Abstract
Hepatitis B reactivation is a well-described complication in patients with inactive chronic hepatitis B receiving chemotherapy. Screening for HBV and preemptive therapy are recommended. However, the rates of HBV screening, prophylaxis and reactivation during rituximab-containing chemotherapy are unknown.
Patients and methods
We performed a retrospective study of patients with non-Hodgkin lymphoma (NHL) who received rituximab between August 1997 and September 2009. We evaluated patients for hepatitis B serologies, antiviral prophylaxis and hepatitis B reactivation during or up to 6 months after chemotherapy.
Results
One thousand four hundred twenty nine patients underwent rituximab-containing chemotherapy for NHL. Hepatitis B serologies were documented in 524 (36.6%) patients. Of these, 20 (3.8 %) were HBsAg positive and 10 (50%) experienced HBV reactivation. Only half (5/10) had HBV serology documented prior to reactivation. Only 3/8 (37.5%) of patients with newly documented HBsAg positivity received antiviral prophylaxis. Virologic breakthrough occurred in 2 of the patients on chronic therapy, in one of three inactive carriers on prophylaxis and in 2 of 5 patients not receiving prophylaxis. Reactivation developed in another 5 patients not previously screened for hepatitis B. One patient developed ALF and died. Reactivation did not occur in 25 patients with isolated positive core antibody.
Conclusions
At tertiary care institutions hepatitis B serologies are infrequently assessed prior to rituximab-based chemotherapy and prophylaxis is uncommon. Greater adherence to recommendations for screening and prophylaxis is necessary. This suboptimal screening rate could be even lower in community hospitals and could result in significant harm to unscreened and unprophylaxed patients.
Keywords: Chemotherapy, HBV Reactivation, Hepatitis B, HBV prophylaxis, Non-Hodgkin lymphoma, Rituximab
INTRODUCTION
Chronic infection with hepatitis B virus (HBV) affects 1.25 million persons in the United States and over 350 million worldwide.(1,2) The morbidity and mortality from chronic hepatitis B due principally to the host immune response to viral infection(3) is high, with 15 to 40% developing chronic liver disease or hepatocellular carcinoma.(4,5)
Even quiescent hepatitis B infection, as seen in chronic inactive carriers or patients with resolved infection, can reactivate in the setting of immunosuppression or chemotherapy. Reactivation of hepatitis B is marked by an abrupt onset of hepatitis B replication, with an elevated HBV DNA level and elevated aminotransferases. Reactivation is most commonly subclinical but can result in severe disease, including acute liver failure and death.(6)
Rates of hepatitis B reactivation vary widely in different studies, ranging from 20% to 55% in inactive carriers undergoing immunosuppressive chemotherapy.(7, 8, 9, 10,) Fortunately, antiviral prophylaxis has been shown to be effective in reducing the rates of reactivation. Lamivudine prophylaxis dramatically reduces reactivation rates between 0% to 11.5%.(11,12)
Because reactivation is common and can have devastating consequences, since 2003(13,14) multiple societies (NIH, AASLD, APASL, EASL) have published recommendations calling for universal screening for hepatitis B infection with hepatitis B surface antigen for all patients planning chemotherapy.(4, 5, 7, 15) In addition, antiviral prophylaxis for patients with positive surface antigen is currently recommended during and for a variable period following the completion of chemotherapy.(4,5,13) (Table 1) However, the rates of adherence to these recommendations are unknown and their impact on rates of reactivation of hepatitis B remains unclear.
Table 1.
Summary of current guidelines recommendations of HBV patients undergoing chemotherapy or immunosuppressive therapy
| Guideline | HBV Screening | HBsAg-Positive Prophylaxis |
HBsAg-Negative and Anti-HBc positive |
|---|---|---|---|
| APASL Consensus 2008 (7) | Screen with HBsAg before chemotherapy. | Prophylactic lamivudine until 12 weeks after end of chemotherapy. Other antivirals can be used. |
Closely monitor. Start Rx if reactivation develops |
| AASLD Guidelines 2009 (5) | Persons needing immunosuppressive therapy, screen with HBsAg and Anti-HBc | Low HBV DNA, Rx for 6 months after finish therapy High HBV DNA until Rx endpoints Tenofovir/entecavir preferred |
Not enough information for routine prophylaxis. Monitoring recommended Initiate Rx if HBV DNA becomes detectable. |
| EASL Guidelines 2009 (15) | All candidates, screen with HBsAg and Anti-HBc | Nucleos(t)ide analogs until 12 months after end of chemotherapy. Lamivudine in low HBV DNA Entecavir/Tenofovir for High HBV DNA |
Closely monitor with ALT and HBV DNA. Start Rx if reactivation develops. |
| NIH Consensus 2009 (4) | Screening high risk populations | Start antiviral before immunosuppressive therapy and maintain throughout the course of therapy | NA |
APASL, Asian Pacific Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the study of liver diseases; NIH, National Institutes of health; Rx, treatment. NA, Not available data.
This table is oriented to be simple and synthesized the current guidelines of HBV in the setting of chemotherapy or immunosuppressive therapy. For complete information refer to the original reference.
Since the previous reports of hepatitis B reactivation, chemotherapy for hematologic malignancies has evolved. Rituximab, a genetically engineered chimeric murine/human monoclonal antibody (mAb) directed against the CD20 antigen expressed on the surface of normal and malignant B-lymphocytes(16), has become a mainstay of chemotherapy for non-Hodgkin’s lymphoma.(17) Rituximab combined with standard chemotherapy is associated with higher response rates.(18, 19) The current label for rituximab contains a black box warning about the risk of HBV reactivation. However, the impact of the addition of rituximab on the incidence of HBV reactivation remains unknown.
We therefore sought to (1) assess the rates of screening for hepatitis B infection in patients with non-Hodgkin’s lymphoma starting rituximab-based chemotherapy and (2) assess rates of antiviral prophylaxis among surface antigen positive patients. In addition, we sought to evaluate the rates of hepatitis B reactivation in patients receiving rituximab based chemotherapy and the impact of antiviral prophylaxis.
PATIENTS AND METHODS
We performed a retrospective search of patients seen at the Massachusetts General Hospital (MGH) using the Research Patient Database Registry (RPDR) query tool. The RPDR is a centralized clinical registry for data summaries: demographics, providers, diagnoses, procedures, laboratory information, medications, etc. We searched for all patients with a diagnosis of non-Hodgkin lymphoma (NHL) who had received at least one dose of rituximab between August 1997 (the year of FDA approval) and September 2009.
