite side (Fig 3B; supplementary Fig S5A–C online and supplementary Movie S4 online), resulting in apical off-centred positioning of the midbody. The association of the midbody to AJs formed with one adjacent cell is confirmed by transmission electron microscopy (Fig 3C; supplementary Fig S5F online). The levels of AJ components E-CAD, α-catenin and Armadillo (Arm, Drosophila β-catenin) are reduced at the side where the CR is uncoupled (yellow arrows in Fig 3B; supplementary Fig S5B,C online), suggesting that anisotropic distribution of AJs anchoring points correlates with the asymmetry of midbody localization within the apical side. Consistently, symmetric organization of the AJ at the plane of membrane invagination correlates with centred midbody positioning, which is observed when the midbody remains connected to or disengages of AJs at both sides (supplementary Fig S5D online). Both apical off-centred and centred midbody positioning are followed by the formation of an AJ at the interface between daughter cells (cyan arrows in Fig 3B; supplementary Fig S5D online).
Figure 3.
Adherens junctions determine asymmetric cytokinesis, positioning the midbody. (A) Longitudinal and (B) surface projections of follicle cells expressing E-Cad-Tomato and Sqh-GFP. The apical side of the CR remains closely associated with AJs formed with an adjacent cell (white arrows), whereas E-Cad levels are reduced on the opposite side (yellow arrows). The new AJs are established at the interface between daughter cells upon ring constriction (cyan arrow). See also supplementary Movies S3 and S4 online. (C) TEM image shows the midbody in close contact with AJs formed between the dividing and an adjacent cell. A lower magnification is presented in supplementary Fig S5F online. (D) In arm4 mutant cells (RFP absence), the midbody (Sqh:GFP) is not positioned apically (yellow arrows). Selected frames from supplementary Movie S5 online are shown on the right. (E) Quantification of the normalized CR position along the AB axis at the end of constriction versus final position in arm4 and arm4; E-Cad-α-cat-GFP follicle cells. (F) Mosaic arm4 egg chambers expressing Sqh:GFP or Jupiter-GFP. At the clone border, wild-type cells always direct the midbody towards the wild-type neighbour (arrows) (100%, n=19). See also supplementary Movie S7 online. (G) S2 cells expressing Ed-GFP or Ed-GFP-Ecadi and stained with the indicated proteins. (H) S2 cells expressing Ed-GFP or Ed-GFP-ECADi, and Cherry-Tubulin. Time-lapse sequence taken from supplementary Movie S8 online shows midbody recruitment to the place of adhesion in cells expressing Ed-GFP-ECADi (arrow). (I) Quantification of the distance between induced adhesion site and midbody localization at the moment when midbody microtubules compact (t=0) and after 15 min (t=15). Only cells where the axis of division was nearly parallel to the adhesion surface (<30°) were quantified. Scale bar, 5 μm (A,B,D,F,G) and 500 nm (C). AJ, adherens junction; CR, contractile ring, E-Cad, E-cadherin; GFP, green fluorescent protein.
AJ formation requires Arm, which links E-cadherin to α-catenin and subsequently to the intracellular actin cytoskeleton [12, 17, 18]. We generated arm clones to further investigate if AJ function is required to position the midbody apically. arm mutant cells disrupt midbody positioning on the putative apical domain, defined as the surface that contacts the germline (Fig 3D, yellow arrows, Fig 3E; supplementary Movie S5 online). To bypass the requirement of Arm to connect DE–Cad to α-catenin, we used a DE-Cad-α-Cat fusion knocked into the endogenous DE–Cad (shg) locus [19]. When overexpressed [20] or using an endogenous promoter, this fusion construct rescues the integrity of arm mutant cells (supplementary Fig S6 online). As it also rescues apical midbody positioning (Fig 3E; supplementary Fig S6C online), we conclude that restoring AJ function by a direct connection between E-cadherin and the intracellular cytoskeleton is sufficient for midbody positioning, independently of Arm.
