Abstract
Toll-like receptors (TLRs) enable metazoans to mount effective innate immune responses to microbial and viral pathogens, as well as to endogenous host-derived ligands. It is understood that genetic background of the host can influence TLR responsiveness, altering susceptibility to pathogen infection, autoimmunity and cancer. Macrophage stimulatory protein (MSP), which activates the receptor tyrosine kinase recepteur d'origine nantais (RON), promotes key macrophage functions such as motility and phagocytic activity. MSP also acts via RON to modulate signaling by TLR4, which recognizes a range of pathogen or endogenous host-derived molecules. Here, we show that RON exerts divergent control over TLR4 activity in macrophages from different mouse genetic backgrounds. RON potently modulated the TLR4 response in macrophages from M2-prone FVB mice, as compared with M1-skewed C57Bl6 mice. Moreover, global expression analysis revealed that RON suppresses the TLR4-dependent type-I interferon gene signature only in FVB macrophages. This leads to attenuated production of the potent inflammatory mediator, tumor necrosis factor-α. Eliminating RON kinase activity markedly decreased carcinogen-mediated tumorigenesis in M2/Th2-biased FVB mice. We propose that host genetic background influences RON function, thereby contributing to the variability in TLR4 responsiveness in rodents and, potentially, in humans. These findings provide novel insight into the complex interplay between genetic context and immune function.
Keywords: RON, macrophage, TLR4, interferon
Toll-like receptors (TLRs) have a crucial role in enabling the innate immune system to respond effectively to infectious agents, and to endogenous intracellular proteins released from necrotic cells, oxidatively modified lipids and extracellular matrix proteins. TLRs bind to ligands containing specific pathogen- or danger-associated molecular patterns and transduce signals to orchestrate activation of innate immune cells such as macrophages, dendritic cells and natural killer cells.1, 2, 3 Previous studies in rodent and human models have established that distinct genetic backgrounds can dictate differential responsiveness to TLR activation.4, 5, 6 Indeed, the differences in TLR signaling outcome between individual subjects may affect immune competence as well as susceptibility to autoimmune disease or cancer. How genetic context influences TLR signaling outcomes remains poorly understood.
Receptor tyrosine kinases are a family of cell surface receptors that regulate diverse cellular functions, including proliferation, differentiation, survival and motility.7 Aberrant receptor tyrosine kinase signaling, arising through genetic or epigenetic alteration, often contributes to malignant cell transformation.8, 9, 10 The receptor tyrosine kinase recepteur d'origine nantais (RON) is highly expressed in several human epithelial cell malignancies.11, 12, 13, 14 RON is also expressed by tissue-resident macrophages in the lung, liver and peritoneal cavity.12, 15 The cognate ligand for RON is a macrophage-stimulatory protein (MSP), which regulates a number of key macrophage functions via RON including; motility, phagocytic activity and the production of various cytokines and chemokines.16, 17, 18 Importantly, mice deficient in RON kinase activity are hypersensitive to bacterial lipopolysaccharide (LPS)—a key ligand for TLR4—suggesting that RON can sculpt innate immune responses elicited through TLR4 activation.19, 20 Studies using tissue-resident peritoneal macrophages further show that RON stimulation can attenuate TLR4-induced pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin (IL)-12 and nitric oxide (NO).18, 20, 21 RON activation in macrophages also increases the expression of scavenger receptors and of the enzyme arginase-I, which hydrolyzes arginine to urea and ornithine.18, 22
The M1/M2 paradigm provides a useful conceptual framework for understanding macrophage function. Macrophages from genetically diverse subjects exhibit different M1 versus M2 phenotypic characteristics. For example, it is well documented that individuals vary in their responsiveness to LPS.23 In humans and mice, this variability can be explained in part by polymorphism in the TLR4 gene itself.24, 25 However, more complex downstream signaling thresholds in the TLR4 pathway also may contribute to the variation in the response to ligands such as LPS.6, 26 M2 macrophages have been implicated in supporting tissue repair, as well as promoting tumor growth and metastasis.27, 28, 29, 30, 31
The importance of M2 macrophage polarization in the host response to pathogen or trauma-associated tissue inflammation and tumorigenesis led us to explore how host genetic background might impact the ability of RON to regulate TLR4 responsiveness and M2 versus M1 differentiation. To investigate this, we compared inflammatory outcomes in macrophages from M1-predisposed C57Bl6 or M2-prone FVB mice.32 Our studies reveal striking divergence in the ability of RON to regulate the TLR4 pathway that is highly dependent on host genetic background. In addition, we identified a novel function of RON to repress the type-I interferon (IFN) gene signature in M2-predisposed macrophages activated through TLR4. Translated in vivo, we show that RON kinase deficiency resulted in a decreased susceptibility to carcinogen-induced papilloma and fibrosarcoma development in FVB mice. Taken together, our findings suggest that therapeutic approaches to modulate the RON pathway in autoimmune disease and cancer may benefit from consideration of how host genetic background can influence immune responses.
