Key Points
rVWF is safe, well tolerated, and has a PK profile generally comparable to pdVWF, but promotes enhanced stabilization of endogenous FVIII.
Abstract
Safety and pharmacokinetics (PK) of recombinant von Willebrand factor (rVWF) combined at a fixed ratio with recombinant factor VIII (rFVIII) were investigated in 32 subjects with type 3 or severe type 1 von Willebrand disease (VWD) in a prospective phase 1, multicenter, randomized clinical trial. rVWF was well tolerated and no thrombotic events, inhibitors, or serious adverse events were observed. The PK of rVWF ristocetin cofactor activity, VWF antigen, and collagen-binding activity were similar to those of the comparator plasma-derived (pd) VWF-pdFVIII. In vivo cleavage of ultra-large molecular-weight rVWF multimers by ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; the endogenous VWF protease) and generation of characteristic satellite bands were demonstrated. In 2 subjects with specific nonneutralizing anti-VWF–binding antibodies already detectable before rVWF infusion, a reduction in VWF multimers and VWF activity was observed. Stabilization of endogenous FVIII was enhanced following post–rVWF-rFVIII infusion as shown by the difference in area under the plasma concentration curve compared with pdVWF-pdFVIII (AUC0-∞) (P < .01). These data support the concept of administering rVWF alone once a therapeutic level of endogenous FVIII is achieved. This trial was registered at www.clinicaltrials.gov as #NCT00816660.
Introduction
von Willebrand disease (VWD) is an inherited bleeding disorder caused by a deficiency or dysfunction of the largest soluble multimeric plasma glycoprotein, von Willebrand factor (VWF), which is encoded by a gene spanning 178 kb of genomic DNA on chromosome 12.1,2 VWF has 2 main functions in hemostasis. As an adhesion protein it captures platelets at sites of vascular injury, and it also stabilizes factor VIII (FVIII) through formation of a noncovalent VWF-FVIII complex.3,4 This dual role is reflected in the commonly encountered clinical manifestations of VWD, including mucocutaneous hemorrhages (epistaxis, easy bruising, gynecologic and gastrointestinal bleeding), excessive bleeding after major surgery, and hemarthroses in severely affected patients.
Current therapeutic strategies to prevent or control bleeding in VWD patients involve either replacement of VWF with human plasma-derived (pd) coagulation factor concentrates containing both FVIII and VWF (pdVWF-pdFVIII), elevation of the FVIII and VWF plasma concentrations through the release of endogenous VWF from endothelial cells with desmopressin, or use of adjuvant agents that promote local hemostasis and wound healing without altering the plasma concentration of VWF. Treatment options depend on the type and severity of VWD, as well as the intensity of the hemostatic challenge.5
Replacement therapy is typically required for clinically significant bleeding events and for providing hemostatic coverage for major surgery in patients who have severe quantitative (types 1 or 3) or qualitative (type 2) VWF deficiencies and are unresponsive or intolerant to desmopressin.6 Infusion of sufficient exogenous VWF promotes an increase in endogenous FVIII to hemostatic levels.7,8 Human pdVWF-pdFVIII products are commonly used to achieve rapid normalization of both VWF and FVIII required in the treatment of acute hemorrhage or prior to urgent surgery.5,9
Plasma-derived FVIII concentrates containing VWF have inherent limitations, including a lack of the multimers of larger molecular weight normally found in plasma, considerable variation in the VWF multimer composition, and a wide range of VWF:FVIII ratios among lots of the same product. VWF produced by recombinant technology could offer a new perspective in the treatment of VWD by eliminating risks that may be associated with products derived from human plasma while maintaining efficacy and product consistency.5,10
A recombinant human VWF (rVWF) has been developed in a genetically engineered Chinese hamster ovary (CHO) cell line that coexpresses VWF and FVIII genes.11 The highly pure (>99% purity) rVWF product has a homogeneous and intact VWF multimer distribution because it is not exposed throughout manufacturing to the VWF protease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). It has been postulated that this consistent VWF multimer distribution composition may promote a more predictable therapeutic effect than seen with plasma-derived products.10
In this study, we investigated the safety and tolerability of the novel rVWF at a fixed ratio with rFVIII compared with a marketed pdVWF-pdFVIII concentrate.
Materials and methods
Design
This was a prospective, controlled, randomized, first-in-human clinical study of the safety, tolerability, and pharmacokinetics (PK) of rVWF combined at a fixed ratio (1.3:1) with rFVIII in subjects with severe VWD. The trial was conducted in accordance with the Declaration of Helsinki and good clinical practice and approved by the responsible ethics committees and institutional review boards.
A 1.3:1 ratio (VWF:ristocetin cofactor activity [RCo] to FVIII coagulant activity (FVIII:C) was chosen for rVWF-rFVIII based on published recoveries of ∼1.9 international unit (IU)/dL per IU per kilogram for plasma-derived (pd) VWF and 2.4 IU/dL for pdFVIII, respectively, with the intent to correct both activities to ∼100% with an initial rVWF-rFVIII dose of 50 IU/kg VWF:RCo.12-14 Dosing for pdVWF-pdFVIII was based on published reports on the licensed plasma-derived complex Humate-P/Haemate P (CSL Behring), confirming reliance on the average ratio of VWF:RCo to FVIII:C which is ∼2:1 pdVWF:pdFVIII.
Initial cohorts received either 2, 7.5, or 20 IU/kg VWF:RCo. After the safety and tolerability of the lower doses was established, the 50 IU/kg VWF:RCo dose was administered to subjects in the PK cohort.
The PK cohort included a randomized,15 blinded dual-crossover design, in which subjects received a single dose each of rVWF-rFVIII (50 IU/kg VWF:RCo and 38.5 IU/kg FVIII:C) and pdVWF-pdFVIII (50 IU/kg VWF:RCo and 25 IU/kg FVIII:C) in random order (8-day washout).
Patients
Male and female adults with hereditary type 3 VWD (≤3 IU/dL VWF:antigen [Ag]) or severe type 1 or type 2A VWD (VWF:RCo ≤10% and FVIII:C <20%), a Karnofsky16 score of ≥70% (to maintain treatment compliance throughout the duration of the trial), and previous multiple exposures to pdVWF-pdFVIII concentrates were eligible. In accordance with the Helsinki protocol, each patient gave written informed consent prior to enrollment.
Treatments
rVWF is a purified glycoprotein synthesized by the genetically engineered CHO cell line that expresses both rFVIII and rVWF.11 The cell-culture and purification processes used in the manufacture of rVWF do not use any additives of human or animal origin. rVWF, which is not yet licensed, and rFVIII (ADVATE; Baxter) were reconstituted separately and the components mixed prior to infusion. The pdVWF-pdFVIII (Humate-P/Haemate P; CSL Behring) used as a comparator is purified from the cold-insoluble fraction of pooled human fresh-frozen plasma and is considered an intermediate-purity FVIII concentrate containing VWF. Humate-P/Haemate P was selected as the comparator as it is the only commercially available pdVWF-pdFVIII product licensed for treatment of VWD in all countries in which the study was conducted.
Safety assessments
Subjects were under continued surveillance in hospital for the first 24 hours postinfusion. Safety was evaluated through clinical assessments, hematology, serum chemistry, urinalysis, coagulation assessments, antibodies against VWF, FVIII, CHO cell protein, murine immunoglobulin G (IgG), and recombinant furin; viral serology, cardiac troponin, soluble P-selectin (sP-selectin), and D-dimers. Adverse events (AEs) were recorded by the subject in a diary until 30 days postinfusion. Patients were also monitored for the occurrence of thrombotic thrombocytopenic purpura (TTP)–like events.
