Abstract
The use of herbal medications is increasing significantly in the UK and there is a perception that herbal preparations are without adverse effects. This case report highlights the potential risks of black cohosh, which is one of the most commonly used herbal products. This is a case report of a 60-year-old Caucasian lady who presented with subacute liver failure secondary to taking black cohosh. This was further confirmed by liver biopsy and she subsequently deteriorated and underwent liver transplantation. Available evidence supports an association between black cohosh and risk of hepatotoxicity. In current literature, there have only been four previously reported cases of hepatotoxicity associated with black cohosh, which required liver transplantation. We submit that our patient represents the fifth case. We recommend that patients taking this supplement should have close monitoring of their hepatic function, especially in the presence of other risk factors.
Background
The use of herbal medications is increasing significantly in the UK and around the world, and there is a perception that herbal preparations are without adverse effects. I decided to write up this case because it highlights the potential risks of black cohosh, which is one of the most commonly used herbal products. It almost costs the life of a woman who innocently took the product for menopausal symptoms. The Public Assessment Report from the Medicines and Healthcare products Regulatory Agency (MHRA) in 2006 emphasised the importance of informing users of the potential hepatotoxic risk of using black cohosh. However, I feel that the current herbal medications market is not tightly regulated enough and hence can put the general public at risk. By raising awareness and providing accurate information, people will be able to make more informed choices of what they consume over the counter. Educating health professionals will also enable them to be able to offer the right advice to patients.
Case presentation
A 60-year-old Caucasian lady presented initially to her general practitioner (GP) with a 2-day history of pruritis and dark urine. She did not have any symptoms of jaundice, abdominal pain or bleeding. She had no risk factors for acquiring a viral hepatitis; there were no history of tattoos, blood transfusions, intravenous drug use, recent foreign travel or sick contacts. However, she admitted to taking black cohosh for menopausal symptoms 2 weeks prior to the GP visit. She had a medical history of hypothyroidism and migraine which was managed with levothyroxine 100 μg daily and propranolol 20 mg daily. She had no drug allergies and no family history of liver disease. She worked as a receptionist at a GP practice and lived with her husband at home. She was a non-smoker and drank only occasional alcohol. She denied the use of any other herbal or over the counter medication. On presentation, she was deeply jaundiced, but there was no evidence clinically of ascites or encephalopathy.
Investigations
Her blood results at presentation showed bilirubin 474 µmol/L (normal range 3–20), alkaline phosphatase 151 IU/L (normal 30–130), aspartate transaminase 2385 IU/L (normal 10–50) and international normalised ratio 1.57. A comprehensive acute liver failure blood screen was negative including viral serology for hepatitis A, B, C and E, Epstein-Barr, cytomegalovirus, herpes simplex and adenovirus, immunoglobulins, autoantibodies (including antibodies to extractable nuclear antigens). An ultrasound scan of the liver with Dopplers of the hepatic and portal veins was reported as normal and a vascular phase CT of the liver with liver volumetric analysis excluded the presence of malignancy, lymphadenopathy and pre-existing chronic liver disease and portal hypertension. The liver was collapsed down with a liver volume of 1109 cm3 (figure 1). The working diagnosis at this point was subacute liver failure, most likely secondary to black cohosh. She was referred early to a liver transplant unit for further assessment.
Figure 1.
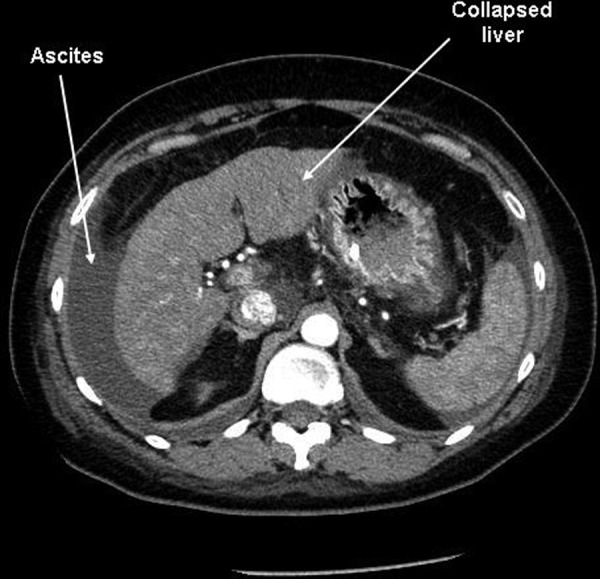
Abdominal CT of patient showing collapsed liver and ascites.