Results
RON differentially regulates TLR4 responsiveness in M2 versus M1-predisposed macrophages
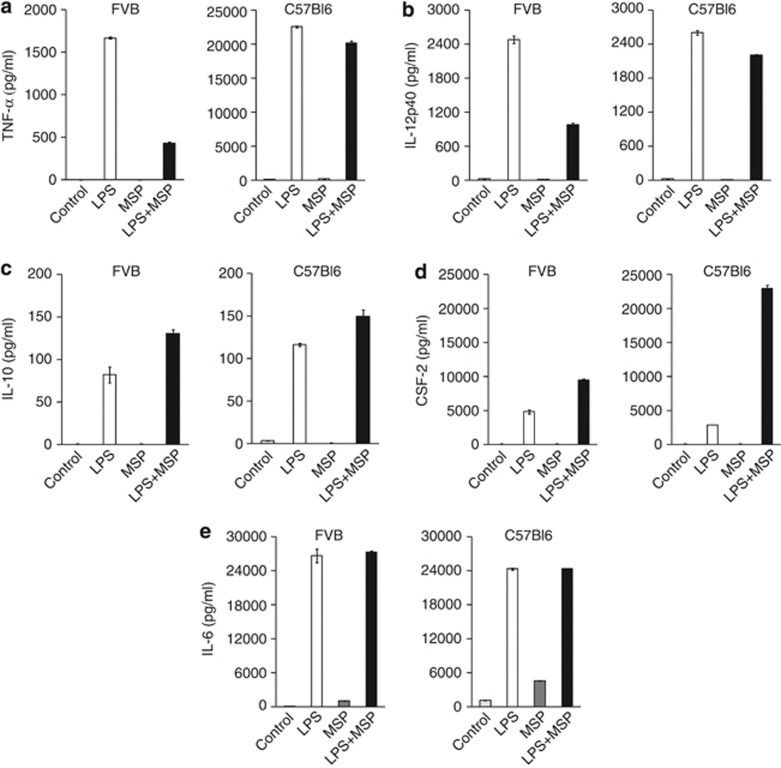
MSP suppressed the production of cytokines and chemokines by peritoneal macrophages from outbred CD-1 mice stimulated with the TLR4 agonist LPS.17, 18 To examine the modulation of TLR4 function by RON in different inbred genetic backgrounds, we isolated peritoneal macrophages from M1/Th1- (C57Bl6) or M2/Th2- (FVB) biased mice and analyzed the output of cytokines and chemokines in response to LPS. Irrespective of strain background, TLR4 stimulation induced production of a range of cytokine and chemokine factors (Supplementary Tables S1 and S2). Compared with FVB mice, M1-prone C57Bl6 macrophages showed higher basal levels of certain cytokines and chemokines, but all were enhanced by LPS stimulation. Three distinct patterns emerged in TLR4-activated macrophages co-stimulated with MSP: (1) MSP markedly suppressed LPS-induced TNF-α and IL-12p40 in FVB macrophages but not in C57Bl6 (Figures 1a and b). (2) MSP increased LPS-induced IL-10 and colony stimulating factor (CSF), irrespective of macrophage strain background (Figures 1c and d). (3) Finally, cytokines, like IL-6, were not significantly altered by RON signaling in either macrophage background (Figure 1e). A complete list of MSP-regulated cytokines and chemokines in FVB and C57Bl6 macrophages is provided as supplementary data (Supplementary Table S3). The impact of MSP on TLR4-mediated responsiveness was exerted at the transcriptional level, as evidenced by monitoring mRNA levels over a time course, and modulation was entirely dependent on intact RON kinase activity (Supplementary Figure S1). Consistent with chemokine and cytokine protein determinations, MSP failed to suppress TNF-α and IL-12p40 transcript levels in LPS-stimulated C57Bl6 macrophages but markedly enhanced CSF-2 transcription (Supplementary Figure S2). These results confirm known aspects and uncover some novel features of RON's ability to impact TLR4 responsiveness in tissue-derived macrophages. Importantly, they reveal that strain background can significantly influence the effect of RON on the TLR4 pathway.