Hemostasis assays
VWF:RCo activity was measured with the automated VWF:RCo activity assay (Dade Behring Coagulation System). The detection limit of the assay was extended to 8 IU/dL by increasing the sample plasma volume and changing the dilution media from NaCl to severe VWF-deficient plasma. A semiquantitative manual assay (Siemens) was applied to quantify VWF:RCo activities below 8 IU/dL.17 VWF:Ag was assessed using a sandwich enzyme-linked immunosorbent assay (ELISA) using polyclonal anti-human VWF antibodies (Diagnostica Stago). A commercial ELISA (Technozym VWF:CB ELISA; Technoclone) was used to determine VWF collagen-binding activity (VWF:CB). FVIII activity was measured with an activated partial thromboplastin time (APTT)–based 1-stage clotting assay. The detection limit for the VWF:Ag, VWF:CB, and FVIII assays was 1 IU/dL. The VWF:FVIII-binding capacity of FVIII to VWF relative to normal human plasma was tested on 2 rVWF clinical trial lots (87% and 108%, respectively) using an ELISA-based assay.
ADAMTS13 assay
Endogenous ADAMTS13 activity was calculated by analysis of fluorescence over time using the synthetic fluorogenic VWF73 peptide as substrate. VWF73 was identified as the 73-amino acid minimal region of the VWF A2 domain required for ADAMTS13 cleavage.18
Electrophoretic analysis
Qualitative analysis of VWF multimers was performed using sodium dodecyl sulfate (SDS) agarose gel electrophoresis followed by western blotting and sensitive luminescence 2-step detection as previously described.11 VWF degradation products generated by ADAMTS13-mediated cleavage were measured by SDS–polyacrylamide gel electrophoresis (PAGE) under reducing conditions followed by western blotting and immunostaining with a horseradish peroxidase (HRP)–labeled polyclonal rabbit anti-human VWF antibody with enhanced chemiluminescence detection.
Neutralizing antibodies inhibiting FVIII and VWF
Neutralizing antibodies to VWF:RCo, VWF:CB, and FVIII-binding (VWF:FVIIIB) activities were measured by assays based on the Nijmegen modification of the Bethesda assay established for quantitative analysis of FVIII inhibitors.19 The screening test for inhibitors was performed on a mixture of patient and normal plasma by incubation for 2 hours at 37°. The detection limit for anti-VWF inhibitors was set to 1 Bethesda unit/mL for all 3 assays, based on their respective performance characteristics.
Nonneutralizing anti-VWF–binding antibodies
The presence of nonneutralizing anti-VWF–binding antibodies was determined with an ELISA using polyclonal anti-human Ig antibodies (IgG, IgM, and IgA) as described for FVIII.20 Binding antibodies were analyzed in 2 steps: antibody screening followed by confirmation of specificity in a competition assay for samples with a titer of 1:80 or higher.
Genetic analyses
Mutations of the VWF gene were analyzed using nucleotide numbering, amino acid numbering, and nomenclature of mutations according to previous recommendations.21,22 High-molecular-weight genomic DNA was used for the amplification of VWF coding exons 2 through 52 by polymerase chain reaction (PCR) as previously described.23 Candidate mutations were confirmed by sequencing both strands.
Pharmacokinetic assessments
PK parameters, area under the concentration–time curve (AUC [hours × units per deciliter]), plasma half-life (T1/2 [hours]), clearance (Cl [milliliters per kilogram per hour]), mean residence time (MRT [hours]), volume of distribution at steady state (Vss [deciliters per kilogram]), maximum plasma concentration (Cmax [units per deciliter]), and incremental recovery (IR [units per deciliter]/[units of VWF:RCo per kilogram] for VWF); [units per deciliter]/[units of FVIII:C per kilogram] for FVIII). were assessed for VWF:RCo, VWF:Ag, VWF:CB, and FVIII:C at 60 and 15 minutes preinfusion; 15, 30, 60 minutes and 1, 3, 6, 9, 12, 24, 28, 32, 48, 72, and 96 hours postinfusion.
Statistics
VWF:RCo, VWF:Ag, VWF:CB, and FVIII:C levels over time, as well as PK parameters and plasma half-life (calculated from the biphasic log‐linear model) were summarized using descriptive statistics. The total AUC0‐∞ was calculated by the linear trapezoidal rule up to the last quantifiable concentration with a tail area correction. Terminal T1/2 was determined from the terminal rate constant obtained by log-linear fitting by the least squares deviation criterion to the last 5 quantifiable concentrations above preinfusion level. MRT was calculated as total area under the moment curve divided by the total AUC corrected for the duration of the infusion. Systemic Cl was calculated as dose per body mass (kilogram) divided by the AUC0‐∞. IR was calculated as Cmax divided by dose per body mass. Descriptive statistics were also used to assess safety in terms of product-related AEs.
Results
Thirty-three unique subjects were allocated to treatment. Thirteen were treated in a lower-dose cohort, of whom 6 continued in the crossover cohort; an additional 20 were enrolled in the crossover cohort only. All 26 subjects were randomized, 1 of whom did not receive treatment; therefore, 25 subjects were treated in the crossover cohort (22 with type 3 VWD and 3 with severe type 1). Three of these 25 subjects withdrew after treatment. Of the remaining 22 subjects (Table 1), 3 who had a positive binding antibody titer prior to rVWF-rFVIII treatment were excluded from the PK analysis. Safety analyses are presented for all 32 unique subjects who were treated with rVWF-rFVIII (Table 2).
Table 1.
Baseline subject characteristics
| Subject | Gender | Age, y | VWD gene mutation | Mutation location exon/domain | |
|---|---|---|---|---|---|
| Nucleotide substitution | Amino acid substitution | ||||
| 1 | M | 27 | IVS4-7del7bp_ins13bp/c.2438dupG | splice/p.M814HfsX5 | 18/D′ |
| 2*† | F | 45 | c.7603C>T/c.7603C>T | p.R2535X/p.R2535X | 45/C2 |
| 3 | M | 38 | c.311_312delAG/c.2771G>A | p.Q104RfsX19/p.R924Q | 5/D1; 21/D3 |
| 4 | M | 26 | c.2879G>C/c.7352G>A | p.R960P/p.C2451Y | 21/D3; 43/C1 |
| 5*‡ | M | 47 | c.1147G>T/c.1147G>T | p.E383X/p.E383X | 11/D2 |
| 6 | M | 57 | c.1147G>T/c.1147G>T | p.E383X/p.E383X | 11/D2 |
| 7 | F | 60 | c.8419_8422dupTCCC/c.8419_8422dupTCCC | p.P2808LfsX24/p.P2808LfsX24 | 52/CK |
| 8 | M | 19 | c.8419_8422dupTCCC/c.8419_8422dupTCCC | p.P2808LfsX24/p.P2808LfsX24 | 52/CK |
| 9 | F | 18 | c.1130G>A/c.2516delG | p.W377X/p.G839EfsX4 | 10/D1 |
| 10 | M | 52 | c.2435delC/c.2435delC | p.P812RfsX31/p.P812RfsX31 | 18/D′ |
| 11 | M | 39 | c.1047delC/c.2900G>A/c.8322C>G | p.C350AfsX107/p.G967D/p.C2774W | 10/D1; 21/D3; 52/CK |
| 12 | M | 26 | c.2157delA/IVS45+7C>T | p.D720TfsX21/splice | 16/D2 |
| 13 | F | 37 | c.7085G>T/c.7603C>T | p.C2362F/p.R2535X | 42/C1; 45/C2 |
| 14 | F | 34 | c.1751 C>T + 729_735OLEE | p.C584F + 1366X | 15/D2 |
| 15 | M | 38 | IVS40+1G>T/c.1147G>T | splice/p.E383X | 10/D1 |
| 16 | F | 21 | 1VS 13 + 1G>T/IVS13 +1 G>T | splice/splice | — |
| 17 | F | 32 | c. 6222insAACC/c.6911G>A | NA/p.C2304Y | 36/D4; 40/B1-2 |
| 18 | M | 26 | c.2435delC/IVS28N+3AX | p.812RfsX31/splice | 18/D′ |
| 19 | F | 46 | c.2435delC/c.2435delC | p.P812RfsX31/p.P812RfsX31 | 18/D′ |
| 20 | F | 28 | c.2753C>G | p.S918X | 21/D3 |
| 21*§ | F | 23 | c.8419_8422dupTCCC/c.8419_8422dupTCCC | p.P2808LfsX24/p.P2808LfsX24 | 52/CK |
| 22*‡ | F | 22 | c.8241_8249del9bp/c.8241_8249del9bp | p.2748delHisTyrCys/p.2748delHisTyrCys | 52/CK |
| 23*§|| | M | 19 | c.6790C>T/c.6790C>T | p.Q2264X/p.Q2264X | 38/D4 |
Subjects had nonneutralizing anti-VWF–binding antibody titer while on treatment.