The lymphoma search included all patients with diffuse large B-cell lymphoma, Burkitt’s lymphoma, mantle cell lymphoma, marginal zone lymphoma, follicular lymphoma, or other malignant lymphomas and unspecified malignant neoplasms of lymphoid tissue. For each subject we searched the medical record for the assessment of HBV serology including HBsAg, anti-HBs antibody, HBeAg, anti-HBe antibody, anti-HBc antibody, anti-HBc IgM, HBV DNA level and liver biochemical tests. In addition, we evaluated all patient notes to determine whether screening for hepatitis B had been performed outside of our system and had been documented in the medical record. Medication lists and medical records were reviewed for the use of antiviral medications.
All patients with positive HBV serologies were evaluated for the development of possible reactivation. Hepatitis B reactivation was defined as: 1) an alanine aminotransferase (ALT) increase to ≥ 8 upper limit of normal (ULN; 40 U/L women, ULN; 55 U/L men) and 2) elevated HBV DNA (above baseline) during or for 6 months following the cessation of chemotherapy, 3) the absence of clinical or laboratory features of acute infection with hepatitis A, C, D, autoimmune hepatitis, Wilson’s disease or other systemic infection. We used the AASLD 2009 definitions(5) of chronic hepatitis B (HBsAg-positive > 6 months, HBV DNA >20,000 IU/ml, persistent or intermittent elevations of ALT’s) and inactive HBsAg carrier state (HBsAg-positive, HbeAg negative, anti-HBe positive, HBV DNA < 2000 IU/ml and persistently normal ALT levels) to classify each case.
HBV serologies (HBsAg, anti-HBs, anti-HBc) were performed by Abbott Architect 2000SR CMIA assay; HBV DNA was performed using the COBAS® AmpliPrep/COBAS® TaqMan® HBV Test, v2.0 with one of three different assays depending on the year of performance (HBV DNA Quant, PCR SL reference range <0.01 pg/ml; HBV DNA Quantitative PCR, assay range 300–200,000 DNA copies/ml; HBV DNA quantitative PCR assay range 60–38,000 IU/ml). In order to convert pg/ml to DNA copies/ml, we used the following conversion: 150,000 DNA copies/ml = 1 pg/ml. In order to convert DNA copies/ml to IU/ml, we used the following conversion: 0.190 IU/ml = 1 DNA copy/ml.(Specialty Labs.) This study was approved by the Partners Human Research Committee.
STATISTICAL ANALYSIS
Continuous variables are presented as median and range; categorical variables are shown in percentages. We used two-sample t-test or Wilcoxon rank sum test for comparison of continuous variables whenever appropriate. For univariate comparisons of dichotomous or categorical variables we used Fisher’s exact test. A two-sided p-value of less than 0.05 was considered to be statistically significant. For identification of independent predictors of HBV reactivation, we used multivariate logistic regression modeling. We used SAS software version 9.1.3 (SAS Institute Inc., Cary, NC) for data analysis.
RESULTS
One thousand four hundred twenty-nine patients met our inclusion criteria. Patients were predominantly white (88%) and male (56.5%) with a mean age of 63.36 ±15.43. (Table 2) We found HBV serologic data or documentation of hepatitis B testing in 524 (36.6%) patients. Of these 524, 20 patients (3.8 %) tested HBsAg positive.
Table 2.
Patient Demographics
| Characteristic | Demographic data |
Number (%) |
|---|---|---|
| Number of patients | 1429 | |
| Gender | Male | 810 (56.6 %) |
| Female | 619 (43.3 %) | |
| Age | 63.36 ± 15.43 | |
| Race | Whites/Caucasian | 1258 (88%) |
| Hispanic | 48 (3.3%) | |
| African-American | 43 (3 %) | |
| Asian | 32 (2.2%) | |
| Unknown/not recorded | 32 (2.2%) | |
| Other | 16 (1.1%) | |
| Vital status | Not report as deceased | 986 (69 %) |
| Deaths | 443 (31%) |
Data are expressed as mean ± standard deviation (SD) and/or absolute numbers
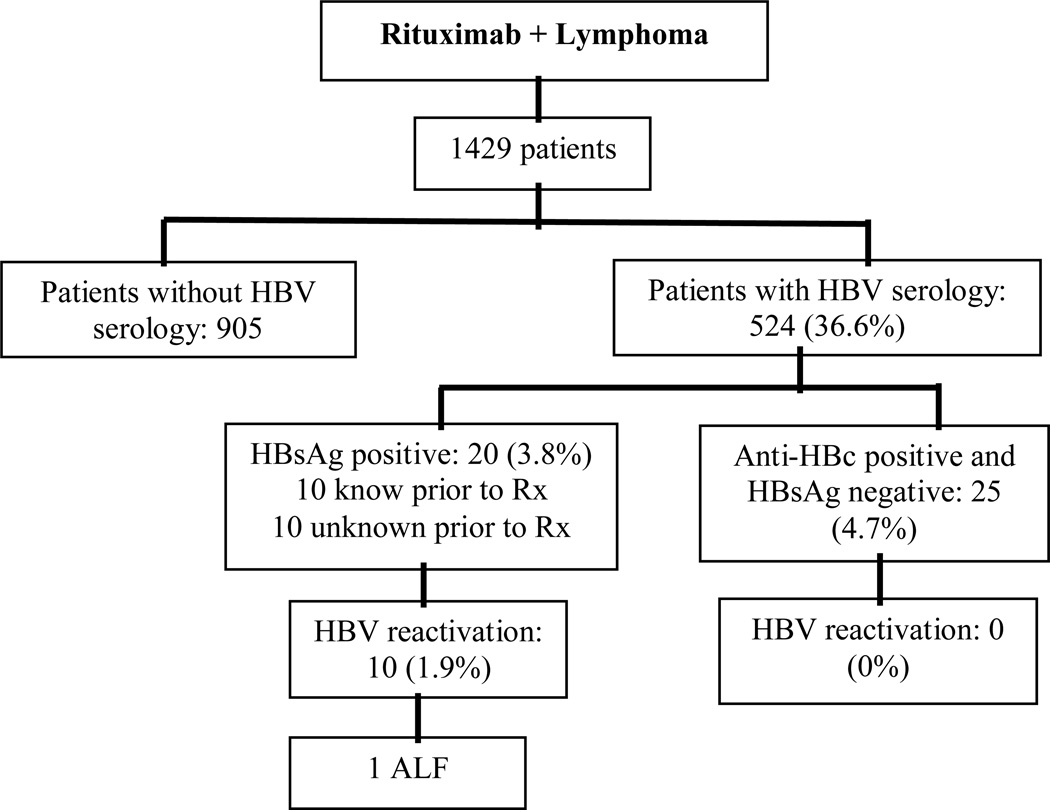
In addition we identified 25 patients (4.7%) who were HBsAg-negative, anti-HBs negative and anti-HB core positive (markers of prior exposure to HBV). (Figure 1) These patients did not have ALT flares during chemotherapy and in follow-up and the ones who had HBV DNA available (12 patients) were always undetectable (< 60 IU/ml). None of these patients received prophylaxis, nor did any of them meet criteria of HBV reactivation.
