Abstract
Cerebral venous thrombosis (CVT) has usually been ascribed to prothrombotic conditions, oral contraceptives, pregnancy, malignancy, infection, head injury or mechanical precipitants. The case reported here illustrates two rare causes of CVT observed in the same patient: the presence of antiphospholipid antibodies associated with an asymptomatic cryptogenic organising pneumopathy (COP) which were considered the origin of the venous cerebral thrombosis and heparin-induced thrombocytopenia (HIT) which was responsible for the worsening of the thrombosis observed a few days after the introduction of treatment. Moreover, we provide here additional positive experience in the treatment of both, CVT and HIT, by fondaparinux with bridging to warfarin given their successful evolution under this anticoagulant option.
Background
Cerebral venous thrombosis (CVT) associated to antiphospholipid syndrome (APS) and heparin-induced thrombocytopenia (HIT) is extremely rare. This first condition is usually treated with anticoagulant. The use of heparin can be complicated by HIT, which can potentially aggravate the thrombus. This situation is poorly described in the literature and the management is even less reported.
Case presentation
A 67-year-old woman presented with transient headache, vertigo, tinnitus and right hemifacial paraesthesia with propagation down to the ipsilateral arm. Her medical history included left endocochlear deficit following acoustic trauma 15 years ago. She was treated for 10 years by hormonal replacement therapy for postmenopausal symptoms.
The general, ear nose and throat and neurological examination yielded no other abnormality except left hearing loss. Cerebral MRI with venography (MRIV) showed a subacute non-occlusive CVT located to the right transverse and sigmoid sinus, as well as the internal jugular vein (figure 1). Full-dose anticoagulation with unfractionated heparin (UFH) was immediately given intravenously until switch to enoxoparin (Ep), a low-molecular weight heparin (LMWH). She was then managed as an outpatient with injectible nadroparin (Np) along with to acenocoumarol (Ac) to achieve international normalised ratio of 2.0–3.0, which were controlled by her family doctor. The patient reported that 2 h after each injection of Np, transient vertigo nausea and vomiting appeared. The symptomatology was described as more intense following each injection. She was readmitted in the department of neurology for further investigations.
Figure 1.
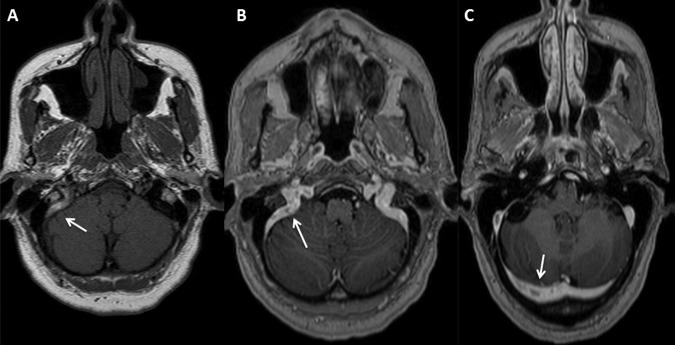
MRI performed at admission. (A) MRI without gadolinium injection showing a spontaneous T1 hyperintensity of the right sigmoid and the beginning of the transverse sinus. (B and C) MRI with injection of gadolinium. Here, we can notice hypointensity in the sinus compatible with non-occlusive thrombosis.
Investigations
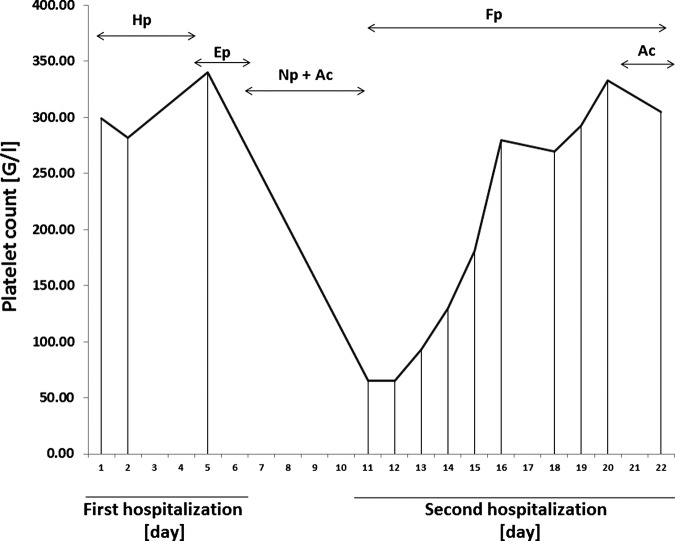
On her arrival, thrombocytopenia was noted with a low platelet count of 65 109/L (figure 2); the cerebral MRIV showed a worsening of the previously described CVT, which became quasiocclusive although always restricted to the same portion as was previously observed (figure 3). Anti-PF4 antibody was positive which was consistent with a HIT under LMWH and explained the evolution of the CVT. The ‘4 T's’ pretest probability of HIT score was 7/8.