Subject had a specific nonneutralizing anti-VWF–binding antibody titer (1/640) at termination, following treatment with pdVWF-pfFVIII.
Subjects had an unspecific titer (<1:40) for nonneutralizing anti-VWF–binding antibodies (only a titer of 1:80 or above could be confirmed in the competition assay).
Subjects had a preexisting, specific nonneutralizing anti-VWF–binding high antibody titer (1/1280) at screening.
All subjects shown in this table, except #23, had VWD type 3 and were in the randomized crossover PK cohort. Subject 23 had VWD type 1 and received rVWF-rFVIII with 20 IU/kg VWF:RCo; however, baseline characteristics are of interest for this subject due to his preexisting anti-VWF–binding antibodies.
Table 2.
Summary of demographic characteristics
| Dose group* | VWD type† | N | Gender, F/M | Median age, y (range) | Median weight, kg (range) |
|---|---|---|---|---|---|
| 50 IU (PK crossover) | Type 3 | 22‡ | 11/11 | 33 (18-60) | 74.8 (43.8-154.4) |
| Severe type 1 | 3 | 2/1 | 25 (19-47) | 82.1 (70.4-130.4) | |
| 20 IU | Type 3 | 5 | 0/5 | 34 (19-55) | 79 (64-131.4) |
| 7.5 IU | Type 3 | 5 | 2/3 | 26 (21-39) | 86.2 (52-135.4) |
| 2 IU | Type 3 | 3 | 1/2 | 38 (26-44) | 130 (101.5-144.8) |
| Total | 32 | 15/17 | 33 (18-60) | 81.1 (43.8-144.8) | |
Subjects in the 50 IU PK crossover group received 50 IU/kg VWF:RCo + 38.5 IU/kg rFVIII and 21-25 IU/kg pdFVIII in random order with an 8-day washout period. Subjects in the 20 IU group received 20 IU/kg VWF:RCo + 15.4 IU/kg rFVIII; those in the 7.5 IU received 7.5 IU/kg VWF:RCo + 5.8 IU/kg FVIII; and those in 2 IU received 2 IU/kg VWF:RCo + 1.5 IU/kg FVIII.
Type 3 VWD: ≤3 IU/dL VWF:Ag; severe type 1 VWD: <10% VWF:RCo; and <20% FVIII.
Six subjects in the PK crossover dose group were also enrolled once in a lower-dose group.
VWF PK
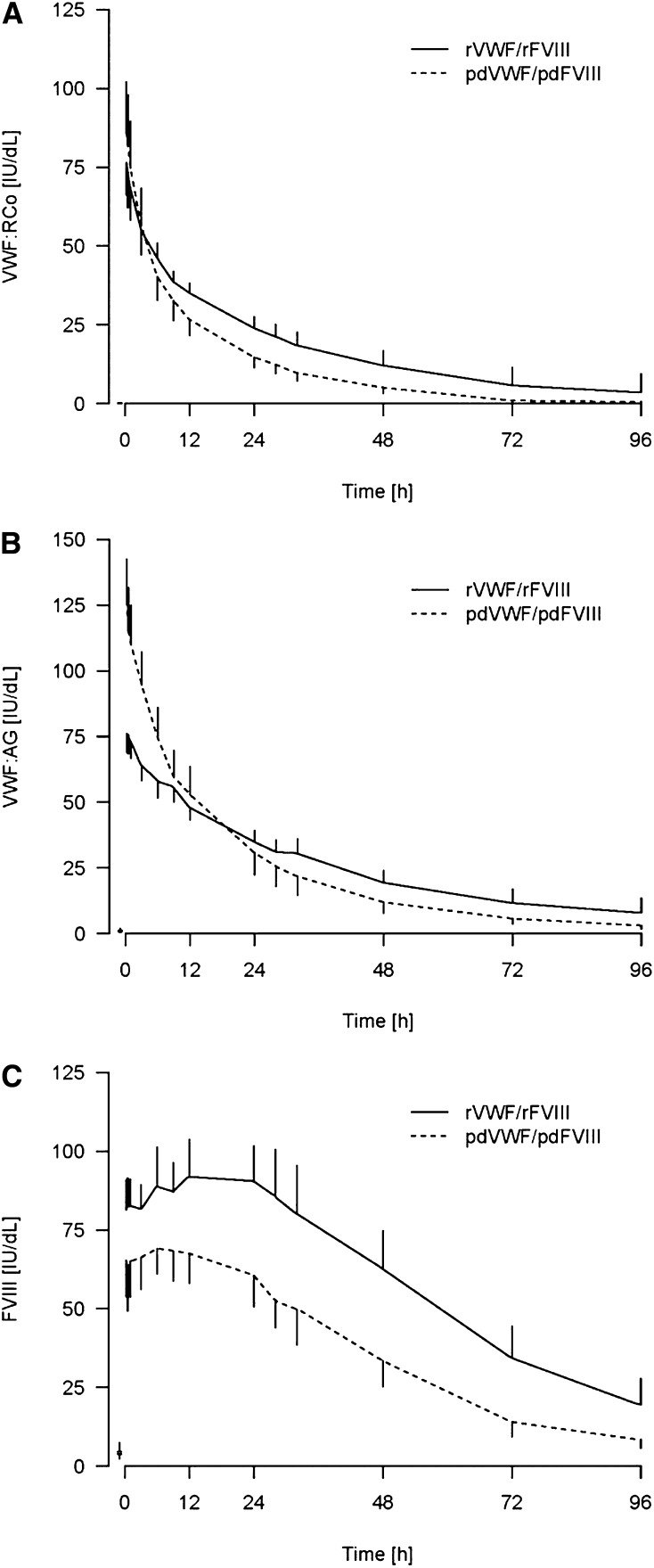
A high level of rVWF activity was observed after infusion (Figure 1A). Maximum VWF:RCo levels were reached in most subjects in <1 hour after infusion. VWF:Ag levels were initially higher with pdVWF-pdFVIII than with rVWF-rFVIII, implying a higher specific activity for the rVWF as the products were dosed at equivalent VWF:RCo activities. However, between 12 and 24 hours, mean VWF:Ag levels with pdVWF-pdFVIII declined to below those of rVWF-rFVIII (Figure 1B). A trend toward a slower clearance for rVWF-rFVIII was observed (Table 3); the mean clearance was 2.2 (2.5) mL/kg per hour for rVWF-rFVIII vs 3.4 (0.90) mL/kg per hour for pdVWF-pdFVIII (P = .09). A longer VWF:Ag T1/2 was also observed with rVWF-rFVIII (25.5 [6.7] hours) than with pdVWF-pdFVIII (17.9 [3.5] hours). The increased specific activity of rVWF as compared with pdVWF was also substantiated by the longer mean VWF:CB T1/2 with rVWF:rFVIII (24.4 hours) compared with VWF:CB T1/2 (16.4 hours) with pdVWF-pdFVIII (calculated based on VWF:RCo dose).
Figure 1.
VWF:RCo, VWF:Ag, and FVIII:C over time after administration of rVWF-rFVIII vs pdVWF-pdFVIII.
Table 3.