Figure 1.
RON modulates TLR4-dependent cytokine production of peritoneal macrophages from FVB or C57Bl6 mice. Peritoneal macrophages from FVB or C57Bl6 were stimulated with Ultrapure LPS (100 ng ml−1) or MSP (100 ng ml−1) separately, or in combination. After overnight culture (20 h), conditioned medium from treatment groups was analyzed for cytokine and chemokine production using a fluorescent-based multiplex assay: (a) TNF-α, (b) IL-12p40, (c) IL-10, (d) CSF-2 and (e) IL-6. Values represent the mean±s.d. of samples from at least two independent experiments analyzed in triplicates.
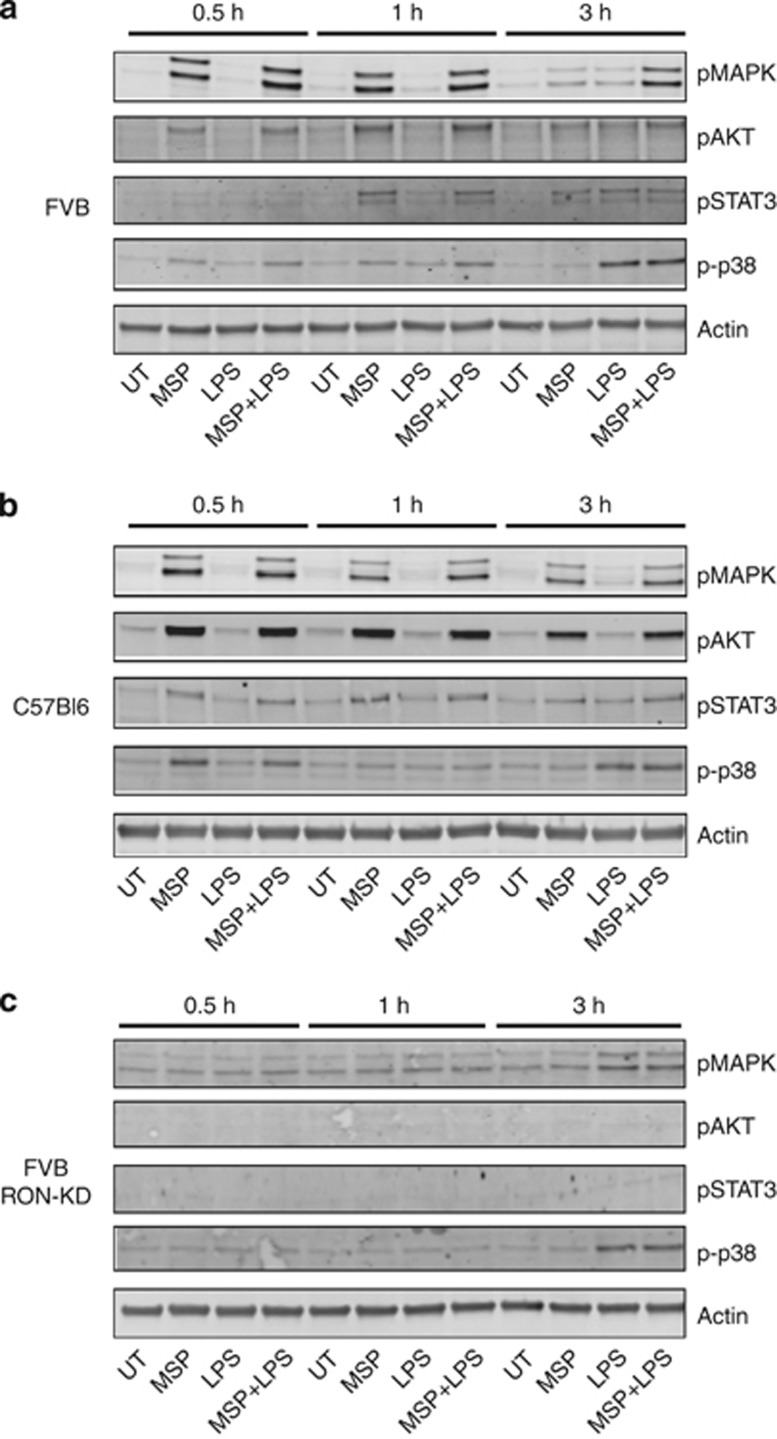
RON activates common downstream signaling pathways in macrophages, irrespective of strain background
To examine if engagement of distinct signaling nodes downstream of RON might account for the divergent regulation of the TLR4 pathway in FVB versus C57Bl6 macrophages, we analyzed the phosphorylation of known downstream mediators: p42/44 MAPK, AKT and STAT3.21, 33, 34 In parallel, we examined phosphorylation of p38 MAPK, a downstream event in the TLR4 signaling axis in macrophages (Figures 2a and b).35 MSP rapidly induced phosphorylation of p42/44 MAPK, AKT and STAT3 in macrophages independent of strain background, although with some apparent delay in the kinetics of STAT3 phosphorylation in FVB-derived cells. MSP also induced rapid and transient phosphorylation of p38 MAPK, but it had no impact on TLR4-induced p38 MAPK phosphorylation, which occurred at later time points. The kinase domain of RON was essential for signaling pathways activated by MSP in FVB and C57Bl6 macrophages (Figure 2c and data not shown). The total protein levels of p42/44 MAPK, AKT, p38 MAPK and STAT3 did not change significantly over the time course evaluated (data not shown). Given the similarities in downstream RON signaling, independent of macrophage strain background, we reasoned that additional features of the RON signaling pathway might explain the divergent modulation of the TLR4 pathway observed in M2-predisposed FVB macrophages (Figure 1).
Figure 2.