Figure 2.
Evolution of platelet count. The graph shows platelet concentration in blood demonstrating the evolution of thrombocytopenia. The normal range is between 150 and 350 (109/L). From admission to day 4, the patient received intravenous unfractionated heparin (Hp). On day 5, Enoxaparin (Ep), a low-molecular weight heparin (LMWH) was administrated until discharge. Nadroparin (Np) and Acenocoumarol (Ac) were given on day 6 until readmission, when thrombocytopenia was revealing. The treatment was pursued with fondaparinux (Fp) associated to a vitamin K antogonist (Ac) during the last 2 days of hospitalisation.
Figure 3.
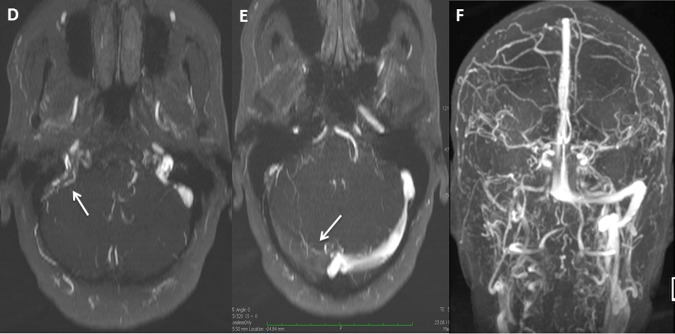
Worsening of cerebral venous thrombosis after anticoagulant therapy. (D–F) MRI with venography performed at readmission (6 days after administration of low-molecular weight heparin). These images show a lack of signal in the right transverse, sigmoid and jugular sinus veins. We can notice a worsening of the previously described cerebral venous thrombosis which became occlusive.
Regarding the aetiology of the CVT, different markers of prothrombotic conditions on laboratory tests were analysed and an elevated anti-β2-glycoprotein-1 IgM was found suggesting an APS. Investigations looking for an immunological or neoplasic problem were thus performed. The chest X-ray showed small nodules which were confirmed by CT scan whereas total body PET CT demonstrated the presence of four hypermetabolic nodules. A bronchoscopy with biopsy for cytological and histological examination demonstrated atypical alveolar cells with chronic inflammatory lymphomacrophagic elements. There was no evidence of malign cells.
Differential diagnosis
The most likely diagnosis in descending order was therefore an asymptomatic cryptogenic organizing pneumonia (COP), an undefined inflammatory process and less likely a bronchioloalveolar carcinoma or a lymphoproliferative disorder.
Treatment
Concerning the pulmonary nodules, an empirical treatment with co-amoxiclav and prednisone was given to act on the inflammatory, autoimmune or infectious processes.
With regard to HIT, LMWH and Ac were discontinued and replaced by subcutaneous fondaparinux (Fp).
Outcome and follow-up
As for the origin of the APS and CVT, the thoracic CT scan showed disappearance of all pulmonary nodules after treatment by corticosteroid and antibiotherapy.
The discontinuation of LMWH and introduction of an alternative anticoagulant lead to the normalisation of the platelet count and cessation of the symptoms. Before discharge, a vitamin K antagonist was prescribed.
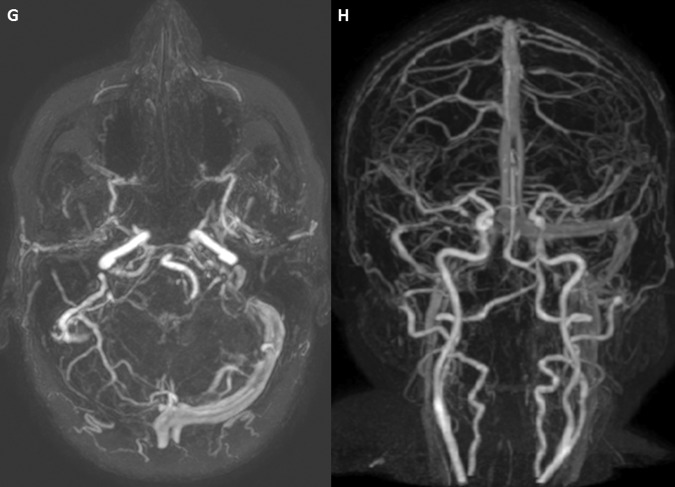
At 1 year follow-up, the patient is asymptomatic and MRIV shows partial repermeabilisation of the right transverse and sigmoid sinus (figure 4).