Summary of PK analysis in patients with type 3 VWD
| Parameter | N* | Mean (SD) | ||
|---|---|---|---|---|
| Product | VWF:RCo | VWF:Ag | FVIII:C | |
| AUC0-∞ | ||||
| rVWF-rFVIII | 17 | 1459.0 (475.3) | 2278.8 (714.8) | 5343.5 (2519.9) |
| pdVWF-pdFVIII | 15 | 1164.6 (527.4) | 2254.6 (1065.3) | 3312.1 (1414.6) |
| Cmax | ||||
| rVWF-rFVIII | 17 | 79.0 (22.0) | 80.0 (13.7) | 94.8 (24.0) |
| pdVWF-pdFVIII | 15 | 88.5 (32.8) | 126.7 (32.3) | 70.7 (17.0) |
| Tmax | ||||
| rVWF-rFVIII | 17 | 0.8 (0.6) | 1.4 (2.0) | 15.7 (11.4) |
| pdVWF-pdFVIII | 15 | 0.6 (0.1) | 0.6 (0.1) | 8.8 (8.9) |
| IR (in vivo) | ||||
| rVWF-rFVIII | 17 | 1.7 (0.7) | 2.2 (0.7) | 2.3 (0.6) |
| pdVWF-pdFVIII | 15 | 1.6 (0.6) | 1.9 (0.4) | 2.9 (0.8) |
| MRT | ||||
| rVWF-rFVIII | 17 | 23.6 (9.7) | 33.4 (11.4) | 38.5 (13.0) |
| pdVWF-pdFVIII | 15 | 18.8 (6.1) | 22.1 (5.5) | 32.58 (7.58) |
| Noncompartmental half-life | ||||
| rVWF-rFVIII | 17 | 16.4 (6.7) | 23.2 (7.9) | 26.7 (9.0) |
| pdVWF-pdFVIII | 15 | 13.0 (4.2) | 15.3 (3.8) | 22.6 (5.3) |
| T1/2 | ||||
| rVWF-rFVIII | 16 | 16.3 (7.1) | 25.5 (6.7) | — |
| pdVWF-pdFVIII | 15 | 14.4 (6.7) | 17.9 (3.5) | — |
| Cl | ||||
| rVWF-rFVIII | 17 | 4.1 (3.1) | 2.2 (2.5) | 2.6 (7.5) |
| pdVWF-pdFVIII | 15 | 5.2 (1.5) | 3.4 (0.9) | 0.9 (0.4) |
| AUC per dose-kg | ||||
| rVWF-rFVIII | 17 | 31.3 (13.4) | 38.1 (15.4) | 126.7 (193.3) |
| pdVWF-pdFVIII | 15 | 20.5 (5.6) | 49.9 (17.6) | 27.1 (10.6) |
| Vss | ||||
| rVWF-rFVIII | 17 | 75.9 (22.0) | 55.4 (17.7) | 32.6 (9.7) |
| pdVWF-pdFVIII | 15 | 96.3 (35.4) | 71.3 (15.6) | 27.4 (8.4) |
| Dose-kg | ||||
| rVWF-rFVIII | 17 | 47.2 (7.5) | 60.2 (9.6) | 5.8 (17.7) |
| pdVWF-pdFVIII | 15 | 56.4 (18.6) | 46.6 (19.3) | 125.6 (49.0) |
Subjects were treated in random order with a single 50 IU of VWF:RCo/kg infusion each of rVWF-rFVIII and pdVWF-pdFVIII with an 8-day washout period.
AUC0-∞, area under the plasma concentration curve from zero to infinity (h × U/dL); T1/2, half-life (hours); MRT, mean residence time (hours); Cl, clearance (mL/kg per hours); Vss, volume at a steady state (dL/kg); Cmax, maximum concentration (U/dL); Tmax, time to maximum concentration (hours); IR, incremental recovery ([U/dL]/[U VWF: RCo/kg] for VWF); ([U/dL]/[U FVIII: RCo/kg] for FVIII); AUC per dose/kg measured in h × U/dL/(U/kg); dose/kg in U/kg.
The PK analysis population consists of all subjects in the randomized crossover cohort who completed treatment with both the recombinant and plasma-derived products in the assigned order. Subjects with a nonneutralizing anti-VWF–binding antibody titer prior to rVWF-rFVIII treatment (subjects 5, 21, and 22) were excluded from the PK analysis.
FVIII PK
rVWF-rFVIII at a dose of 50 IU of VWF:RCo/kg was associated with a higher degree of sustained FVIII:C than with pdVWF-pdFVIII, as demonstrated by the difference in FVIII AUC0-∞ (P < .01). The mean peak FVIII:C level (Cmax) postinfusion of rVWF-rFVIII was 94.8 IU/dL vs 70.7 IU/dL with pdVWF-pdFVIII (Table 3), reflecting differences in the quantity of FVIII that was initially infused. However, there was a greater secondary rise in endogenous FVIII with rVWF-rFVIII than with the pdVWF-pdFVIII concentrate (Figure 1C); FVIII:C was significantly higher (P < .01) at 72 hours after administration with rVWF-rFVIII than with pdVWF-pdFVIII.
Effect of nonneutralizing anti-VWF–binding antibodies on VWF kinetics
Four of 39 VWD patients (10%) screened had preexisting nonneutralizing binding antibodies against VWF measured by ELISA. Incidentally, 1 of these 4 subjects also had neutralizing antibodies against VWF:CB at screening and was excluded from the study per protocol exclusion criterion. Of the 3 subjects with preexisting binding antibodies who received treatment, 2 (#21, #23) had a specific high titer (1:1280), and 1 (#22) had a low titer of 1/20 in the screening assay, which could not be confirmed as specific as it is below the detection limit of the method.20 The nonneutralizing anti-VWF–binding antibody titer identified in subject 21 increased by 2 titration steps postinfusion of pdVWF-pdFVIII and an increase by 1 titration step was observed postinfusion of rVWF-rFVIII in subject 23 (Table 4).
Table 4.
Antibody titers in subjects with preexisting high specific binding anti-VWF antibodies
| Subject | Gender | Age, y | Binding anti-VWF Ab titer | ||
|---|---|---|---|---|---|
| Screening | Preinfusion 2 | Study termination | |||
| 21 | F | 23 | 1:1280 | 1:5120* | 1:5120 |
| 23 | M | 19 | 1:1280 | NA† | 1:2560 |
Postinfusion with pdVWF-pdFVII and preinfusion with rVWF-rFVIII.
Subject 23 received only rVWF-rFVIII (not enrolled in the crossover cohort).
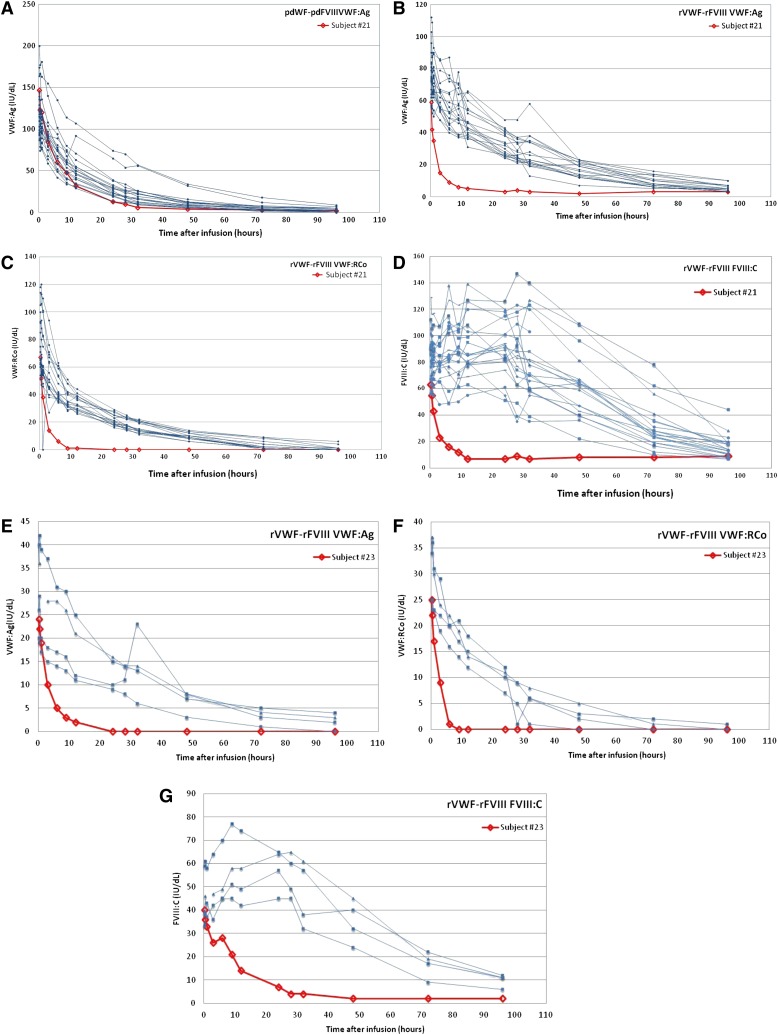
A high titer of nonneutralizing anti-VWF–binding antibodies was associated with a lower postinfusion VWF:Ag activity and concomitant reductions in VWF:RCo, VWF:CB, and FVIII:C activities (Figure 2). The PK of VWF:Ag, VWF:RCo, VWF:CB, and FVIII in subject 23 were similar to those observed for subject 21; however, it should be considered that subject 23 received a lower dose of VWF:RCo (20 IU of VWF:RCo/kg).