Signaling networks downstream of RON are conserved in peritoneal macrophages from FVB and C57Bl6 mice. Peritoneal macrophages from wild-type FVB (a), C57/B6 (b) or from RON-KD mice (c) were stimulated with 100 ng ml−1 of LPS or MSP alone, or in combination. Macrophage lysates were analyzed at the indicated times for p38 MAPK, p42/44 MAPK, AKT and STAT3 phosphorylation by western blotting, with actin serving as a loading control Total p38 MAPK, p42/44 MAPK, AKT and STAT3 levels were similar under all conditions (data not shown). Results shown are representative of at least two independent experiments.
RON potently represses the TLR4-mediated IFN gene signature in FVB macrophages
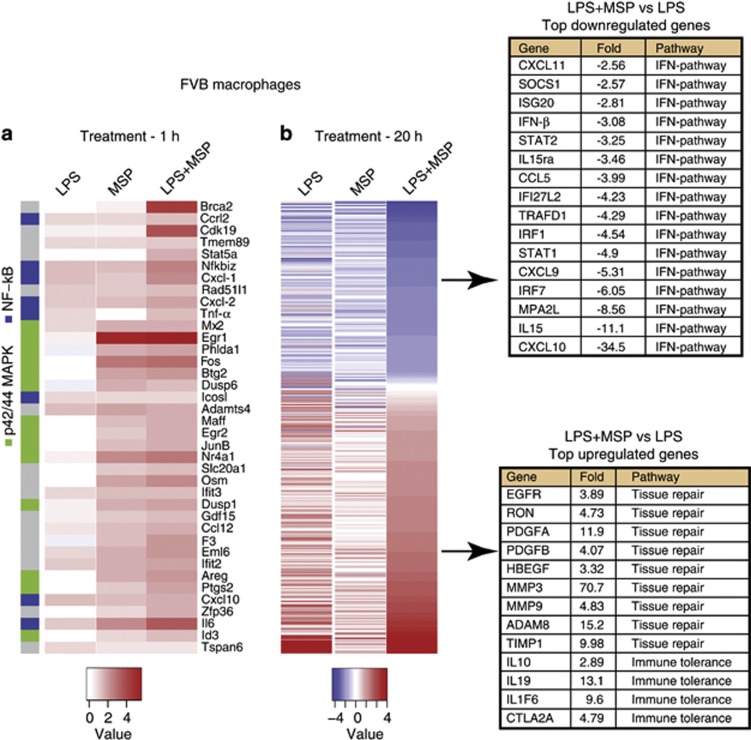
Negative feedback loops serve to control the magnitude and duration of TLR activity, thereby preventing an excessive inflammatory response.3 A hallmark of TLR4 signaling is activation of nuclear factor-κB (NF-κB), IFN regulatory factor (IRF) and their corresponding transcriptional targets.3, 36 To explore RON's ability to impact the TLR4 pathway in more detail, we performed a global gene expression analysis in M2-prone FVB macrophages. As expected, TLR4 activation induced many NF-κB-regulated target genes within an hour, including IL-6, TNF-α and NF-κBiz (Figure 3a—y-axis (blue)).37, 38 Moreover, consistent with the induction of p38 MAPK phosphorylation by MSP in FVB macrophages, several NF-κB-regulated genes were also upregulated by MSP including IL-6, CXCL-2 and CXCL-10. A prominent feature of the early transcriptional response to MSP alone was the p42/44 MAPK gene signature, highlighted by the expression of transcription factors such as EGR1, FOS and NR4A1, and phosphatases such as DUSP1 and DUSP6 (Figure 3a—y-axis (green)). At 1 h, MSP had little effect on TLR4-mediated transcription, supporting that RON did not impact the early phase of TLR4-induced NF-κB target genes. By 20 h, LPS had induced the expression of numerous pro-inflammatory cytokines and chemokines associated with M1 macrophage differentiation, such as IL-12p40 (5.3-fold), IL-1β (206-fold), IL-23α (4.8-fold) and CXCL-10 (2.2-fold) (Figure 3b