A transjugular liver biopsy was performed which showed confluent multiacinar parenchymal collapse with ductular reaction and non-specific inflammation. Three weeks after admission she deteriorated rapidly, becoming confused with grade 3 hepatic encephalopathy, requiring intensive care admission and intubation. Her arterial ammonia level was 171 μmol/L, compared to 33 μmol/L only a week ago. She was then super-urgently listed for a liver transplant while receiving protocolised management of acute liver failure.1
Figure 2.
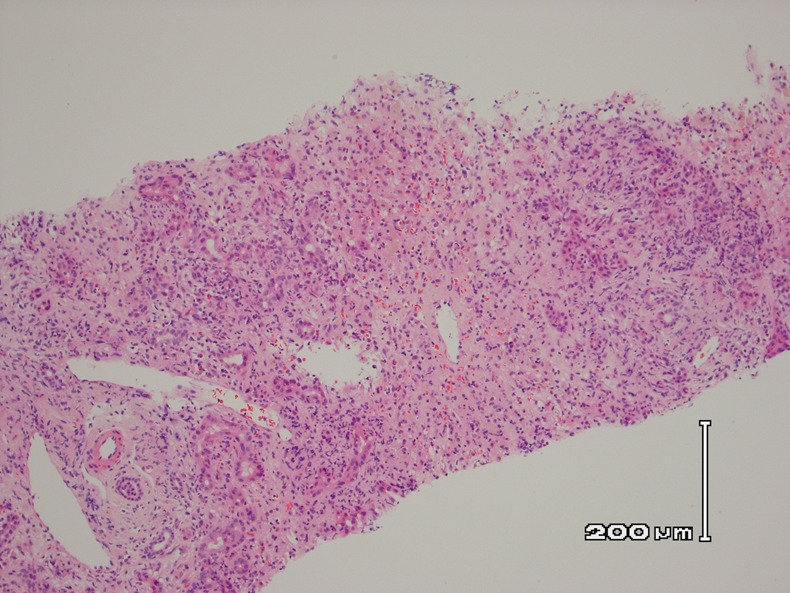
Histology from the liver biopsy showing necrotic inflammation.
Figure 3.
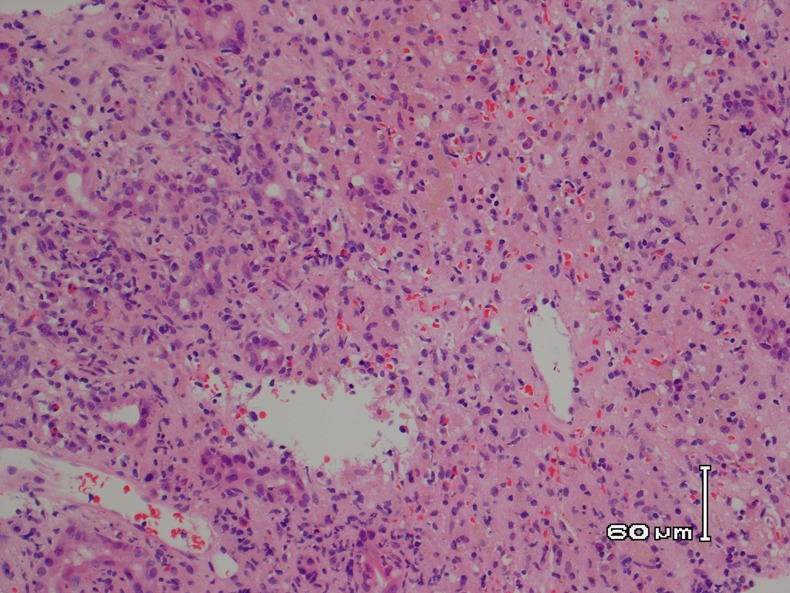
Histology from the liver biopsy showing necrotic inflammation.
Treatment
The patient had an orthotopic liver transplantation 48 h after listing and made a successful recovery. She was discharged from hospital 2 weeks later.
