Abstract
A man in his late 60s presented with symptoms for a few months of itching and head ache after shower. Physical examination was unremarkable except for ruddy complexion and splenomegaly. Complete blood count showed haemoglobin of 18.1 g/dL and haematocrit of 56.6%. To rule out secondary causes of erythrocytosis, such as congenital heart disease with a right to left shunt, a transthoracic echocardiogram was performed, which showed normal left ventricular function with an apical area of dyskinesis and a large left ventricular apical thrombus measuring 3.0 cm×2.0 cm. Further laboratory investigations showed low erythropoietin level and Jak V617F mutation consistent with the diagnosis of polycythemia vera. He was treated with aspirin, enoxaparin, phlebotomy and hydroxyurea with no reported complications during the stay.
Background
Review of the literature revealed that while thrombosis is not uncommon with polycythemia vera (PV), intracardiac thrombosis is rare and is seen usually in patients who have other risk factors for intracardiac thrombosis, such as valve disease, prosthetic valve, device lead or cardiomyopathy.
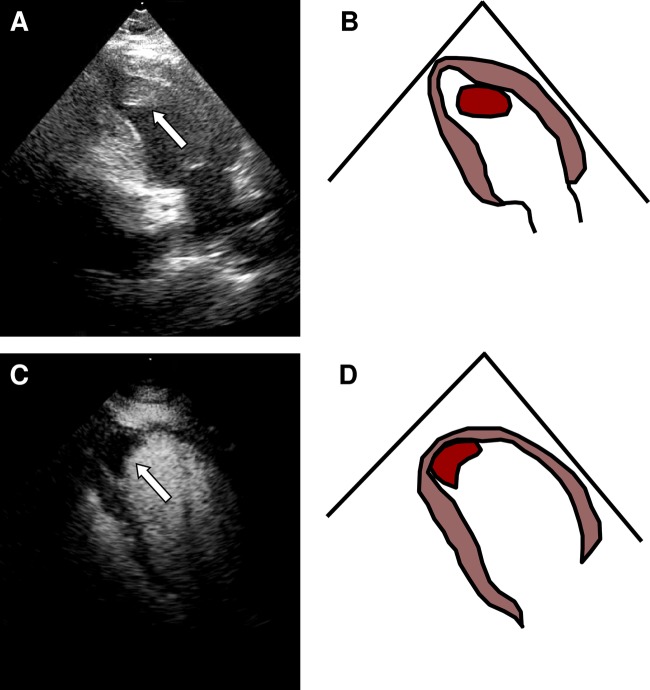
In this man, the left ventricular thrombus was detected incidentally (figure 1). Intracardiac thrombosis is an important but rare cause of mortality and morbidity and our report highlights the possibility that intracardiac thrombosis is underdetected in patients with PV.
Figure 1.
(A–D) Left ventricular apical thrombus (indicate by arrow).
Case presentation
A man from the Middle East, in his late 60s, presented with itching after taking a shower, tinnitus and vertigo for the last 1 year. He denied any symptoms of shortness of breath, headache, chest pain, diplopia or blurry vision. His medications included aspirin 81 mg/day for primary prevention of coronary artery disease and antihistamines as needed for itching. He had history of hypertension and is on treatment with hydrochlorothiazide. He denied any history of coronary artery disease, malignancy or family history. Physical examination was unremarkable except for ruddy complexion and splenomegaly.
Investigations
Complete blood count was repeated here in the USA confirming haemoglobin of 18.1 g/dL and haematocrit of 56.6%. Leucocyte and platelet counts were normal. ECG showed no significant ischaemic changes. Further lab investigations showed low erythropoietin level and JAK2 mutation.
His oxygen saturation was 98% or above on room air. He was initially seen by a haematologist who recommended an echocardiogram to rule out undiagnosed congenital heart disease with right to left shunt. A transthoracic echocardiogram showed normal left ventricular global function with apical dyskinesis and a large left ventricular apical thrombus measuring 3.0 cm×2.0 cm (videos 1 and 2). Ejection fraction was 60%.
Intracardiac thrombosis in polycythemia vera.
Intracardiac thrombosis in polycythemia vera with perflutren lipid microsphere contrast.
PV was subsequently diagnosed on the basis of an increased haemoglobin level, a low serum erythropoietin level, presence of a JAK2 mutation and exclusion of disorders causing secondary erythrocytosis (such as hypoxia, familial polycythemia, high-affinity haemoglobins, truncated erythropoietin receptor or tumour). To evaluate for obstructive coronary artery disease, he had a nuclear stress test that showed an apical infarct but no significant ischaemia.