Figure 1.
Flow diagram of serology/reactivation data
(ALF= Acute Liver Failure)
Of the 1429 patients we found another 41 patients with ALT flares (≥ 400 IU/ml). Nine patients did not have HBV serology or HBV DNA assessed, so we cannot know the exact etiology of their flares. Of the other 32 patients, all were HBsAg negative and all who had anti-HBc, HbeAg and anti-HBe testing performed were negative; however, few had HBV DNA testing performed.
HBsAg-positive cases
The 20 HBsAg-positive cases had a mean age of 61.2 ±14.9 years, were predominantly male (80%) and white (60%). The risk factors for HBV transmission were: sexual (3), health care worker (3), injection drug use (3), perinatal transmission (2), previous surgery (2) and unknown (7). The most frequent oncologic diagnosis was diffuse large B-cell lymphoma (DLBCL) in 12/20 (60%). The care providers of the 20 HBsAg-positive patients were 16 from the Hematology/Oncology cancer center and four from Oncology. In five cases they made referral to the GI/Hepatology Unit (four because of the reactivation).
Patients with a positive surface antigen were classified as either inactive HBsAg carriers (n=7) or as chronically infected with hepatitis B (n=3). Ten patients did not have any hepatitis B serologies checked prior to the development of reactivation and thus their status is unknown. All ten patients did have documented normal ALT levels prior to the initiation of chemotherapy.
HBV Prophylaxis
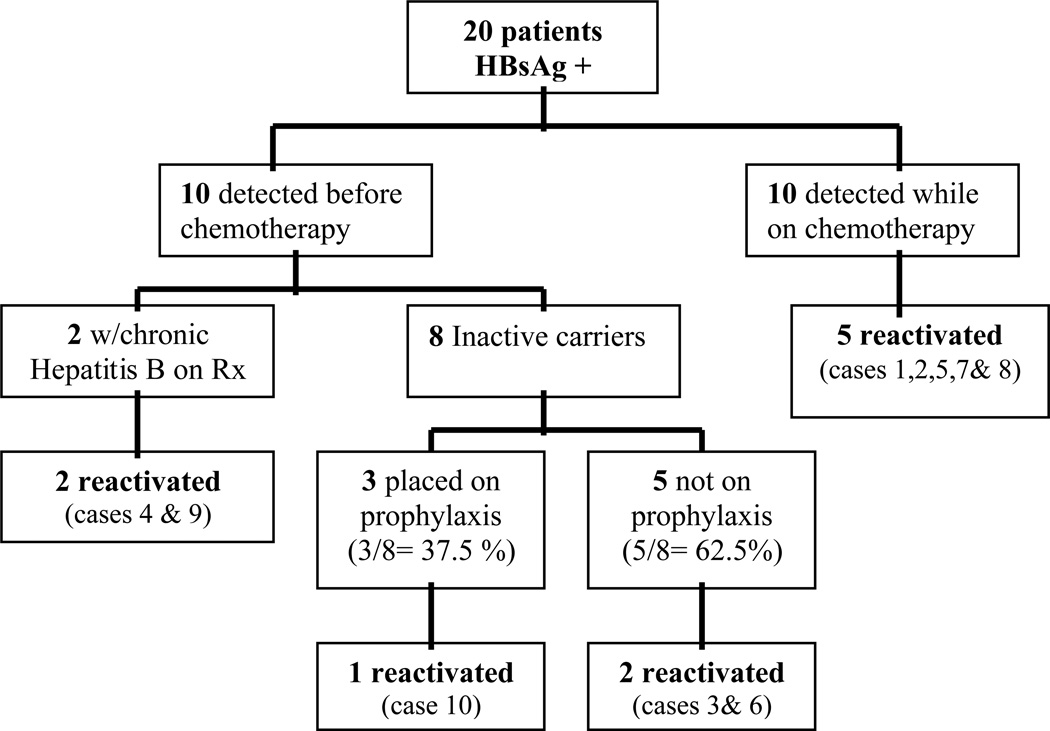
Of the 20 HBsAg-positive patients, 10 had a positive HBV surface antigen detected prior to the initiation of chemotherapy. Two were receiving long-term lamivudine therapy for chronic hepatitis B for five years and 14 months (case 4 and case 9) respectively. We have separated these two cases from those eligible for HBV prophylaxis, and case 4 specifically, is classified as a treatment failure and probable lamivudine resistance because of the high HBV DNA (38,000 IU/ml). Thus, eight patients were eligible for HBV prophylaxis. However, only three (37.5%) received prophylaxis as guidelines recommend,(13, 14) two inactive carriers receiving lamivudine (case 10 and case 18) in 2004, and one receiving tenofovir (case 20, inactive carrier, HBV DNA <300 IU/ml) in 2009. All had prophylaxis initiated 2 weeks prior to the start of chemotherapy. Of the remaining 5 patients who were candidates but did not receive HBV prophylaxis four underwent chemotherapy between 2004 and 2009 and only one patient received chemotherapy before 2003, suggesting that guidelines were not actively followed. (Figure 2) Of the 8 patients who were eligible for prophylaxis, 1 was treated with chemotherapy during 1997–2002 period but did not receive prophylaxis. The remaining 7 patients were treated with chemotherapy during 2003–2009; only 3 received prophylaxis as recommended by international guidelines.
Figure 2.