Figure 2.
PK for subjects with specific pretreatment nonneutralizing anti-VWF–binding antibodies. Subject #21: (A) VWF:Ag after pdVWF-FVIII infusion, (B) VWF:Ag after rVWF-rFVIII infusion, (C) VWF:RCo after rVWF-rFVIII infusion, and (D) FVIII:C after rVWF-rFVIII infusion. Subject #23: (E) VWF:Ag after rVWF-rFVIII infusion, (F) VWF:RCo after rVWF-rFVIII infusion, and (G) FVIII:C after rVWF-rFVIII infusion.
The 2 subjects (#21 and #23) who had high-titer specific pretreatment nonneutralizing anti-VWF–binding antibodies were evaluated for VWF mutations. One of these subjects (#23) expressed a nonsense substitution of cytosine by thymidine (c.6790C>T) in the VWF cDNA sequence resulting in a glutamine residue substitution (p.Q2264X) in the D4 domain. This type of mutation was not identified in other subjects evaluated in this study. A duplication (c.8419_8422dupTCCC) which induced substitution of a proline residue (p.P2808LfsX24) in the CK domain was identified in the second subject (#21) but was also observed in 2 other subjects who had no detectable anti-VWF–binding antibodies.
Multimeric pattern of rVWF following infusion
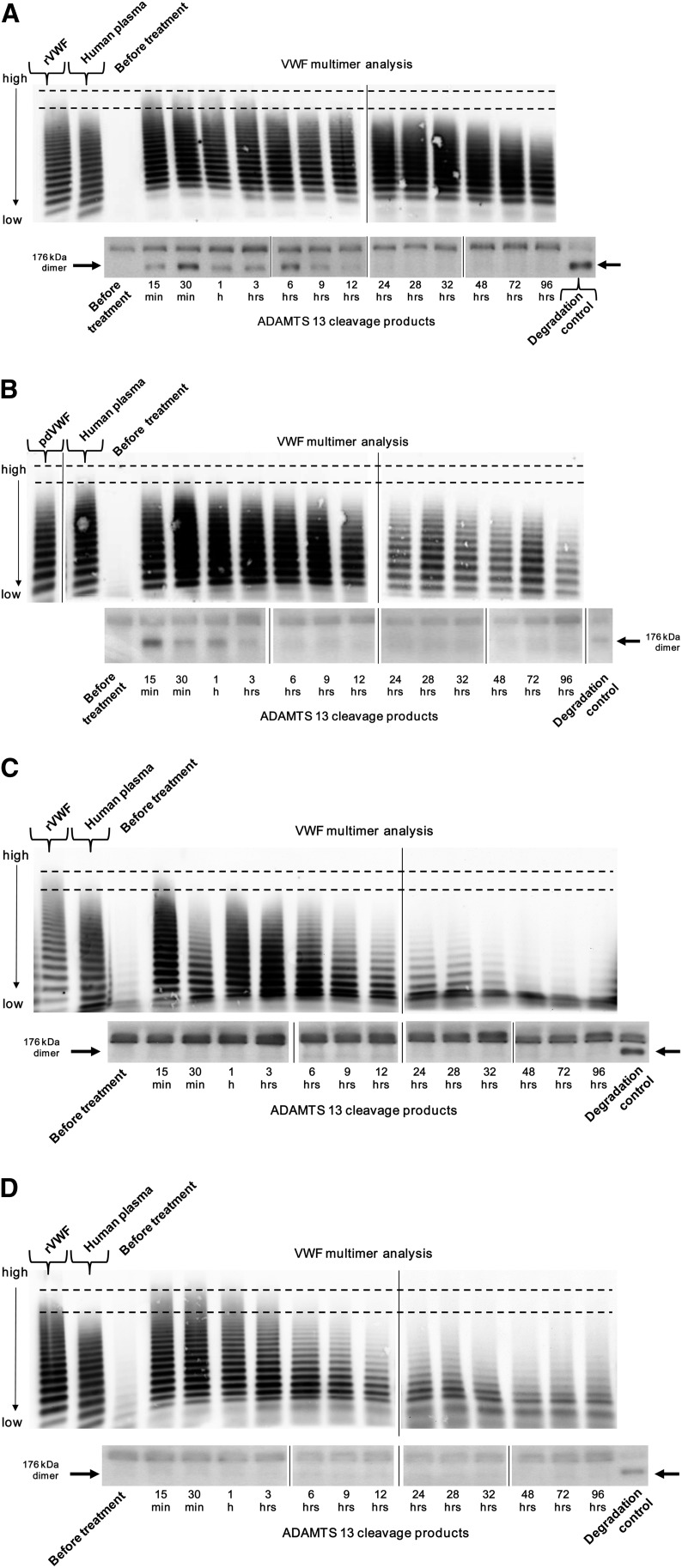
Postinfusion cleavage of VWF subunits by endogenous ADAMTS13 was analyzed semiquantitatively by observing the kinetics of the 176-kDa cleavage products. Most subjects had normal or elevated levels of endogenous ADAMTS13 activity at screening (range: 89% to 244% in type 3 patients; 134% to 156% in type 1 patients). As expected, the appearance of rVWF fragments cleaved by ADAMTS13 was associated with a progressive decrease in VWF multimeric size. Cleavage products were detected in 21 of 22 type 3 VWD patients treated with rVWF-rFVIII in the crossover cohort, with patterns comparable to those observed with pdVWF-pdFVIII. Enzymatic degradation was rapid, as indicated by the appearance of typical VWF cleavage products 15 minutes postinfusion, which was the earliest time point examined (Figure 3). A faster loss of high-molecular-weight VWF multimers was observed in the 2 subjects (#21 and #23) with high specific pretreatment binding antibody titers (Figure 3). Subunit cleavage could not be documented after infusion of rVWF in 1 subject (#21), who had nonneutralizing anti-VWF–binding antibodies (1:1280) pretreatment.
Figure 3.
VWF multimers and degradation products postinfusion of rVWF-rFVIII. Dotted horizontal lines indicate the zone where bands of ULMW multimers appear. Due to limitations in space, it was not possible to run all samples taken from a single patient during the PK study on 1 gel. Therefore, to show the distribution of multimers and fragments over time, it was necessary to combine different gels into 1 picture. Vertical lines have been inserted to indicate repositioned gel lanes. (A) Typical VWF multimer pattern (upper gels; low-resolution agarose [1% Seakem]; samples adjusted to VWF:Ag content) and fragments cleaved by ADAMTS13 (lower gels; SDS-PAGE followed by immunoblot with polyclonal anti-VWF antibody; samples were applied undiluted) postinfusion of rVWF-rFVIII. (B) Typical VWF multimer pattern (upper gels; low-resolution agarose [1% Seakem]; samples adjusted to VWF:Ag content) and fragments cleaved by ADAMTS13 (lower gels; patient plasma diluted to 0.05 VWF:Ag U/mL with VWD plasma; 0.025 mU VWF:Ag per lane applied) postinfusion of pdVWF-pdFVIII. (C) Subject #21, with specific pretreatment nonneutralizing anti-VWF–binding antibodies (upper gels: low-resolution agarose [1% Seakem]; samples adjusted to VWF:Ag content; lower gels: SDS-PAGE followed by immunoblot with polyclonal anti-VWF antibody; samples were applied undiluted). (D) Subject #23, with specific pretreatment nonneutralizing anti-VWF–binding antibodies.