Treatment
Given his left ventricular thrombus and risk for recurrent thrombosis, he was continued treatment with aspirin 81 mg/day, and added enoxaparin (low-molecular weight heparin (LMWH)) 100 mg subcutaneously twice daily, phlebotomy and hydroxyurea 500 mg daily. Our patient declined Warfarin due to frequent blood draws associated with it; however, the available treatment options were discussed in detail. In addition, he was also started on metoprolol XL 25 mg/day and atorvastatin 40 mg/day to lower his risk of future adverse cardiac events. No complications were noted during his visit.
Outcome and follow-up
No embolic events were noted during his stay. He is scheduled to have an echocardiogram in 3 months to assess for resolution of the left ventricular thrombus.
Discussion
Intracardiac thrombosis is a rarely described manifestation of PV and is more common in patients with cardiac predisposing factors for thrombosis, such as valve disease, prosthetic valve, device lead or cardiomyopathy. Although our patient did not have any such risk factors, in retrospect he had unrecognised apical infarct which might have contributed to the formation of thrombus in the setting of PV.
PV is one of the chronic myeloproliferative disorders, characterised by erythrocytosis and resultant manifestations, predominantly thrombotic events and related complications.1
Multiple factors contribute to increased thrombosis in PV—increased haematocrit, thrombocytosis, leucocytosis, impaired fibrinolytic activity, platelet activation, leucocyte activation, endothelial damage, interactions between platelets and endothelium, increased whole-blood viscosity and various modalities of therapy.2 Cardiovascular manifestations are varied and a major cause of mortality and morbidity in PV.3
One report describes a patient with PV and bicuspid aortic valve who had thrombosis on the aortic valve requiring surgery and bioprosthetic valve placement.4 The same patient had thrombosis on the bioprosthetic aortic valve 18 months later.4 Mechanical mitral valve thrombosis despite therapeutic anticoagulation has been described in a patient with PV, who was treated with antiplatelet, anticoagulant and cytoreductive therapies and phlebotomy for 4 years before succumbing to a fatal recurrence.5 Device leads such as a pacemaker lead can predispose to right atrial thrombosis in these patients.6
Intraventricular thrombosis in either the left or the right ventricle can cause heart failure.7 8 In a report from 1980, massive organised left ventricular thrombus caused refractory heart failure in a patient with PV who died 6 months later despite periodic phlebotomy.7 In a more recent report, massive thrombosis in the right ventricle with pulmonary valvular involvement led to heart failure, necessitating surgical removal of the thrombus, tricuspid valve repair and pulmonary valve replacement.8
Another report describes a patient with PV and a large mobile right atrial thrombus associated with bilateral pulmonary embolism, who was successfully treated with anticoagulation alone.9 Extensive thrombosis of the portal, suprahepatic and inferior vena cava following splenectomy in a patient with PV was associated with a large, free-floating thrombus in the right atrium and pulmonary embolism.10 This patient was successfully treated with anticoagulation, thrombolysis and cytoreductive therapy.10
Intracardiac thrombosis in multiple locations—left ventricle, right ventricle, aortic valve and inferior vena cava—has been described in a patient with PV.11 This patient was successfully treated with thrombolytic therapy.11 Extensive thrombosis within all four cardiac chambers associated with severe cardiac dysfunction was noted during a coronary artery bypass procedure in a patient with PV.12 This was managed intraoperatively by four chamber thrombectomy.12
Thus far, there are no specific clinical guidelines established for management of intracardaic thrombus in PV. However, there are multiple choices available for anticoagulation, which vary by the country and institution. In the USA, the accepted choice for oral therapy are warfarin, dabigatran, rivaroxaban and apixaban and parenteral choices are unfractionated heparin, LMWH administration with varying pros and cons for each. Our patient was treated with aspirin 81 mg/day, enoxaparin (LMWH) 100 mg subcutaneously twice daily, phlebotomy and hydroxyurea 500 mg daily with no complications or embolic events during the visit.
Our patient's hypercoagulable state from PV, in combination with left ventricular apical dysfunction presumably from an unrecognised myocardial infarction, predisposed him to intracardiac thrombus formation. Patients with PV have a high incidence of coronary artery disease and myocardial infarction13 and proposed mechanisms include coronary thrombosis13 and occlusive intimal proliferation.14 While a variety of thrombotic events have been noted to occur in 20–50% of patients with PV,15 intracardiac thrombosis has rarely been reported and is, in our opinion, likely underdetected. Systematic studies are warranted to establish the prevalence of, risk factors and treatment for, intracardiac thrombosis in patients with PV.