Outcome of HBsAg-positive patients
Of the 20 HBsAg positive patients who received chemotherapy, 10 patients presented with HBV reactivation (Cases 1 to 10). In two cases (Cases 3 and 4), chemotherapy was interrupted (at 5 and 3 weeks respectively) because of the ALT flare but ultimately resumed. The other 8 patients did not experience delay or interruption of their chemotherapy. Three of these latter cases (Cases 6, 7 and 8) presented with ALT flares after completion of the planned chemotherapy (presumptive delayed reactivation).
All patients received at least one dose of rituximab (Rituxan®, Biogen Idec/Genentech) given at 375 mg/m2. In most cases rituximab was combined with chemotherapy and administered on the first day of the cycle, 3 to 5 days before the remainder of the regimen. In the majority of cases rituximab and remaining chemotherapy were given for 14 to 21 days of each cycle. First-line chemotherapy was CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone/prednisolone) or CHOP-like regimens. The mean number of rituximab doses was 5.3 (range 1–16 doses).
HBV reactivation cases
Review of the records disclosed that 10 patients (50%) of the 20 HBsAg-positive fulfilled the criteria for HBV reactivation. (Figure 1) Eight were male, six were white, 2 African American, 1 Asian and 1 Hispanic. Five of the 10 (50%) cases of HBV reactivation developed jaundice with a mean total bilirubin of 6.11 mg/dl (0.7–16 mg/dl). The mean baseline ALT before chemotherapy was 28.7 U/L (11–53 U/L) and all the reactivation cases had an ALT above 400 U/L (mean ALT 1036 ±557 U/L) during HBV reactivation. (Table 3) The type of chemotherapy used varied, but was predominantly rituximab + CHOP (R-CHOP) in 4 cases. Other regimens included rituximab + EPOCH in two, rituximab + ICE in one, rituximab-decitabine in one, rituximab-bendamustine + Methotrexate in one, and rituximab alone in another. The mean time between the first dose of rituximab and reactivation was 130.5 days. The mean time between the last dose of rituximab in the cycle and the reactivation was 62.4 days and the mean time between the last dose of any chemotherapy and reactivation was 51.6 days. (Table 4)
Table 3.
Clinical/serologic characteristics of the HBsAg-positive patients
| No. | Known prior to chemo |
HBV status prior chemo |
HBV reactivation |
Pre ALT (U/L) |
Peak ALT (U/L) |
HBeAg prior to chemo |
Anti- HBe Prior to chemo |
HBVDNA prior to chemo |
HBV Rx prior to chemo |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Unknown | Yes | 53 | 1560 | NA | NA | NA | No |
| 2 | No | Unknown | Yes | 15 | 506 | NA | NA | NA | No |
| 3 | Yes | Inactive carrier | Yes | 31 | 1971 | Neg | Pos | 1500 IU/ml | No |
| 4 | Yes | Chronic HBV | Yes | 47 | 424 | Pos | Neg | 38000 IU/ml | Chron. LAM |
| 5 | No | Unknown | Yes | 20 | 1004 | NA | NA | NA | No |
| 6 | Yes | Chronic HBV | Yes | 26 | 406 | Pos | Neg | 38000 IU/ml | No |
| 7 | No | Unknown | Yes | 17 | 660 | NA | NA | NA | No |
| 8 | No | Unknown | Yes | 22 | 1479 | NA | NA | NA | No |
| 9 | Yes | Inactive carrier | Yes | 11 | 1505 | Neg | Pos | 285 IU/ml | Chron. LAM |
| 10 | Yes | Inactive carrier | Yes | 40 | 853 | Neg | Pos | 300 IU/ml | Proph LAM |
| 11 | No | Unknown | No | 83 | 83 | NA | NA | NA | No |
| 12 | No | Unknown | No | 20 | 92 | NA | NA | NA | No |
| 13 | No | Unknown | No | 27 | 35 | NA | NA | NA | No |
| 14 | Yes | Inactive carrier | No | 29 | 32 | Neg | Pos | 300 IU/ml | No |
| 15 | No | Unknown | No | 37 | 102 | NA | NA | NA | No |
| 16 | No | Unknown | No | 13 | 74 | NA | NA | NA | No |
| 17 | Yes | Inactive carrier | No | 21 | 121 | Pos | Neg | 300 IU/ml | No |
| 18 | Yes | Inactive carrier | No | 48 | 52 | Pos | Pos | 300 IU/ml | Proph LAM |
| 19 | Yes | Chronic HBV | No | 137 | 310 | Neg | Pos | 20000 IU/ml | No |
| 20 | Yes | Inactive carrier | No | 14 | 31 | NA | Neg | 300 IU/ml | Proph TNF |
NA= Not available. ALT= alanine aminotransferase; LAM= Lamivudine;TNF=Tenofovir; Chron. LAM = Use of chronic Lamivudine before chemotherapy. Proph = use of prophylaxis before chemotherapy. Anti-HBc =total antibody to hepatitis B core antigen. HBV DNA reported in IU/ml.
Table 4.
Virologic and clinical outcomes of the HBV reactivation cases
| No | Reason for serology |
HBV status prior chemo |
Baseline HBV DNA prior chemo |
Peak HBV DNA |
Time from 1st rituximab to reactivation |
Time from last chemo to reactivation |
Antiviral Prophylaxis |
Treatment of reactivation |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Evaluation of thrombocy topenia | Unknown | NA | 18,000 IU/ml | 161 days | 76 days | No | None | ALF/Death |
| 2 | Acute ALT elevation | Unknown | NA | 387,600 IU/ml | 97 days | 22 days | No | Lost to Follow up | Lost to follow up |
| 3 | Know inactive carrier | Inactive carrier | 1500 IU/ml | 7980000 IU/ml | 76 days | 34 days | No | LAM 100mg + Tenofovir | Recovery |
| 4 | Acute ALT elevation | Chronic HBV | 38,000 IU/ml | 494,000 IU/ml | 222 days | 101 days | No, only chronic LAM | LAM 100mg + Tenofovir | Recovery |
| 5 | Acute ALT elevation | Unknown | NA | 102,600 IU/ml | 87 days | 80 days | No | Entecavir 0.5 mg | Recovery |
| 6 | HBV(+) in semen | Chronic HBV | 28,000 IU/ml | >38,000 IU/ml | 155 days | 24 days | No | Lost to follow up | Lost to follow up |
| 7 | Acute ALT elevation | Unknown | NA | 17,900 IU/ml | 234 days | 84 days | No | Entecavir 0.5 mg | Recovery |
| 8 | Acute ALT elevation | Unknown | NA | 22,900 IU/ml | 156 days | 44 days | No | Entecavir 0.5 mg | Recovery |
| 9 | Risk factor | Inactive carrier | 285 IU/ml | 17,955 IU/ml | 39 days | 39 days | No, only chronic LAM | LAM 150mg/d | Recovery |
| 10 | Preventive | Inactive carrier | 2900 IU/ml | >38,000 IU/ml | 78 days | 12 days | Yes: LAM | Entecavir 0.5 mg | Recovery |
Cases diagnosed before to chemotherapy were: Number 3,4,6,9 and 10.