In order to assess whether AJs have a direct role in midbody positioning, we used two genetic approaches that modulate the asymmetric position of AJs within the follicle cells. First, we generated dlg mutant clones, which disrupt polarity, giving rise to multiple layers of cells, and to the misplacement of AJs along the AB axis (supplementary Fig S7A online). Live imaging revealed that midbody positioning is also disrupted, particularly when cells can form misplaced AJs with a more basal cell (supplementary Fig S7B online and supplementary Movie S6 online). Second, we analysed midbody position in wild-type cells dividing alongside arm clones, and so unable to form AJs at the border with the mutant clone (Fig 3F; supplementary Movie S7 online). Strikingly, the midbody is always directed away from the arm clone, to a wild-type adjacent cell, suggesting that AJs recruit the midbody at the end of follicle cell division. To further test this hypothesis, we examined whether the polarized intracellular assembly of AJ components was sufficient to recruit the midbody in otherwise non-polarized S2 cells. The adhesion molecule Echinoid (Ed)-GFP can induce polarized distribution of proteins in S2 cells via its restricted recruitment to the adhesion sites [21]. A fusion with the intracellular domain of DE-Cad (Ed-GFP-E-Cadi) recruits the intracellular AJ components Arm, α-catenin and Baz to the polarized sites of adhesion, whereas it does not interfere with the localization of other polarized components of epithelial cells such as aPKC, Dlg and activated myosin (Fig 3G). Cells expressing Ed-GFP-E-Cadi form polarized adhesion sites that recruit the midbody, whereas this recruitment is significantly less efficient when Ed-GFP is expressed (Fig 3H,I and supplementary Movie S8 online). This result suggests that polarized assembly of AJs is sufficient to determine the position of the midbody, independently of other polarizing systems that work on epithelial cells.
Midbody mispositioning alters epithelial architecture
We next addressed whether the position of the midbody could influence the formation of the new apical interface between daughter cells. Using fluorescently labelled LifeAct to follow actin dynamics, we observed a transient F-actin accumulation that starts around the midbody, and precedes the formation of the apical AJ interface between daughter cells (supplementary Fig S8A,C online). This is consistent with the transient enrichment around the midbody of the regulator of fast actin polymerization, Arp2/3 complex [22] (supplementary Fig S8B online). Also, ectopic positioning of the midbody in arm mutant cells leads to actin accumulation wherever it is positioned along the AB axis (supplementary Fig S8D online), suggesting that the midbody provides a positional cue that orients actin polymerization wherever it is positioned, and regardless of the association to AJs.
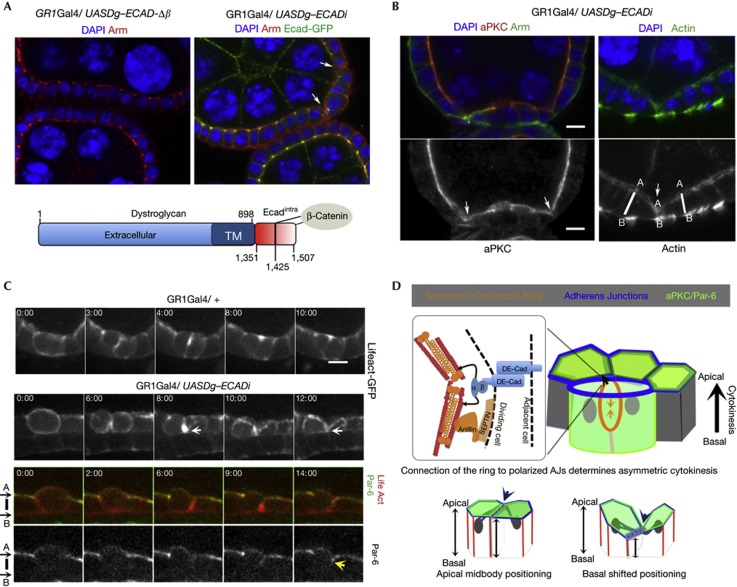
To assess the effect of midbody mispositioning, we targeted intracellular AJ components to the basal side via a fusion between the basal transmembrane protein Dystroglican (Dg) [23] and the intracellular domain of DE-Cad (Ecadi). Expression of this transgene in early oogenesis recruits the AJ component Arm to the basal side of the follicle cells, but maintains normal apical AJs marked by the localization of a GFP knock-in in the DE-Cad locus (Fig 4A). Although epithelial organization is mostly maintained, we observed the occasional formation of epithelial invaginations, characterized by a basal shift of the apical interface between two cells (Fig 4A,B, arrows). This phenotype is due to ectopic intracellular localization of AJ components, as expression of transgenes that abolish the Cadherin–Catenin interaction does not affect epithelial organization (Fig 4A). More importantly, live imaging reveals that when Dg-Ecadi expression positions the midbody and its related actin polymerization basally, the apical surface is basally shifted relatively to the AB axis of the tissue (Fig 4C,D). Thus, ectopic midbody localization disrupts epithelial shape, affecting the AB length of the daughter cell interface.