Safety
Results of the safety evaluation indicate that rVWF-rFVIII is well tolerated in patients with VWD. No inhibitors to VWF or FVIII were detected after any of the treatments with rVWF-rFVIII or pdVWF-pdFVIII and no patients experienced anaphylaxis. There were no reported signs or symptoms indicative of thrombosis, as determined by examination of clinical and subclinical parameters. No serious adverse reactions occurred after either treatment type.
The rates of related AEs (number of AEs/total number of subjects) in subjects with type 3 VWD treated with 50 IU of VWF:RCo IU/kg were 0.24 (rVWF-rFVIII) vs 0.18 (pdVWF-pdFVIII). Eight nonserious AEs associated with rVWF-rFVIII administration (tremor, pruritis, psychomotor hyperactivity, hypertension, nausea, variable sP-selectin values, and dizziness) occurred in 4 subjects; all adverse reactions were mild and occurred within 72 hours postinfusion. Symptoms of AEs associated with pdVWF-pdFVIII were headache and dizziness; a positive nonneutralizing anti-VWF–binding antibody titer after administration of pdVWF-pdFVIII was also reported by the investigator as an AE; however, the presence of these antibodies could not be verified in the confirmatory assay.
Discussion
VWF produced by recombinant technology could offer a novel option for the treatment of VWD by eliminating risks associated with products derived from human plasma.5,10 In our prospective, randomized clinical trial we investigated the safety, tolerability, and PK of rVWF combined with rFVIII infused for the first time in humans.
Effective treatment of VWD by VWF replacement therapy is based on the correction to therapeutic levels of VWF:RCo and FVIII:C. VWF:RCo and VWF:CB are surrogates for the primary hemostatic functions of VWF.24 In our study, comparable VWF:RCo activity was demonstrated following infusion with rVWF-rFVIII and a marketed pdVWF-pdFVIII. VWF:Ag levels were initially higher with pdVWF-pdFVIII than with rVWF-rFVIII, demonstrating a greater initial specific activity of rVWF. The higher specific activity of rVWF than in pdVWF-pdFVIII complex products has been described previously.25 The VWF:CB results in our study are also consistent with previous findings which showed that the specific collagen-binding activity of the rVWF-rFVIII study product (defined as the ratio VWF:CB/VWF:Ag) is higher (1.14 ± 0.16) when compared with pdVWF-pdFVIII (0.84) or pdVWF (0.81 ± 0.06).25
Higher ratios of VWF:RCo/VWF:Ag correspond to a higher relative amount of ultra-large molecular weight (ULMW). Therefore, the higher levels of sustained VWF:RCo and VWF:Ag observed after treatment with rVWF-rFVIII and the increased specific activity of rVWF (reflected in the longer mean VWF:CB T1/2 with rVWF-rFVIII as compared with pdVWF) are associated with an improved multimeric profile (increased ULMW multimers). Finally, as ULMW multimers are absent in VWF preparations derived from human plasma, it can be assumed that the improved VWF-specific activity of rVWF is associated with the presence of ULMW multimers.
Immediately following infusion, FVIII levels were markedly higher with rVWF-rFVIII than with pdVWF-pdFVIII, reflecting differences in the infused dose (38.5 IU/kg vs 21-25 IU/kg) of FVIII. However, after 72 hours, FVIII AUC0-∞ and FVIII:C were still higher (P < .01) after infusion with rVWF-rFVIII than with pdVWF-pdFVIII. rFVIII has a T1/2 of ∼12 hours in patients with hemophilia A.26 The difference in the VWF:FVIII infusion ratio would not be expected to significantly influence the observed endogenous FVIII levels in treated subjects after 48 to 60 hours, so that higher FVIII levels at 72 and 96 hours observed in subjects treated with rVWF:rFVIII principally reflect the stabilization of endogenous (synthesized) FVIII most likely by an increased capacity of rVWF compared with pdVWF (Figure 1C).
We hypothesize that the observed differences in FVIII stabilization are a reflection of the differences in VWF:Ag clearance,27-29 although this warrants further investigation. In the circulation, FVIII is fully bound to VWF which acts as its carrier protein prolonging its T1/2 and protecting it from proteolytic inactivation. Thus, the relatively longer FVIII half-life could stem from a longer VWF T1/2 and improved VWF multimeric profile. Although it could be expected that an increased proportion of VWF molecules of the highest molecular weight should be more effective in binding FVIII molecules (as each monomer of VWF has 1 binding domain able to bind 1 FVIII molecule), only 1% to 2% of the available VWF monomers are occupied by FVIII in vivo.30 However, the probability of encountering FVIII bound to any 1 multimer is expected to increase with the multimer length.25,31 Furthermore, upon characterization of rVWF, preparations containing only low-molecular-weight multimers had a decreased FVIII-binding capacity when assessed under in vitro conditions, where the VWF-FVIII complex is formed in the presence of excess FVIII.25
Our findings suggest that rVWF, administered independent of rFVIII, could maintain sufficient FVIII activity to treat a bleeding episode once the initial FVIII level has reached a therapeutic threshold. Although only a single dose level of VWF (50 IU of VWF:RCo/kg) was examined in the crossover cohort, the stabilization of endogenous FVIII induced by VWF has been shown to be independent of the VWF:RCo dose infused in studies of other VWF products.13,32 In 2 prospective studies, a single dose of Wilfactin, a high purity plasma-derived VWF concentrate with low FVIII:C content administered to type 3 VWD patients at 100 or 60 IU VWF:RCo/kg, induced similar progressive postinfusion increases in FVIII:C despite the difference in VWF dose.13,32
Plasma VWF consists of a series of multimers with a size range of 600 to >20 000 kDa. The ULMW fraction of VWF multimers is highly hemostatic, but thrombogenic risk under certain circumstances needs to be considered. The ULMW multimers present in vivo are, however, transient, due to rapid proteolysis of the multimers by the plasma metalloprotease ADAMTS13.5,33-35 Proteolysis by ADAMTS13 occurs on endothelial cell membranes after VWF release from Weibel-Palade bodies, as well as in the plasma.36 In pdVWF concentrates, the quantity of the larger, most hemostatically active multimers of VWF is decreased compared with human plasma as a result of prior exposure to ADAMTS13. The ULMW multimers in rVWF remain intact prior to administration because this product does not come into contact with ADAMTS13 during expression in vitro.11 In addition, the observed increase in ULMW VWF multimers following administration of rVWF was associated with prolonged T1/2 of both VWF and FVIII activities and an increase in specific collagen-binding activity. Increased ULMW multimers are known to enhance platelet agglutination as they have more available binding sites than smaller VWF multimers and are thus more functionally active which increases their hemostatic potential. Therefore, this effect could also induce improved overall hemostatic function in the treated patients. While the ULMW multimer portion of rVWF could induce microvascular thrombosis after persistent exposure, the analyses performed in our study demonstrated a rapid proteolytic degradation of the rVWF multimers in the circulation. Furthermore, there was no evidence of overt or subclinical thrombotic events observed in this study. These risks are to be evaluated further in ongoing phase 3 studies of rVWF in the treatment of bleeding episodes and surgical prophylaxis.
rVWF-rFVIII was well tolerated in this study consisting principally of type 3 VWD patients. There were no allergic type hypersensitivity reactions; AEs were mild and transient, and occurred at rates comparable to those observed with pdVWF-pdFVIII concentrates in our study as well as in the literature.7,14 Well-known risk factors such as neutralizing antibodies (inhibitors) against VWF or FVIII were not detected in any patient after either treatment.37
While no posttreatment inhibitors were observed in our study population, noninhibitory anti-VWF–binding antibodies were present in 2 subjects at screening prior to treatment. The detection and associated effect of these binding antibodies on VWF activity has not been described previously. Neither of these subjects displayed evidence of any other concomitant disease associated with atypical or nonspecific autoantibodies. The clearance of VWF:Ag was more rapid in these than in other patients without such antibodies, which was similarly reflected in the levels of VWF:RCo, VWF:CB, and FVIII:C. Further clinical study will be needed to determine whether the presence of nonneutralizing anti-VWF–binding antibodies exerts adverse clinical effect in the setting of the treatment of bleeding events.