ALT= alanine aminotransferase, LAM= Lamivudine, ALF= Acute Liver Failure.
In order to convert reported data in pg/ml to DNA copies/ml, we used the following conversion: 150,000 DNA copies/ml = 1 pg/ml. In order to convert reported data in DNA copies/ml to IU/ml, we used the following conversion: 0.190 IU/ml = 1 DNA copy/ml.
One patient with reactivation (0.1%) developed acute liver failure with jaundice (total bilirubin 12.5 mg/dl), coagulopathy, asterixis, encephalopathy and ultimately died (Case 1). In this case the diagnosis of HBV reactivation was supported by liver biopsy.
Known status prior to chemotherapy
In only 5 of 10 patients who reactivated was HBV serology documented prior to reactivation (Cases 3, 4, 6, 9, 10); in the other five (Cases 1, 2, 5, 7, 8), serologies were discovered only during reactivation (development of jaundice or elevated ALT). In these five cases whose serologies were documented at the time of reactivation, all were negative for anti-HBc IgM, suggesting reactivation rather than de novo HBV infection. None of these patients had been on antiviral therapy.
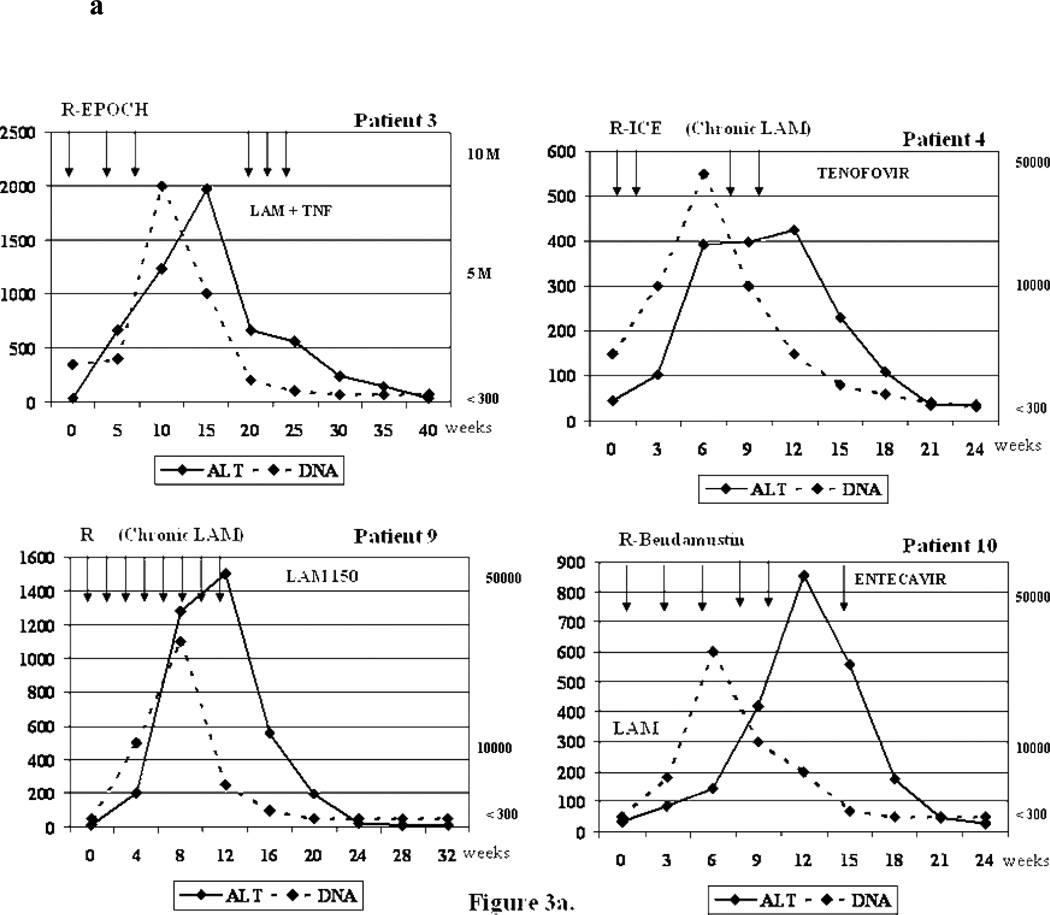
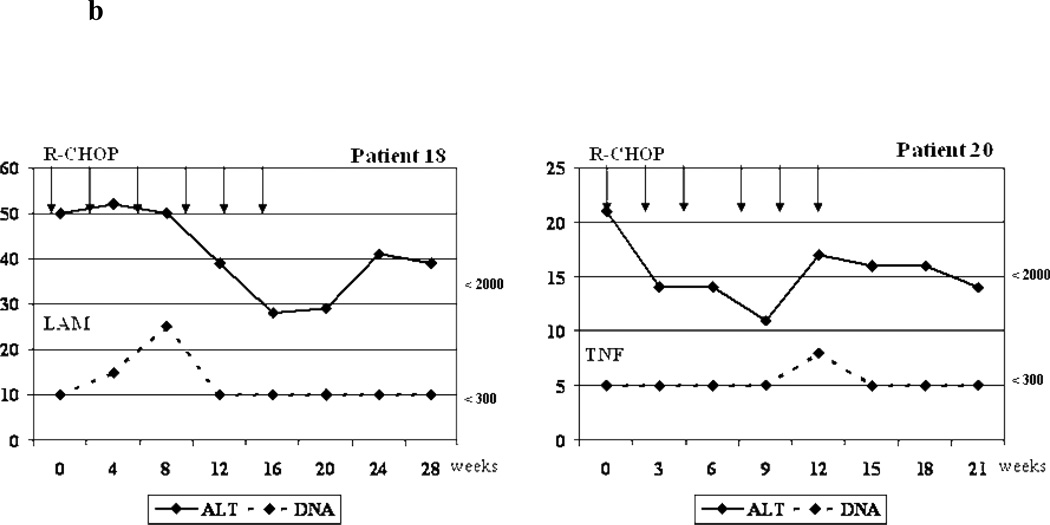
Of the remaining 5 patients whose HBV surface antigen positivity was known before chemotherapy, two (Cases 4 and 9) were on long-term lamivudine therapy (chronic HBV) and one (Case 10) began lamivudine prophylaxis before chemotherapy. The remaining two who reactivated (Cases 3 and 6) were not on antiviral therapy. (HBV DNA from 1500 IU/ml to 7,980,000 IU/ml and 28,000 to >38,000 IU/ml respectively) (Figure 2, Figure 3a and Figure 3b)
Figure 3.
a. Biochemical and virologic outcome of four representative reactivation cases.