Figure 4.
Ectopic midbody position disrupts epithelial architecture. (A) Expression of UASDg-ECadi recruits Arm to the basal side of epithelial cells without disrupting AJs marked with DE-Cad-GFP. Invaginations are seen within the follicle epithelium (arrows) in Dg-DE-Cadi-expressing cells. A scheme of the fusion between the extracellular and transmembrane domains of Dg and the intracellular domain of DE-Cad is shown. UASDg-ECadi-Δβ deletes the β-catenin/Arm binding site. (B) Confocal images of Dg-ECADi-expressing egg chambers stained for the indicated markers show that the epithelial invaginations occur between cells that shift basally the position of the apical side (marked with aPKC) along the tissue AB axis. (C) Time-lapse projections of cells with the indicated genotypes and expressing LifeAct-GFP or LifeAct-Ruby and Par-6-GFP show that a basal shift in the wave of actin polymerization places more basally the interface that contacts the germline, reducing the length of the AB axis. See also supplementary Movie S10 online. Scale bar=5 μm. (D) Model illustrates that polarized AJs determine asymmetric constriction of a nearly symmetric actomyosin ring (top) and the effect of midbody misposition on epithelial architecture (bottom). AJ, adherens junction; aPKC, atypical protein kinase C; GFP, green fluorescent protein.
Overall, we found that asymmetric positioning of the midbody in follicle cells does not rely on the intrinsic asymmetry of CR components as described for the first division of the C. elegans zygote [9], but requires the association of the CR to AJs formed with the neighbouring cells. This model is consistent with the finding in Drosophila spermatocytes that the Cadherin–Catenin complex can anchor the actomyosin ring to the plasma membrane during cytokinesis as an alternative to the actin-binding protein Anillin [24]. During cytokinesis, the force generated for contractile ring constriction must counterbalance the global stiffness of the cortex [25]. It is therefore reasonable to consider that the apical anchoring of AJs to a CR with symmetric intrinsic forces results in anisotropic mechanical resistance of the cortex to contractile actomyosin activity, leading to asymmetric constriction (Fig 4D, top). Although it is unclear how AJs’ intracellular components are coupled to the actomyosin ring, α-catenin is likely to be central to this interaction, either via its ability to connect AJ clusters to actin filaments [12, 26], or through other components of the CR, such as the RhoGEF Ect2 [27].
Three studies that examine cytokinesis in Drosophila embryonic [28] and dorsal thorax pupal epithelium [29, 30] were published during the revision of this manuscript. Anchorage of the CR to AJs also seems to underline asymmetric cleavage in these epithelial contexts, centring the CR on the apical domain [28, 30]. This is then followed by disengagement of the CR from neighbouring AJs that facilitate the extension of the interface between daughter cells [28–30]. Interestingly, the CR maintains engagement to one of the neighbouring AJs in most follicle cells, revealing that dual disengagement is not critical for the extension of a new apical interface in the follicle epithelium. In line with the observation that the CR also displays asymmetric AJ engagement if cadherin levels are increased in embryonic epithelia [28], adhesion forces might be sufficiently strong within the follicle epithelium to sustain attachment to the CR. This might be related to the ability of N-cadherin to participate in AJ formation during the stages of follicle cell division [18], whereas simultaneous N-cadherin and E-cadherin expression is unusual in other tissues [31].