Variability in the risk of developing antibodies to exogenously administered VWF is likely to be, at least in large part, explained by genetic factors as the VWF molecular defects are extremely heterogeneous.38-43 Large deletions of the VWF gene or homozygous nonsense mutations are frequently associated with the presence of inhibitory VWF alloantibodies neutralizing the action of exogenously administered VWF.38-43 However, no specific mutations have been discovered that are associated with nonneutralizing anti-VWF–binding antibodies. In the present study, the mutations observed in the 2 subjects who had high-titer specific pretreatment binding antibodies are both located in domains responsible for dimerization (D4 and C-terminal), which were also suggested to be additional binding sites for ADAMTS13.42,44 The 2 subjects with high specific pretreatment binding antibody titers uniquely displayed a more rapid antigen clearance as reflected by the loss of HMW VWF multimers and lack of cleavage products. This observation may be due to decreased sensitivity to ADAMTS13 proteolysis as a result of anti-VWF antibodies binding at cleavage sites.
In summary, results of this clinical trial show that rVWF-rFVIII is safe, and has a PK profile comparable to pdVWF-pdFVIII, but with an enhanced stabilization of endogenous FVIII, indicating that rVWF administered without the further addition of FVIII has the potential to maintain sufficient VWF and FVIII activities for an extended period of time. This property of rVWF is potentially favorable for use in clinical situations such as the postoperative period and continuous prophylaxis. Importantly, the ability to separately titrate the quantity of rVWF and rFVIII infused offers the potential to better optimize the quantity of FVIII and VWF that a patient receives, reducing the theoretical risk of thrombotic events due to high FVIII levels.45 Further investigation of the first recombinant VWF in a larger clinical trial is ongoing to substantiate the initial findings and define dosing strategies in the prevention and treatment of bleeding in VWD patients. An incidental finding of this study was the first demonstration that 2 patients with severe VWD had nonneutralizing anti-VWF antibodies preinfusion, and that these antibodies hampered the pattern of recovery of rVWF. The frequency of this abnormality and its clinical significance should be tested in larger cohorts of VWD patients.
Supplementary Material
Acknowledgments
The authors thank the following individuals for their contributions to the conduct of the trial at the investigative sites: Ingrid Pabinger, Charles Hay, John Pasi, Will Lester, Desmond Craigh, Giancarlo Castaman, Antonella Bertomoro, Vlad Radulescu, Sue Robinson, Judith C. Lin, Peter Kouides, Leonard Valentino, Nirda Roiguez, Vivek Sharma, as well as Reinhard Schneppenheim and Florian Oyen for VWF gene mutation analysis; Peter Quehenberger for coagulation parameter testing; Hans-Dieter Volk for testing of foreign proteins; and Bio Analytical Research Corporation for blood count, virology, and clinical chemistry analyses. The authors also thank the rVWF clinical study team for operational and administrative support: Smita Barua, Roger Berg, Martin Blum, Yuliana Brankova, Erik Bjornson, Lin Connely, Simone Deutschel, Jorge Escobar, Herbert Gritsch, Birgit Reipert, Frank Horling, Sakir Mutevelic, Thomas Scholze, and Katalin Varadi.
This work was supported by Baxter Healthcare Corporation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.M.S. and B.M.E. designed the research; B.M.E. and W.-Y.W. supervised the research; T.M.S., B.M.E., P.M.M., J.C.G., and M.C. wrote the manuscript; P.M.M., C.K., C.M., E.R., A.S., I.B., M.V.R., J.C.G., T.T.Y., and R.K. performed the research; T.M.S., W.E., M.C., P.L.T., and B.M.E. interpreted the data; W.E. performed the statistical analysis; P.L.T. contributed vital new reagents or analytical tools; W.-Y.W. also critically revised and approved the final manuscript; and all authors critically revised the manuscript and approved the final manuscript.
Conflict-of-interest disclosure: W.E., M.C., W.-Y.W., P.L.T., and B.M.E. are employees of Baxter. T.M.S. is a former Baxter employee. P.M.M., C.K., C.M., E.R., A.S., I.B., J.C.G., T.T.Y., and R.K. served as investigators in the trial and received honoraria from Baxter. M.V.R. served as an investigator and declares not having received honoraria.
A list of members of the rVWF Ad Hoc Study Group appears in the supplemental Appendix.
Correspondence: Bruce M. Ewenstein, BioScience Division, Baxter Healthcare, One Baxter Way, Westlake Village, CA 91362; e-mail: bruce_ewenstein@baxter.com.
References
- 1.Nichols WC, Ginsburg D. von Willebrand disease. Medicine (Baltimore) 1997;76(1):1–20. doi: 10.1097/00005792-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE, Mannucci PM, Berntorp E, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost. 2000;84(2):160–174. [PubMed] [Google Scholar]
- 3.Weiss HJ, Sussman II, Hoyer LW. Stabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s disease. J Clin Invest. 1977;60(2):390–404. doi: 10.1172/JCI108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise RJ, Dorner AJ, Krane M, Pittman DD, Kaufman RJ. The role of von Willebrand factor multimers and propeptide cleavage in binding and stabilization of factor VIII. J Biol Chem. 1991;266(32):21948–21955. [PubMed] [Google Scholar]
- 5.Mannucci PM. Treatment of von Willebrand's disease. N Engl J Med. 2004;351(7):683–694. doi: 10.1056/NEJMra040403. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg D. Molecular genetics of von Willebrand disease. Thromb Haemost. 1999;82(2):585–591. [PubMed] [Google Scholar]
- 7.Mannucci PM, Chediak J, Hanna W, et al. Alphanate Study Group. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood. 2002;99(2):450–456. doi: 10.1182/blood.v99.2.450. [DOI] [PubMed] [Google Scholar]
- 8.Gill JC, Ewenstein BM, Thompson AR, Mueller-Velten G, Schwartz BA Humate-P Study Group. Successful treatment of urgent bleeding in von Willebrand disease with factor VIII/VWF concentrate (Humate-P): use of the ristocetin cofactor assay (VWF:RCo) to measure potency and to guide therapy. Haemophilia. 2003;9(6):688–695. doi: 10.1046/j.1351-8216.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- 9.Federici AB, Baudo F, Caracciolo C, et al. Clinical efficacy of highly purified, doubly virus-inactivated factor VIII/von Willebrand factor concentrate (Fanhdi) in the treatment of von Willebrand disease: a retrospective clinical study. Haemophilia. 2002;8(6):761–767. doi: 10.1046/j.1365-2516.2002.00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Fischer BE. Recombinant von Willebrand factor: potential therapeutic use. J Thromb Thrombolysis. 1999;8(3):197–205. doi: 10.1023/a:1008906103637. [DOI] [PubMed] [Google Scholar]
- 11.Turecek PL, Mitterer A, Matthiessen HP, et al. Development of a plasma- and albumin-free recombinant von Willebrand factor. Hamostaseologie. 2009;29(suppl 1):S32–S38. [PubMed] [Google Scholar]
- 12.Goudemand J, Parquet-Gernez A, Goudemand M. Purity of factor VIII concentrates. Blood Coagul Fibrinolysis. 1993;4(3):499–500. doi: 10.1097/00001721-199306000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Goudemand J, Scharrer I, Berntorp E, et al. Pharmacokinetic studies on Wilfactin, a von Willebrand factor concentrate with a low factor VIII content treated with three virus-inactivation/removal methods. J Thromb Haemost. 2005;3(10):2219–2227. doi: 10.1111/j.1538-7836.2005.01435.x. [DOI] [PubMed] [Google Scholar]