R-ICE= Rituximab+ ifosfamide, carboplatin, etoposide; R-EPOCH=Rituximab + etoposide, prednisone, vincristine, cyclophosphamide, hydroxydaunorubicin; R-CHOP=Rituximab + cyclophosphamide, doxorubicin, vincristine and prednisone/prednisolone ; ALT= alanine aminotransferase; LAM= Lamivudine; TNF=Tenofovir; DNA= Viral Load (HBV DNA). Arrows = Indicate the time when the cycle of chemotherapy was administrated.
b. Biochemical and virologic outcome of two cases without reactivation that received antiviral prophylaxis.
R-CHOP =Rituximab + cyclophosphamide, doxorubicin, vincristine and prednisone/prednisolone ; ALT= alanine aminotransferase; LAM= Lamivudine; TNF=Tenofovir; DNA= Viral Load (HBV DNA). Arrows = Indicate the time when the cycle of chemotherapy was administrated.
Active treatment for reactivation of the hepatitis B varied among the ten patients. In the seven patients who did not receive antiviral prophylaxis treatment until reactivation, three received entecavir (Cases 5, 7,8), one received lamivudine plus tenofovir (Case 3), and the other 3 patients did not receive treatment (2 lost to follow up, Cases 2 and 6, and 1 died from ALF, Case 1). When the flare was identified in the two patients receiving long-term lamivudine therapy, one had their dose of lamivudine increased from 100 mg to 150 mg daily (Case 9) and the other (case 4) that was classified as a treatment failure and probable lamivudine resistance, had tenofovir added to lamivudine therapy, leading to the conclusion that there was only one failure of lamivudine prophylaxis. (Figure 3a) This one patient receiving lamivudine prophylaxis who developed reactivation (case 10) was switched from lamivudine to entecavir. (Inactive carrier, HBV DNA from <300 to >38,000 IU/ml) (Table 4 and Figure 2).
Characteristics of reactivation and non-reactivation cases
We compared the characteristics of reactivators with non-reactivators in an attempt to identify factors that were associated with reactivation. We analyzed baseline demographic and clinical characteristics of the HBsAg positive patients including gender, age, race, type of oncologic disease, type of chemotherapy, number of rituximab doses, HBV DNA and ALT pretreatment between the reactivation and non-reactivation cases. Using multivariate analysis, we found none of the baseline characteristics of the HBsAg positive patients to be a predictive of reactivation. There were no significant differences in demographic, clinical or virological characteristics between the 2 groups. (Table 5) In addition, as guidelines for hepatitis B screening became widely available following 2002, we evaluated the rate of HBV screening between 1997–2002 and 2003–2009. We found that 164/504 (32.5%) patients were screened during 1997–2002 and 340/504 (67.4%) were screened during 2003–2009. These data indicate that the publication of guidelines has improved screening, although screening rates remain well below the goal of 100%.
Table 5.
Comparison characteristics between reactivation and non-reactivation groups.
| Characteristic | Reactivation N= 10 |
Non-reactivation N= 10 |
P value |
|---|---|---|---|
| Gender (M/F) | 8/2 | 8/2 | p = 1.00 |
| Race (White/Non-white) | 5/5 | 7/3 | p = 0.64 |
| Type cancer (DLBCL/NO-DLBCL) | 7/3 | 5/5 | p = 0.64 |
| Type chemo (R-CHOP/ Another) | 4/6 | 6/4 | p = 0.65 |
| AGE median (range) | 58 (37–78) | 64.5 (25–79) | p = 0.84 |
| Rituximab Doses, median (range) | 5.5 (1.0–12.0) | 4.5 (2.0–16.0) | p = 0.70 |
| Pre ALT median (range) | 24.0 (11.0–58.0) | 28.0 (13.0–189.0) | p = 0.55 |
| HBV DNA prior to chemo median IU/ml (range) | 38000 (17900–7,980,000) | 24320 (60–380,000) | p = 0.19 |
Data are expressed as range (Age, rituximab doses and Pre-ALT)and absolute numbers or median.
Multivariate logistic regression model with Fisher’s exact test and Wilcoxon rank sum test for 2 by 2 tables.
Age by T test for two samples equal variances.
DLBCL= Diffuse large B-cell lymphoma;R-CHOP=Rituximab + cyclophosphamide, doxorubicin, vincristine and prednisone/prednisolone ; ALT= alanine aminotransferase.
DISCUSSION
To our knowledge our study is the first to evaluate institutional adherence to hepatitis B screening guidelines and prophylaxis as well as the incidence of HBV reactivation in the era of rituximab. Importantly, we found that despite widely published guidelines, hepatitis B screening is still not consistently practiced by hematologist-oncologists. Only a third of patients (36%) undergoing rituximab-based chemotherapy had HBV serologies performed either inside our institution or prior documentation of serologies performed outside of our institution. As a result, five cases of HBV reactivation occurred and were not diagnosed until clinical presentation. The rate of reactivation during chemotherapy, among those with known HBsAg positivity, was 50% (one developed ALF and died) two of these five failed to received prophylaxis. Thus, the lack of comprehensive screening and prophylaxis clearly impacted outcomes in these patients.
It is possible that our finding of 36% rate of screening is an underestimate of the true screening rate. For example, patients may have undergone screening prior to being seen at our institution and results forwarded to our oncologists may not have been entered into the medical record. However, the patients who were discovered to have reactivation of the hepatitis B without recorded serologies in our database were not on prophylaxis, suggesting a lack of knowledge about their serologic status.