and Supplementary Tables S4-S6). In contrast, co-treatment of cells with MSP and LPS potently skewed the transcriptional response toward an M2-like macrophage differentiation program, including the upregulation of genes associated with protease pathways, tissue repair and immune suppression (Figure 3b (lower panel) and Supplementary Table S4).39, 40, 41
Figure 3.
Global gene expression analysis reveals the distinct RON pathway effects on TLR4 signaling outcomes. Peritoneal macrophages were isolated from FVB mice and stimulated with or without 100 ng ml−1 LPS, 100 ng ml−1 MSP or the combination of both factors, for 1 or 20 h. Differentially expressed genes are represented in the heat map provided at 1 h (a) or 20 h (b). Colors represent fold-change relative to unstimulated FVB macrophages. Genes corresponding to p42/44 MAPK (green) or NF-κB (blue) pathways are indicated in (a; y-axis). Inset tables show genes upregulated (upper panel) and downregulated (lower panel) following RON and TLR4 co-stimulation.
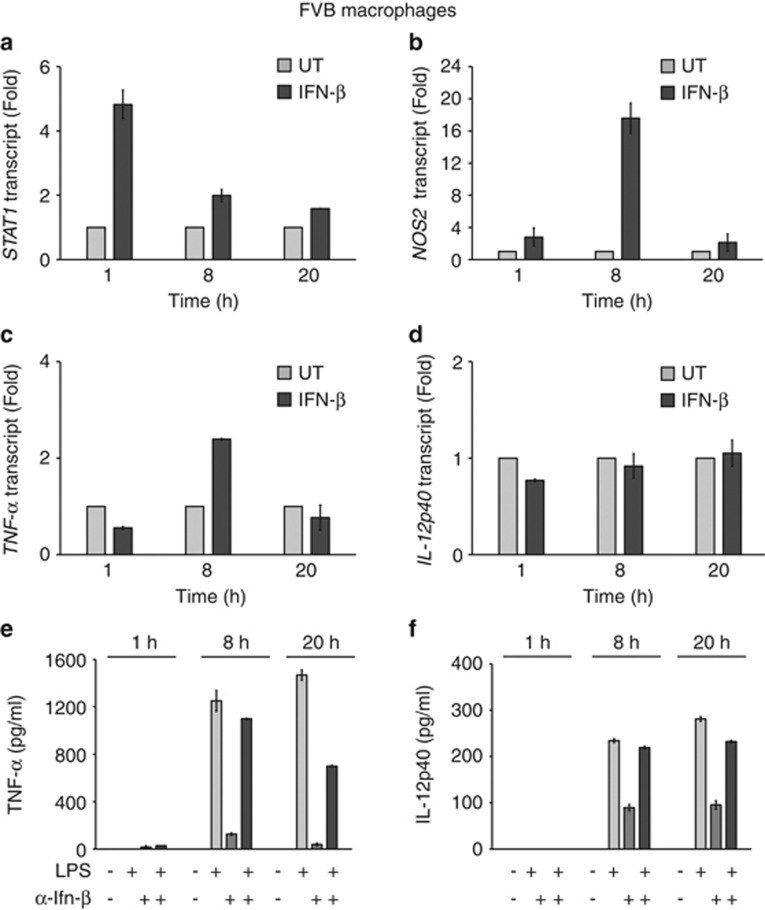
Importantly, our genome-wide transcriptome profiling revealed the previously unknown ability of MSP to attenuate TLR4-induced IFN response genes. Indeed, of the 30 top LPS-induced transcripts downregulated by MSP, 14 were associated with the type-I IFN pathway (Figure 3b (upper panel)). Regulation of the IFN pathway was verified by quantitative PCR analysis (Supplementary Figure S3). Further, we confirmed that repression of the type-I IFN response was entirely dependent on intact RON kinase function (Supplementary Figure S4). In contrast, RON signaling had a significant but weaker impact on the type-I IFN transcriptional response in macrophages from C57Bl6 mice at the earliest time point (8 h) (Supplementary Figure S5). Related to these findings, there was a large kinetic delay in the TLR4-mediated type-I IFN transcriptional response in macrophages from C57Bl6 versus FVB mice (viz, 8 h or 1 h, respectively) (Supplementary Figures S3 and S5). To further explore the effect of RON signaling on the type-I IFN pathway, we analyzed the transcriptional