- 14.Favaloro EJ, Lloyd J, Rowell J, et al. Comparison of the pharmacokinetics of two von Willebrand factor concentrates [biostate and AHF (high purity)] in people with von Willebrand disorder. A randomised cross-over, multi-centre study. Thromb Haemost. 2007;97(6):922–930. [PubMed] [Google Scholar]
- 15.Wichmann BA, Hill ID. Algorithm AS 183. An efficient and portable pseudo-random number generator. J R Stat Soc Ser C Appl Stat. 1982;31(2):188–190. [Google Scholar]
- 16.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 17.Varadi K, Rottensteiner H, Gruber E, et al. Establishment of an automated VWF: RCo assay for measuring plasma samples with low activity [abstract]. J Thromb Haemost. 2011;9(suppl 2):919. [Google Scholar]
- 18.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasper CK. Laboratory tests for factor VIII inhibitors, their variation, significance and interpretation. Blood Coagul Fibrinolysis. 1991;2(suppl 1):7–10. [Google Scholar]
- 20.Whelan SF, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121(6):1039–1048. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 21.Schneppenheim R, Budde U, Krey S, et al. Results of a screening for von Willebrand disease type 2N in patients with suspected haemophilia A or von Willebrand disease type 1. Thromb Haemost. 1996;76(4):598–602. [PubMed] [Google Scholar]
- 22.Mazurier C, Rodeghiero F von Willebrand Factor Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Recommended abbreviations for von Willebrand Factor and its activities. Thromb Haemost. 2001;86(2):712. [PubMed] [Google Scholar]
- 23.Goodeve AC, Eikenboom JC, Ginsburg D, et al. ISTH SSC Subcommittee on von Willebrand factor. A standard nomenclature for von Willebrand factor gene mutations and polymorphisms. On behalf of the ISTH SSC Subcommittee on von Willebrand factor. Thromb Haemost. 2001;85(5):929–931. [PubMed] [Google Scholar]
- 24.Ewenstein BM. Use of ristocetin cofactor activity in the management of von Willebrand disease. Haemophilia. 2001;7(suppl 1):10–15. doi: 10.1046/j.1365-2516.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 25.Turecek PL, Schrenk G, Rottensteiner H, et al. Structure and function of a recombinant von Willebrand factor drug candidate. Semin Thromb Hemost. 2010;36(5):510–521. doi: 10.1055/s-0030-1255445. [DOI] [PubMed] [Google Scholar]
- 26.Ewenstein BM, Collins P, Tarantino MD, et al. Hemophilia therapy innovation: development of an advanced category recombinant factor VIII by a plasma/albumin-free method. Proceedings of a Special Symposium at the XIXth Congress of the International Society on Thrombosis and Haemostasis, July 12-18, 2003, Birmingham, UK. Semin Hematol. 2004;41(1 suppl 2):1–16, discussion 16-18. doi: 10.1016/s0037-1963(04)00017-4. [DOI] [PubMed] [Google Scholar]
- 27.Denis CV, Christophe OD, Oortwijn BD, Lenting PJ. Clearance of von Willebrand factor. Thromb Haemost. 2008;99(2):271–278. doi: 10.1160/TH07-10-0629. [DOI] [PubMed] [Google Scholar]
- 28.Fischer K, Pendu R, van Schooten CJ, et al. Models for prediction of factor VIII half-life in severe haemophiliacs: distinct approaches for blood group O and non-O patients. PLoS ONE. 2009;4(8):e6745. doi: 10.1371/journal.pone.0006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10(12):2428–2437. doi: 10.1111/jth.12008. [DOI] [PubMed] [Google Scholar]
- 30.Vlot AJ, Koppelman SJ, Bouma BN, Sixma JJ. Factor VIII and von Willebrand factor. Thromb Haemost. 1998;79(3):456–465. [PubMed] [Google Scholar]
- 31.Cao W, Krishnaswamy S, Camire RM, Lenting PJ, Zheng XL. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc Natl Acad Sci USA. 2008;105(21):7416–7421. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menache D, Aronson DL, Darr F, et al. Cooperative Study Groups. Pharmacokinetics of von Willebrand factor and factor VIIIC in patients with severe von Willebrand disease (type 3 VWD): estimation of the rate of factor VIIIC synthesis. Br J Haematol. 1996;94(4):740–745. doi: 10.1046/j.1365-2141.1996.d01-1860.x. [DOI] [PubMed] [Google Scholar]
- 33.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varadi K, Rottensteiner H, Vejda S, et al. Species-dependent variability of ADAMTS13-mediated proteolysis of human recombinant von Willebrand factor. J Thromb Haemost. 2009;7(7):1134–1142. doi: 10.1111/j.1538-7836.2009.03453.x. [DOI] [PubMed] [Google Scholar]
- 35.Metzner HJ, Hermentin P, Cuesta-Linker T, Langner S, Müller HG, Friedebold J. Characterization of factor VIII/von Willebrand factor concentrates using a modified method of von Willebrand factor multimer analysis. Haemophilia. 1998;4(suppl 3):25–32. doi: 10.1046/j.1365-2516.1998.0040s3025.x. [DOI] [PubMed] [Google Scholar]
- 36.Skipwith CG, Cao W, Zheng XL. Factor VIII and platelets synergistically accelerate cleavage of von Willebrand factor by ADAMTS13 under fluid shear stress. J Biol Chem. 2010;285(37):28596–28603. doi: 10.1074/jbc.M110.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James PD, Lillicrap D, Mannucci PM. Alloantibodies in von Willebrand disease [published online ahead of print January 7, 2013]. Blood. doi: 10.1182/blood-2012-10-462085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peake IR, Liddell MB, Moodie P, et al. Severe type III von Willebrand’s disease caused by deletion of exon 42 of the von Willebrand factor gene: family studies that identify carriers of the condition and a compound heterozygous individual. Blood. 1990;75(3):654–661. [PubMed] [Google Scholar]
- 39.Mancuso DJ, Tuley EA, Castillo R, de Bosch N, Mannucci PM, Sadler JE. Characterization of partial gene deletions in type III von Willebrand disease with alloantibody inhibitors. Thromb Haemost. 1994;72(2):180–185. [PubMed] [Google Scholar]
- 40.Shelton-Inloes BB, Chehab FF, Mannucci PM, Federici AB, Sadler JE. Gene deletions correlate with the development of alloantibodies in von Willebrand disease. J Clin Invest. 1987;79(5):1459–1465. doi: 10.1172/JCI112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surdhar GK, Enayat MS, Lawson S, Williams MD, Hill FG. Homozygous gene conversion in von Willebrand factor gene as a cause of type 3 von Willebrand disease and predisposition to inhibitor development. Blood. 2001;98(1):248–250. doi: 10.1182/blood.v98.1.248. [DOI] [PubMed] [Google Scholar]
- 42.Baronciani L, Cozzi G, Canciani MT, et al. Molecular characterization of a multiethnic group of 21 patients with type 3 von Willebrand disease. Thromb Haemost. 2000;84(4):536–540. [PubMed] [Google Scholar]
- 43.Baronciani L, Cozzi G, Canciani MT, et al. Molecular defects in type 3 von Willebrand disease: updated results from 40 multiethnic patients. Blood Cells Mol Dis. 2003;30(3):264–270. doi: 10.1016/s1079-9796(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 44.Zanardelli S, Chion AC, Groot E, et al. A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood. 2009;114(13):2819–2828. doi: 10.1182/blood-2009-05-224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannucci PM. Venous thromboembolism in von Willebrand disease. Thromb Haemost. 2002;88(3):378–379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.