As we report, of the 1429 patients we found another 41 patients with ALT flares. Thirty two patients were HBsAg negative but few had HBV DNA testing performed. The other nine patients did not have HBV serology or HBV DNA assessed, and we cannot know the exact etiology of their flares. Overall, 51 patients had ALT flares (41 cases without exact causes of their flares and 10 attributable directly to HBV reactivation), which means that as many as 20% of the flares (10/50) in our region can be referable to Hepatitis B, where HBV prevalence is not high. This example emphasizes the importance of HBV screening in candidates for chemotherapy and the value of carefully monitoring ALT elevations during chemotherapy.
Another important finding in our study was the low rate of HBV prophylaxis administration. Only 3 of 8 patients (37.5%) with known HBV surface antigen positivity received prophylaxis. This finding suggests both that guidelines regarding prophylaxis are not known or followed and that further education of providers is necessary. In addition, we found that 2 patients on long term lamivudine therapy developed reactivation. In both of these patients the HBV DNA was detectable prior to the start of chemotherapy but rose 10 fold during chemotherapy. Efforts to appropriately manage lamivudine resistance prior to the initiation of chemotherapy would also have been warranted.
In a summary of 12 trials in the pre-rituximab era, Kohrt et al.(10) reported a rate of HBV reactivation without lamivudine of 39.8% versus 6.8% with prophylactic lamivudine. Li et al.(20) described HBV-related hepatitis in 51.7% in control versus 17.5% of the patients treated with lamivudine. One recently prospective randomized trial by Hsu et al.(12) HBV carriers with newly diagnosed non-Hodgkin’s lymphoma underwent chemotherapy using CHOP without rituximab. They were randomized to either prophylactic lamivudine on the first day of chemotherapy or to reactive lamivudine when a flare was detected. Higher HBV reactivation rates were seen in the group with therapeutic lamivudine (56%) compared with the prophylactic lamivudine group (11.5% p= 0.001).
There are fewer studies addressing rituximab chemotherapy and HBV reactivation. Pei et al.,(21) in a recent retrospective study, analyzed 115 patients with B cell lymphoma who received rituximab-based regimens, 15 were HBsAg positive. Of these 15, 5 received lamivudine prophylaxis and did not develop HBV-related hepatitis, but 8 of 10 who did not receive prophylaxis experienced HBV-related hepatitis. Unfortunately, most of the rituximab-related HBV reactivation cases in the literature are severe or fatal case reports(22, 23, 24, 25); further analysis of this group is needed.
Fewer data exist on HBsAg-negative, anti-HB core positive patients. Yeo et al.(26) in a prospective randomized trial of 104 anti-HB core positive patients, found a rate of reactivation of 25% with rituximab plus CHOP compared to 0% in patients treated with CHOP without rituximab. This latter study, performed in an endemic area for hepatitis B, suggests that rituximab can induce more HBV reactivation in patients without surface antigen than does conventional chemotherapy. These authors used a three-fold or absolute increase of ALT greater than 100 U/L as a definition of hepatitis. In our study, we did not observe reactivation in the group of HBsAg negative and known anti-HBcore positive patients undergoing rituximab therapy. Our criteria for ALT flares was higher (≥ 8 ULN), and some of the patients did not have available HBV DNA data, thereby limiting our ability to identify asymptomatic reactivations. This difference could explain in part the discrepancy between our result and those of Yeo et al. and underscore the importance of assessment of HBV viral load and serology during and after chemotherapy to detect HBsAg seroreversion.
Reactivation of hepatitis B is important not only because of its potential fatal outcome but because it can be effectively prevented with antiviral therapy. However, the appropriate prophylaxis remains less clear. While therapy with lamivudine reduced the reactivation risk, its efficacy is limited by the frequent appearance of antiviral resistance(27), as was observed in one patient on long term lamivudine and one receiving prophylaxis. As a result, agents such as tenofovir or entecavir likely represent superior choices for prophylaxis, especially when patients have high levels of HBV DNA. It is critical that HBsAg positive persons about to initiate chemotherapy should have a complete serologic and virologic assessment, including HBV DNA, liver biochemical tests, and should start prophylaxis with an effective oral therapy with high barrier to resistance such as tenofovir or entecavir. Although our experience was predominantly based on lamivudine, entecavir or tenofovir offer advantages over lamivudine, in that either agent achieves more rapid and potent HBV DNA suppression than lamivudine and offers a higher barrier to resistance. We recommend that antiviral therapy with entecavir, tenofovir, or lamivudine should be applied prophylactically in HBsAg+ persons about to undergo chemotherapy. Use of entecavir or tenofovir should be particularly favored under conditions in which the HBsAg+ patient has received lamivudine previously or if the pre-chemotherapy HBV DNA is positive.(25,28) Of note, in one of the cases of reactivation (case 9) the treatment by the providers was an increase in the dose of lamivudine from 100 mg to 150 mg; however, this was not appropriate management -- it would have been preferable to add on adefovir or tenofovir.
In this series of patients undergoing chemotherapy, two patients had to interrupt treatment because of the ALT flares, but ultimately completed chemotherapy. There need not be a choice between screening and treating for hepatitis B and treating for lymphoma. Rather, both can be done simultaneously to prevent HBV reactivation as well as delay in chemotherapy. While the retrospective nature of our study prevents us from determining the number and duration of chemotherapy delays, cancer treatment interruption would appear to represent a real risk.
One major question is how much of the reactivation is attributable to chemotherapy as compared to rituximab. Our study cannot address this, since all patients received combined chemotherapy from the outset. Reactivation commonly occurs after the first 2–3 cycles, and the median onset of reactivation is 16 weeks after initiation chemotherapy with a pre-treatment high viral load as the most important risk factor.(7) Our reactivation cases presented at an average of 51 days after the first cycle of any combined regimen, so it will be impossible to define which drug was the principal offender. However, our study confirms that reactivation HBV remains an important issue in rituximab-based chemotherapy.
Our retrospective study has limitations inherent to its study design. However, we used strict guidelines to secure the diagnosis of hepatitis B reactivation and excluded other forms of liver disease where possible. In addition, the successful use of antivirals directed against hepatitis B lends weight to the diagnosis of reactivation hepatitis B. We do acknowledge that our study is limited by the small number of subjects with and without reactivation. Prospective long term trials are needed to more precisely define rates of reactivation.