response in macrophages exposed to recombinant IFN-β. IFN-β rapidly induced its related transcriptional mediators including STAT1/STAT2 and IRF7, as well as downstream targets NOS2 and CXCL-10 (Figures 4a–d, and Supplementary Figure S6A-C). Notably, transcriptional induction of STAT1 by IFN-β was more rapid following LPS exposure (Figure 4a and Supplementary Figure S3C). Eight hours after the addition of recombinant IFN-β, we observed a reproducible twofold increase in TNF-α transcript levels in FVB macrophages (Figure 4c). In contrast, IFN-β had no effect on IL-12p40 or IL-10 transcription, supporting the selectivity of IFN-α/β receptor-mediated TNF-α transcriptional response in FVB macrophages (Figure 4d, Supplementary Figure S6D).
Figure 4.
IFN-β regulates TNF-α expression in peritoneal macrophages isolated from FVB mice. Peritoneal macrophages were untreated or stimulated with 2 nℳ of recombinant mouse IFN-β. At the indicated time, expression of IFN pathway response genes; STAT1 (a), NOS2 (b), cytokines TNF-α (c) or IL-12p40 (d) production were evaluated by quantitative reverse transcriptase-PCR. Peritoneal macrophages pretreated with 6.5 nℳ of a neutralizing antibody against mouse IFN-β were analyzed (1, 8 or 20 h) following the LPS exposure and analyzed for TNF-α (e) or IL-12p40 (f) production. Values represent the average±s.d. samples analyzed as duplicates. Representative data shown is from one of two independent experiments.
To verify our hypothesis that TNF-α produced by TLR4-stimulated FVB macrophages was mediated indirectly via IFN-β production, we used a neutralizing antibody to IFN-β.42 Antibody-pretreated macrophages showed a significant reduction in the amount of TNF-α produced in response to LPS, attenuating production by 50% at 20 h (Figure 4e). Conversely, the anti-IFN-β antibody had no impact on LPS-induced IL-12p40 and IL-10 protein levels (Figure 4 and Supplementary Figure S6E). Taken together our genome-wide transcriptional analysis suggested that RON might reduce TNF-α production by suppressing the early type-I IFN response in FVB macrophages stimulated through TLR4.