Figure 4.
MRI with venography performed at 1 year follow-up. (G and H) The MRI illustrate partial repermeabilisation of the right transverse and sigmoid sinus.
Discussion
CVT has been usually reported with prothrombotic conditions, oral contraceptives, pregnancy, malignancy, infection, head injury or mechanical precipitants. This case report illustrates two rare causes of CVT that happened in the same patient: first an asymptomatic pneumopathy of unknown origin associated with antiphospholipid antibodies (APLa) considered at the genesis of the cerebral thrombosis and second type II HIT, responsible for the cerebral thrombosis worsening. Clinical manifestation of CVT depends on the thrombus localisation and can be grouped in three major syndromes: isolated intracranial hypertension, focal and encephalopathy syndrome. Our patient suffered from headache with vomiting, tinnitus, vertigo and right hemifacial paraesthesia attributed to a right transverse, jugular and sigmoid sinus thrombosis observed on cerebral MRIV.
First of all, hormonal replacement therapy (HRT) was suspected to be responsible for the CVT because as suggested in previous work, while HRT may reduce the risk for arterial disease, it also increases the risk for venous event by altering the threshold for thrombotic events in healthy postmenopausal women. As previously demonstrated the risk of venous thromboembolic events appears higher in the first 6 months to 1 year after initiation of HRT and tends to decrease and even disappear overtime.1 Our patient, however, had taken this treatment for 10 years.
Final diagnosis was that of a COP associated with secondary APS responsible of CVT, which is an unusual presentation. APS is a condition triggered by autoimmune and rheumatic diseases, infections, medications, neoplasms and other associations. APLa are directed against phospholipids or plasma proteins that are bound to phospholipids. They are responsible for the hypercoagulable state, the mechanism of which remains poorly understood. These antibodies include anticardiolipin and anti-β 2-glycoprotein-I as well as lupus anticoagulant antibodies. Other autoantibodies exist and are directed against prothrombin, annexin V, phosphatidylserine, phosphatidylinositol and phosphatidylethanolamine. They must be positive in laboratory examination two times at least 12 weeks apart. Clinical expressions are related to arterial or venous thrombosis inducing deep vein thrombosis, cutaneous abnormalities, thrombocytopenia as well as cerebrovascular disease as the most frequent neurological manifestation.2 Strokes and transient ischaemic attacks (TIAs) are the second most common clinical expression of primary antiphospholipid syndrome following venous thrombosis.3 On the other side, CVT is an extremely rare presentation of APS. This association has been poorly investigated because of the lack of reported cases and up to now, only a few were published. Meanwhile, Carhuapoma et al4 concluded that the presence of anticardiolipin antibody appears to predispose patients to develop CVT at a relatively younger age, to have more extensive cerebral venous system involvement and frequent thrombosis of the deep venous system. As a reminder, this case of CVT exhibited a right transverse and sigmoid sinus thrombus extended to proximal internal jugular vein. Moreover, Cesarman-Maus et al5 showed that antibodies against annexin A2, a binding site for β2- glycoprotein- I that is highly expressed on cerebral endothelium, are significantly associated with autoimmune CVT.
Possible therapies for APS consist in heparin, warfarin, antiplatelet agents and hydroxychloroquine. In our patient, subcutaneous UFH and LMWH were injected in the acute thrombotic phase. In addition, prednisone with co-amoxiclav was introduced for the pulmonary infiltrates. Unfortunately, the patient developed type II HIT responsible for the worsening of the thrombosis with a quasiocclusion of her cerebral sinus by local increase of the thrombus size without length extension.
Type II HIT is an immune reaction against the association of heparin and platelet factor 4, a heparin neutralising protein contained in the α granules of platelets. Heparin-PF4-antibodies activate platelets which stimulate them to produce procoagulant microparticles responsible of arterial and venous thrombosis. Moreover, these thrombocytes undergo aggregation and are removed from the circulation leading to thrombocytopenia.
HIT has been more frequently reported in women and surgical patients, more often with UFH than with LMWH.6 Clinical manifestation of HIT is a >50% platelet fall that occurs 5–10 days after initiation of heparin therapy. Thrombosis tends to occur in veins rather than arteries, deep venous thrombosis and pulmonary embolism being the most frequent presentation. CVT as a complication of HIT is extremely rare. Only few cases of HIT associated to CVT have been reported so far. To our knowledge, our case is the third one describing HIT triggered by LMWH with CVT. In this patient, CVT due to APS was nevertheless already present and its treatment by LMWH exacerbated the thrombus. Richard et al described a similar case of CVT secondary to essential thrombocythemia worsened by treatment with heparin. The patient was successfully treated with Danaparoïd.7 Our case provides further support to the idea that HIT developed under treatment for CVT would aggravate the thrombus present in the cerebral sinus, rather than initiating a new thrombus elsewhere.