Discussion
Black cohosh (Cimifuga racemosa), a member of the buttercup family, is a perennial plant that is native to North America. It is used traditionally as an herbal remedy for rheumatism, rheumatoid arthritis, intercostal myalgia, sciatica, whooping cough, chorea, tinnitus, dysmenorrhoea and uterine colic. At present, it is most commonly used for menopausal symptoms. Estimates suggest that in the UK, 9 million treatment days for this product were purchased in 2004.2 However, it is difficult to assess fully the extent of black cohosh use in the UK as most herbal medicines reach the consumer as unlicensed herbal remedies.
Published literature suggests that large doses of black cohosh might be associated with gastrointestinal irritation, headache, dizziness and vomiting. The long-term safety of black cohosh is not known. Moreover, there has been a growing concern worldwide about the risk of adverse effects on the liver (hepatotoxicity) associated with use of black cohosh. Since 2002, the Medicine and Healthcare products Regulatory Agency (MHRA) has been monitoring reports of adverse liver reactions with the use of black cohosh. This resulted in the UK Public Assessment Report in 2006 which revealed 21 reports of ‘liver reactions’ up to 31 March, 2006. Using the WHO classification criteria, 14 of these 21 reports were shown to support a relation between black cohosh and liver damage. Of these 14 cases, four had hepatitis, nine had abnormal or raised liver function tests and one had jaundice.
The Herbal Medicines Advisory Committee and the Commission on Human Medicines, which are two panels of independent expert advisors to the MHRA, concluded that the available evidence supports an association between black cohosh and risk of hepatotoxicity. In light of the number of reports of liver disorders and the use of this herbal product in the UK, they considered it important to inform users of black cohosh of this potential risk, and that warnings about rare adverse reactions in the liver should be added to the product information for all black-cohosh products.
As of 13 June 2012, the MHRA has received a total of 34 case reports of hepatic adverse events suspected to be associated with black cohosh including another case of subacute liver failure requiring liver transplantation. The patient took black cohosh for 6 weeks and developed symptoms 2 weeks after stopping treatment and she was subsequently admitted to hospital 4 weeks later. Initially, her liver function test results showed improvements (her alanine transaminase (ALT) decreased from 1119 to 339 over 9 days), but then she dramatically deteriorated and her ALT peaked at 7304, 7 days later. Alternative causes such as alcohol and hepatitis A, B and C were excluded and there was no obvious cause identified for the deterioration while she was hospitalised, approximately 2 months after she had stopped taking black cohosh.
In the literature, there have been four previously reported cases of hepatotoxicity associated with the use of black cohosh, which required liver transplantation.3–5 We submit that our patient represents the fifth case. Using the Council of International Organisations of Medical Sciences (CIOMS) classification system (called Roussel Uclaf Causality Assessment Method (RUCAM)), this case has a score of 6, which suggests probable adverse drug reaction.
Conclusion
The use of herbal medications is a commonplace in the UK; as they are frequently considered ‘natural’ products, there is a perception that herbal preparations are without adverse effects. The Public Assessment Report in 2006 emphasised the importance of informing users of black cohosh of the potential risk of hepatotoxicity. This case report further highlights the potential severity of adverse effect of black cohosh use and we recommend that patients taking this supplement should have close monitoring of their hepatic function, especially in the presence of other risk factors.
Learning points.
Although black cohosh is one of the most commonly used herbal preparations, most users underestimate the risks of this product.
Early referral and discussion with a transplant centre is recommended as this could potentially affect the outcome of patients with acute or subacute liver failure.
Current regulations for herbal medications should be reviewed such that the general public can make more informed choices for their health.
Footnotes
Contributors: TYL had the idea for the article, performed the literature search, wrote the article and is the guarantor. The case was managed under DS, with contribution from VA. AC provided medicine information on black cohosh and was involved with the care of the case. AQ was the histopathologist who reviewed the biopsy results and kindly provided the histology images.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet 2010;2013:190–201 [DOI] [PubMed] [Google Scholar]
- 2.Medicines and Healthcare products Regulatory Agency, Black Cohosh: UK Public Assessment Report. 2008
- 3.Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust 2002;2013:440–3 [DOI] [PubMed] [Google Scholar]
- 4.Lontos S, Jones RM, Angus PW, et al. Acute liver failure associated with the use of herbal preparations containing black cohosh. Med J Aust 2003;2013:390–1 [PubMed] [Google Scholar]
- 5.Levitsky J, Alli TA, Wisecarver J, et al. Fulminant liver failure associated with the use of black cohosh. Dig Dis Sci 2005;2013:538–9 [DOI] [PubMed] [Google Scholar]