RON signaling promotes carcinogen-induced tumorigenesis in M2/Th2-prone FVB mice
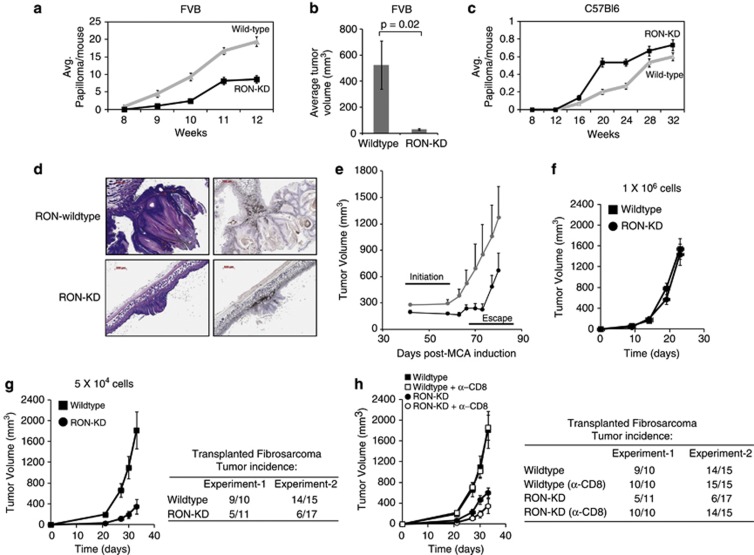
Genetic differences between mouse strains can alter the timing and magnitude of the inflammatory response, thereby impacting inherent susceptibility to pathogens and cancer development.43, 44, 45 Further, type-I IFNs are reported to inhibit de novo carcinogenesis by promoting innate and adaptive antitumor immunity.46, 47, 48 Our findings that RON could modulate the IFN-β pathway in FVB macrophages led us to examine how RON kinase deficiency affects susceptibility of M2/Th2-predisposed FVB mice to carcinogen-induced tumorigenesis. To explore this, we used two carcinogen models known to be dependent on pro-inflammatory pathways, namely 7,12-dimethylbenz-(a) anthracene/12-O-tetradecanoyl phorbol-13 acetate (DMBA/TPA)-induced skin papilloma and methylcholanthrene (MCA)-induced fibrosarcoma.46, 49 Consistent with an earlier study,50 FVB mice lacking RON kinase function displayed a marked reduction in papilloma tumor burden as compared with wild-type controls (Figures 5a and b). In contrast, there was no significant difference in papilloma development between RON-KD and wild-type mice in the C57Bl6 background (Figure 5c). Histological examination of cutaneous papillomas from RON-KD and wild-type FVB mice revealed numerous infiltrating F4/80-expressing macrophages, consistent with their established role in supporting tumorigenesis (Figure 5d). To extend this finding, we evaluated tumor initiation and outgrowth in the MCA-induced fibrosarcoma model. De novo tumor initiation was delayed in RON-KD mice, whereas the outgrowth of established tumors was indistinguishable in wild-type and RON-KD backgrounds, suggesting that RON signaling is important in the early events of fibrosarcoma development (Figure 5e and Supplementary Figure S7A-B). To investigate this hypothesis in more detail, we derived a tumor cell line from fibrosarcoma developed in a wild-type FVB mouse and transplanted a high (1 × 106) or low (5 × 104) cell density into naive wild-type or RON-KD recipients (Figures 5f and g). At the high cell inoculum, tumor growth was indistinguishable in wild-type or RON-KD mice. However, a 20-fold reduction in the seeding cell number resulted in a significant delay in tumor initiation, with >50% of RON-KD remaining tumor free in two independent experiments. This difference in tumor take was completely restored (100%) in RON-KD mice depleted of CD8+ T cells (Figure 5h). However, despite restoration of tumor engraftment in CD8 T-cell-depleted RON-KD mice, tumor growth was significantly restricted, supporting the finding that innate and adaptive immunity combined to reduce tumor growth in the absence of RON signaling.
Figure 5.