The first recommended intervention in HIT is discontinuation of all types of heparin because cross-reaction could occur. Alternative anticoagulant therapy is recommended and cessation alone is not sufficient because of the subsequent risk of thrombosis.8 The other anticoagulants for HIT consist of direct thrombin inhibitors (Lepirudin, Argotroban) and Factor Xa inhibitors (fondaparinux, Danaparoid) with bridging to vitamin K antagonist (warfarin).
Lepirudin and Argotroban are the usually proposed treatment for HIT and they have been shown to effectively prevent new thrombosis.9 However, they are administrated by intravenous route, are expansive, less efficient for warfarin bridging than fondaparinux and require frequent activated partial thromboplastin time measurements. Another approved agent is Danaparoid intravenous bolus followed by infusion; its dosage adjustment should achieve 0.5–0.8 anti-Xa U/mL. In our patient, we used fondaparinux as an alternative anticoagulant treatment. This agent has not been formally approved by the Food and Drugs Administration and several cases have been published so far suggesting HIT associated with fondaparinux. Its use therefore remains controversial. One advantage of this drug nevertheless is that it lowers risk during bridging to warfarin when compared to direct thrombin inhibitor (DTI). For this reasons, the use of DTI in the acute phase of HIT with transition to fondaparinux once the platelet has recovered has been proposed.10 Literature on fondaparinux in HIT is, however, limited and its use should be investigated further. The case reported here is another illustration that HIT and CVT symptomatology may both evolve successfully under treatment by fondaparinux with bridging to vitamin K antagonist.
Learning points.
Autoimmune aetiology of the cerebral venous thrombosis (CVT) may predispose to an autoimmune reaction following heparin treatment, heparin-induced thrombocytopenia (HIT).
This side effect seems to aggravate the cerebral venous thrombus rather than initiating a new thrombus elsewhere.
We provide here a positive experience in the treatment of CVT worsened by HIT with fondaparinux with bridging to warfarin.
Footnotes
Contributors: JH cared for the patient, reviewed the literature, drafted and revised the manuscript and figure. IK cared for the patient and revised the manuscript. MIV performed the MRI and revised the manuscript. IM-M cared for the patient and revised the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pérez Gutthann S, García Rodríguez LA, Castellsague J, et al. Replacement therapy and risk of venous thromboembolism: population based case—control study. BMJ 1997;2013:796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanna G, Bertolaccini ML,, Cuadrado MJ, et al. Central nervous system involvement in the antiphospholipid (Hughes) syndrome. Rheumatology 2003;2013:200–13 [DOI] [PubMed] [Google Scholar]
- 3.Shah NM, Khamashta MA, Atsumi T, et al. Outcome of patients with anticardiolipin antibodies: a 10 year follow-up of 52 patients. Lupus 1998;2013:3–6 [DOI] [PubMed] [Google Scholar]
- 4.Carhuapoma JR, Mitsias P, Levine SR. Cerebral venous thrombosis and anticardiolipin antibodies. Stroke 1997;2013:2363–9 [DOI] [PubMed] [Google Scholar]
- 5.Cesarman-Maus G, Cantú-Brito C, Barinagarrementeria F, et al. Autoantibodies against the fibrinolytic receptor, annexin A2, in cerebral venous thrombosis. Stroke 2001;2013:501–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;2013:1330–5 [DOI] [PubMed] [Google Scholar]
- 7.Richard S, Perrin J, Lavandier K, et al. Cerebral venous thrombosis due to essential thrombocythemia and worsened by heparin-induced thrombocytopenia and thrombosis. Platelets 2011;2013:157–9 [DOI] [PubMed] [Google Scholar]
- 8.Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008;2013(Suppl 6):340S–80S [DOI] [PubMed] [Google Scholar]
- 9.Lubenow N, Eichler P, Lietz T, et al. Lepirudin for prophylaxis of thrombosis in patients with acute isolated heparin-induced thrombocytopenia: an analysis of 3 prospective studies. Blood 2004;2013:3072–7 [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE. Fondaparinux versus direct thrombin inhibitor therapy for the management of heparin-induced thrombocytopenia (HIT)—bridging the River Coumarin. Thromb Haemost 2008;2013:2–3 [DOI] [PubMed] [Google Scholar]