Learning points.
Thrombosis is not uncommon with polycythemia vera (PV) however, intracardiac thrombosis is rare.
Risk factors such as valve disease, prosthetic valve, device lead or cardiomyopathy predispose to the development of intracardiac thrombus.
Intracardiac thrombosis is an important cause of mortality and morbidity and this report highlights the possibility that intracardiac thrombosis is underdetected in patients with PV.
Different treatment approaches remain a matter of speculation. The choice of anticoagulation, thrombolysis or surgery will depend on comorbid conditions and risk factors for bleeding and the choice of therapy may vary by country and institution.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood 2002;2013:4272–90 [DOI] [PubMed] [Google Scholar]
- 2.Kwaan HC, Wang J. Hyperviscosity in polycythemia vera and other red cell abnormalities. Semin Thromb Hemost 2003;2013:451–8 [DOI] [PubMed] [Google Scholar]
- 3.Finazzi G, low-dose aspirin in polycythemia (ECLAP). A prospective analysis of thrombotic events in the European collaboration study on low-dose aspirin in polycythemia (ECLAP). Pathol Biol (Paris) 2004;2013:285–8 [DOI] [PubMed] [Google Scholar]
- 4.Webber GV, Fallon J, Silbiger JJ. Hematoma of a congenitally bicuspid aortic valve in a patient with polycythemia vera and the antiphospholipid antibody syndrome. J Am Soc Echocardiogr 2006;2013:1530 e1–3 [DOI] [PubMed] [Google Scholar]
- 5.Das S, Karachiwala H, Cherian SV, et al. Anticoagulant-resistant thrombophilia in a patient with polycythemia vera: a case report. Blood Coagul Fibrinolysis 2011;2013:746–8 [DOI] [PubMed] [Google Scholar]
- 6.Hendler A, Krakover R, Stryjer D, et al. A right atrial mass in the presence of a permanent pacemaker electrode in a patient with polycythemia vera. Pacing Clin Electrophysiol 1991;2013:2083–5 [DOI] [PubMed] [Google Scholar]
- 7.Ali M, Fayemi AO, Malcolm D, et al. Intraventricular thrombosis in polycythemia vera: a cause of intractable cardiac failure. Am Heart J 1980;2013:520–2 [DOI] [PubMed] [Google Scholar]
- 8.Yuan SM, Shinfeld A, Raanani E. Massive intraventricular thrombus in polycythemia vera. J Cardiac Surg 2009;2013:110–12 [DOI] [PubMed] [Google Scholar]
- 9.Panduranga P, Mukhaini M, Saleem M, et al. Mobile right heart thrombus with pulmonary embolism in a patient with polycythemia rubra vera and splanchnic vein thrombosis. Heart Views 2010;2013:16–20 [PMC free article] [PubMed] [Google Scholar]
- 10.Stanziola AA, Padula S, Carpentieri E, et al. Right heart and pulmonary thromboembolism from extensive splanchnic vein thrombosis after splenectomy for myeloproliferative disease. Heart Lung 2012;2013:188–91 [DOI] [PubMed] [Google Scholar]
- 11.Al-Saif S, Bhat RP, Hijazi A, et al. Left ventricular and aortic valve thrombosis caused by polycythemia rubra vera successfully treated with streptokinase. Am Heart J 1996;2013:397–9 [DOI] [PubMed] [Google Scholar]
- 12.Lima B, Soltesz E. Management of extensive intracardiac thombosis in a patient with polycythemia vera undergoing coronary artery bypass grafting. J Cardiac Surg 2012;2013:320–2 [DOI] [PubMed] [Google Scholar]
- 13.Venegoni P, Schroth G. Myocardial infarction and polycythemia vera: how should we treat it? Catheter Cardiovasc Diagnosis 1994;2013:259–61 [DOI] [PubMed] [Google Scholar]
- 14.Hermanns B, Handt S, Kindler J, et al. Coronary vasculopathy in polycythemia vera. Pathol Oncol Res 1998;2013:37–9 [DOI] [PubMed] [Google Scholar]
- 15.Pearson TC. The risk of thrombosis in essential thrombocythemia and polycythemia vera. Semin Oncol 2002;2013(3 Suppl 10):16–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intracardiac thrombosis in polycythemia vera.
Intracardiac thrombosis in polycythemia vera with perflutren lipid microsphere contrast.