Herszterg et al [29] show that the midbody works as a positional landmark to orient a Rac and Arp2/3-dependent actin flow that is required to extend the AJ interface between daughter cells. Our data suggest that midbody position also orients the site of actin polymerization in the follicular epithelium. In addition, we show that a more basal recruitment of the midbody and the associated actin polymerization misposition the apical interface between new daughter cells (Fig 4D, bottom). Arp2/3-dependent actin polymerization participates in the expansion of new Cadherin adhesive contacts [32]. We propose that by determining the site where new arrays of actin filaments stabilize the formation of AJs, midbody position defines where the daughter cell apical interface is established in relation to the AB axis of the surrounding tissue. Thus, mechanisms that ensure the apical position of the midbody participate in the regulation of the epithelial architecture in the proliferating tissue. Furthermore, asymmetric cytokinesis contributes to apical polarization in mammalian epithelial models [6, 7]. Our results also show that other features of apical polarity, such as the localization of the aPKC/Par-6, must be re-established at the end of follicle cell division. How exactly the inherited cytokinesis machinery orients actin polymerization and the formation of the new AJs, and whether it directly participates in cell repolarization upon division remain key questions to address.
METHODS
Drosophila genetics. Drosophila genetics and the generation of the pUASp-Dg-ECADi and pUASp-Dg-ECADi-Δβ stocks are described in detail in the supplementary Methods online.
Cloning and expression of Ed-GFP-ECADi in S2 cells. The intracellular domain of DE-Cad (157 aa of the C-term domain) flanked by BglII/XbaI sites was amplified from genomic DNA and cloned into pMT-Ed-GFP [21]. Expression was induced with 500 μM CuSO4 for 12–16 h before analysis, and S2 cells were plated on poly-L-lysine-coated culture dishes for live imaging (MatTek). See the details of S2 culture and transfection in supplementary Methods online.
Microscopy and data analysis. Drosophila ovaries or S2 cells were fixed in 4% paraformaldehyde, followed by standard procedures for immunofluorescence. See supplementary Methods online for further details, a list of the primary antibodies used and the description of TEM methods. Images of fixed samples were collected using a Leica TCS SP5 II (Leica Microsystems) confocal microscope and live imaging data sets were collected at 25 °C with a spinning disc confocal system (Andor Revolution XD). Image analysis and assembly were performed using ImageJ and Adobe Photoshop. Images shown are maximum z-series projections, which span the region of interest of the dividing cell. Further details and quantifications are described in the supplementary Methods online. Unpaired Student’s t-tests were performed to assess significance. Statistical analysis and graphs were performed with GraphPad.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank D. St Johnston, J. Knoblish, Y. Hong, P. Rorth, C. Doe, R. Karess, J. Zallen, T. Schupbach, G. Goshima, A. Wilde, D. Glover, J. Brill and Bloomington Stock Center for reagents, A. Carvalho for comments on the manuscript, C. Mendes for technical support and R. Fernandes for assistance with electron microscopy. This work is supported by FEDER Funds through the Operational Competitiveness Programme—COMPETE—and by National Funds through FCT—Fundação para a Ciência e a Tecnologia—under the projects FCOMP-01-0124-FEDER-019740 (PTDC/BIA-BCM/120366/2010) and FCOMP-01-0124-FEDER-019738 (PTDC/BIA-BCM/120132/2010). E.M. was funded by an EMBO fellowship (ALTF-1269-2010) and currently holds a Marie Curie-IEF (FP7-PEOPLE-2010-IEF-275037).