Another limitation of our study is that we were unable to determine how many of the unscreened patients were actually hepatitis B surface antigen positive and thus to establish a true rate of reactivation. However, even if we have overestimated reactivation rates, the identification of 10 episodes of reactivation with one death remains an important finding and warrants redoubled efforts to educate the oncology community about the hazards of the HBV reactivation. Clearly, improved strategies are required to recognize risk factors for hepatitis B virus, to improve screening rates, and to routinely prophylaxis those who harbor chronic hepatitis B.
Our study demonstrates the absolute need for effective communication between hematologists/oncologists and hepatologists. Management of hepatitis B infection has become increasingly complex, especially in the immunocompromised patient population, and requires the input of both specialties to ensure that these patients receive the highest quality care.
In conclusion, our study demonstrates that, in a US tertiary center, adherence to HBV screening and prophylaxis guidelines is suboptimal for patients undergoing therapy with rituximab-based regimens. In this group HBV reactivation is frequent. This suboptimal screening rate could be even lower in community hospitals and could result in significant harm to unscreened and unprophylaxed patients. Efforts must be made to screen all patients undergoing chemotherapy for HBV and give prophylaxis to those who harbor chronic hepatitis B. Assessment of HBV serology, serum ALT and HBV DNA levels is critical and should be closely monitored before, during and after rituximab-based chemotherapy in HBsAg-positive patients; adjustments in regimens may be required in those already on therapy.
ACKNOWLEDGMENT
Jorge Mendez-Navarro has a research scholarship from Health Education Office of Mexican Institute of Social Security (Instituto Mexicano del Seguro Social, IMSS). Kathleen E. Corey has received research support from Bristol Myers Squibb, USA. Raymond T. Chung has received research support from Gilead Sciences.
Abbreviations
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- anti-HBc
antibody to hepatitis B core antigen
- HbeAg
hepatitis B e antigen
- anti-HBe
antibody to hepatitis B e antigen
- ALF
acute liver failure
- NHL
Non-Hodgkin lymphoma
- ALT
alanine aminotransferase
- DLBCL
Diffuse large B-cell lymphoma
- CHOP
cyclophosphamide, doxorubicin, vincristine and prednisone/prednisolone
- ICE
ifosfamide, carboplatin, etoposide
- EPOCH
etoposide, prednisone, vincristine, cyclophosphamide, hydroxydaunorubicin
Footnotes
DISCLOSURES
Hui Zheng, Jae-Young Jang, Lydia Barlow, Wenyu Lin, Run-Xuan Shao, Steven L. McAfee and Hong Zhao have nothing to disclose.
REFERENCES
- 1.Dienstag JL. Hepatitis B Virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 2.Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(Suppl 1):100–107. doi: 10.1111/j.1478-3231.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 3.Vierling JM. The immunology of hepatitis B. Clin Liver Dis. 2007;11:727–759. doi: 10.1016/j.cld.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus development conference statement: management of hepatitis B. Ann Intern Med. 2009;150:104–110. doi: 10.7326/0003-4819-150-2-200901200-00100. [DOI] [PubMed] [Google Scholar]
- 5.Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. AASLD Practice Guidelines. Hepatology. 2009;50:1–36. [Google Scholar]
- 6.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–S165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz LH, Fraser A, Gafter-Gvili A, Leibovici L, Tur-kaspa R. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: systematic review and meta-analysis. J Viral Hepatitis. 2008;15:89–102. doi: 10.1111/j.1365-2893.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 9.Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy-Induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis. 2007;11:965–991. doi: 10.1016/j.cld.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau GK, Yiu HH, Fong DY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in Non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 13.de Franchis R, Hadengue A, Lan G, et al. EASL International Consensus Conference on hepatitis B. 13–14 September,2002: Geneva, Switzerland. Consensus statement. J Hepatol. 2003;39:S3–S25. [PubMed] [Google Scholar]
- 14.Lok AS, McMahon BJ. Chronic Hepatitis B: Update of recommendations. AASLD Practice Guidelines. Hepatology. 2004;39:857–861. doi: 10.1002/hep.20110. [DOI] [PubMed] [Google Scholar]
- 15.EASL Clinical Practice guidelines: Management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Lovric S, Erdbruegger U, Kumpers P, et al. Rituximab as rescue therapy in anti-neutrophil cytoplasmic antibody-associated vasculitis: a single-centre experience with 15 patients. Nephrol Dial Transplant. 2009;24:179–185. doi: 10.1093/ndt/gfn430. [DOI] [PubMed] [Google Scholar]
- 17.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies-historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 19.Li JM, Wang L, Shen Y, et al. Rituximab in combination with CHOP chemotherapy for the treatment of diffuse large B cell lymphoma in Chinese patients. Ann Hematol. 2007;86:639–645. doi: 10.1007/s00277-007-0320-8. [DOI] [PubMed] [Google Scholar]
- 20.Li Y-H, He Y-F, Jiang W-Q, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus Carriers who receive chemotherapy for lymphoma. Cancer. 2006;106:1320–1325. doi: 10.1002/cncr.21701. [DOI] [PubMed] [Google Scholar]
- 21.Pei S-N, Chen C-H, Lee C-M, et al. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol. 2010;89:255–262. doi: 10.1007/s00277-009-0806-7. [DOI] [PubMed] [Google Scholar]
- 22.Simpson N, Simpson P, Ahmed A, et al. Prophylaxis against chemotherapy-induced reactivation of hepatitis B virus infection with lamivudine. J Clin Gastroenterol. 2003;37:68–71. doi: 10.1097/00004836-200307000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Dillon R, Hirschfield GM, Allison ME, Rege KP. Fatal reactivation of hepatitis B after chemotherapy for lymphoma. BMJ. 2008;337:756–758. doi: 10.1136/bmj.39490.680498.BE. [DOI] [PubMed] [Google Scholar]
- 24.Law JK, Ho JK, Hoskins PJ, Erb SR, Steinbrecher UP, Yoshida EM. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: Potential implications for future prophylaxis recommendations. Leuk Lymphoma. 2005;46:1085–1089. doi: 10.1080/10428190500062932. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez MJ, Buti M, Homs M, Palacios A, Rodriguez-Frias F, Esteban R. Successful use of entecavir for a severe case of reactivation of hepatitis B virus following polychemotherapy containing rituximab. J Hepatol. 2009;51:1091–1096. doi: 10.1016/j.jhep.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Yeo W, Chan TC, Leung N, et al. Hepatitis B virus reactivation in Lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 27.Yeo W, Johnson P. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–220. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 28.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]