Author contributions: E.M. conducted all the experimental work; E.M. and C.S. designed and analysed the experiments. E.M. and C.S wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Fededa JP & Gerlich DW (2012) Molecular control of animal cell cytokinesis. Nature Cell Biol 14: 440–447 [DOI] [PubMed] [Google Scholar]
- Kechad A Jananji S Ruella Y & Hickson GR (2012) Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr Biol 22: 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R (1996) Cytokinesis in Animal Cells. Cambridge, UK: Cambridge University Press, [Google Scholar]
- Fleming ES Zajac M Moschenross DM Montrose DC Rosenberg DW Cowan AE & Tirnauer JS (2007) Planar spindle orientation and asymmetric cytokinesis in the mouse small intestine. J Histochem Cytochem 55: 1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S & Karsenti E (1994) Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol 126: 1509–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB Kaji N Durgan J & Hall A (2008) Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183: 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter MA Pfarr CS Pieczynski J Whiteman EL Hurd TW Fan S Liu CJ & Margolis B (2009) Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 20: 4652–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V Marzesco AM Corbeil D Huttner WB & Wilsch-Brauninger M (2007) Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox AS Lewellyn L Desai A & Oegema K (2007) Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev Cell 12: 827–835 [DOI] [PubMed] [Google Scholar]
- Papusheva E & Heisenberg CP (2010) Spatial organization of adhesion: force-dependent regulation and function in tissue morphogenesis. EMBO J 29: 2753–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D & Ahringer J (2010) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141: 757–774 [DOI] [PubMed] [Google Scholar]
- Harris TJ & Tepass U (2010) Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 11: 502–514 [DOI] [PubMed] [Google Scholar]
- Baker J & Garrod D (1993) Epithelial cells retain junctions during mitosis. J Cell Sci 104(Pt 2): 415–425 [DOI] [PubMed] [Google Scholar]
- Airoldi SJ McLean PF Shimada Y & Cooley L (2011) Intercellular protein movement in syncytial Drosophila follicle cells. J Cell Sci 124: 4077–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y & Riechmann V (2007) The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol 17: 1349–1355 [DOI] [PubMed] [Google Scholar]
- Matsui T et al. (1996) Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15: 2208–2216 [PMC free article] [PubMed] [Google Scholar]
- Cox RT Kirkpatrick C & Peifer M (1996) Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J Cell Biol 134: 133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G Smith C McGlade J & Tepass U (2000) Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J Cell Biol 151: 891–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J Zhou W Dong W Watson AM & Hong Y (2009) From the Cover: directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc Natl Acad Sci USA 106: 8284–8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquelet A & Rorth P (2005) Regulatory mechanisms required for DE-cadherin function in cell migration and other types of adhesion. J Cell Biol 170: 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA Hirono K Prehoda KE & Doe CQ (2009) Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138: 1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley ED & Welch MD (2006) The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 7: 713–726 [DOI] [PubMed] [Google Scholar]
- Deng WM Schneider M Frock R Castillejo-Lopez C Gaman EA Baumgartner S & Ruohola-Baker H (2003) Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130: 173–184 [DOI] [PubMed] [Google Scholar]
- Goldbach P Wong R Beise N Sarpal R Trimble WS & Brill JA (2010) Stabilization of the actomyosin ring enables spermatocyte cytokinesis in Drosophila. Mol Biol Cell 21: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN & Spudich JA (2004) Mechanics and regulation of cytokinesis. Curr Opin Cell Biol 16: 182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M Rauzi M Lenne PF & Lecuit T (2008) A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453: 751–756 [DOI] [PubMed] [Google Scholar]
- Ratheesh A et al. (2012) Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 14: 818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot C & Lecuit T (2013) Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev Cell 24: 227–241 [DOI] [PubMed] [Google Scholar]
- Herszterg S Leibfried A Bosveld F Martin C & Bellaiche Y (2013) Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev Cell 24: 256–270 [DOI] [PubMed] [Google Scholar]
- Founounou N Loyer N & Le Borgne R (2013) Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev Cell 24: 242–255 [DOI] [PubMed] [Google Scholar]
- Halbleib JM & Nelson WJ (2006) Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20: 3199–3214 [DOI] [PubMed] [Google Scholar]
- Verma S Shewan AM Scott JA Helwani FM den Elzen NR Miki H Takenawa T & Yap AS (2004) Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J Biol Chem 279: 34062–34070 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.