Chemical-induced carcinogenesis is delayed in the absence of a functional RON in FVB but not in C57/Bl6 mice. Wild-type or RON-KD mice (15 animals per group) on FVB (a) or C57Bl6 (c) backgrounds were treated with DMBA/PMA, as described in the Methods. The number of papillomas appearing over time (a) and average tumor volume (b) in FVB mice are shown in comparison with tumor number in C57Bl6 animals (c). Error bars represent the mean±s.e.m. (d) Infiltrating F4/80-expressing macrophages within papilloma samples collected from wild-type and RON-KD mice were evaluated by immunohistochemical analysis (see Methods). (e) The growth of de novo MCA-induced fibrosarcomas was monitored in RON wild-type and RON-KD FVB mice. Error bars represent the mean±s.e.m. (f, g) A fibrosacoma cell line derived from an FVB mouse was transplanted at two cell densities (high—5 × 10e6 or low—5 × 10e4) into wild-type or RON-KD mice and monitored for growth (n=10–17 animals per group). Error bars represent the mean±s.e.m. The table summarizes tumor incidence in wild-type or RON-KD mice in n=2 separate experiments. (h) Growth of fibrosarcoma cells (5 × 10e4) was evaluated in RON wild-type and RON-KD mice treated with 10 mg per kg of anti-CD8 (clone 2.43) or an isotype control (rat IgG2b) antibody before and during fibrosarcoma-cell transplantation, as described in Methods. Error bars represent the mean±s.e.m. (n=10–17 animals per group). The table summarizes tumor incidence in wild-type or RON-KD mice without or with CD8-T-cell depletion in n=2 separate experiments. Tumor growth data in (f), (g) and (h) are representative of two or more independent experiments.
Discussion
A dynamic relationship exists between the genetic background of the host, quiescent immune system status and susceptibility to pathogenic infection, autoimmunity and carcinogenesis.44, 47, 51, 52 In rodents, this relationship is highlighted by the inherent differences in the sensitivity among inbred strains to tumor development following exposure to the same carcinogenic insult.45 The relative susceptibility of a given strain is a heritable trait, an observation supported by the identification of susceptibility loci associated with pathogenic infection and carcinogenesis. Many genetic factors act in a cell-autonomous manner during tumor formation.45, 53 However, it remains less clear how immune signaling networks interface with cell-autonomous genetic traits to modify cancer susceptibility.
The mechanistic details of RON signaling in malignant epithelial cells have been previously reported.54, 55 Additional studies have more recently revealed that RON can modify macrophage responsiveness to TLR4 stimulation.13, 17, 18, 56 Immune cells stimulated by TLR4 ligands evoke a spectrum of cellular changes, which are highly dependent on cell lineage and host background. For example, quiescent macrophages exposed to LPS typically polarize toward an M1 phenotype, as characterized by production of pro-inflammatory factors such as TNF-α, IL-12p40, type-I IFNs and reactive oxygen and nitrogen species. However, if pre-exposed to cytokines such as IL-4, they produce a different set of M2-linked mediators in response to LPS, namely factors associated with the resolution of inflammation or tissue repair, including IL-10, CCL2, CCL17 and TGF-β.31, 57 These observations support the notion that transcriptional regulatory circuits downstream of TLR4 can be dynamically reprogrammed, such that the same input results in distinct functional outcome. Indeed, TLR4-deficient mice are more susceptible to a range of pathogenic infections and show differential tumor susceptibility, depending on the carcinogenic insult.58, 59, 60, 61, 62 Therefore, genetic background in the context of cross talk with the TLR4 pathway may explain certain heritable differences in vaccine responsiveness, susceptibility to pathogens or carcinogenesis in rodents and, more importantly, in humans.
Here, we sought to understand how host genetic background influenced the regulatory effect of the RON pathway on TLR4 signal transduction.17, 18, 63 To explore this, we compared quiescent peritoneal macrophages from FVB with those from C57Bl6 mice, considered quintessential M2/Th2 and M1/Th1 strains, respectively.32 In agreement with recent reports, we found that RON signaling potently modified a number of characteristic M1-associated chemokine and cytokine outputs in M2-prone FVB macrophages.17, 18, 63 However, the impact of RON on TLR4 responsiveness was markedly less pronounced in macrophages isolated from C57Bl6 mice. RON activated common signaling features irrespective of strain background, albeit with some kinetic differences in STAT3 phosphorylation, with the importance of this kinetic difference requiring further evaluation. RON activation by MSP failed to induce a significant cytokine or chemokine response, with the exception of a limited amount of IL-6 production. However, RON signaling in the context of TLR4 significantly modulated a number of chemokines and cytokines. In particular, RON potently modified a number of characteristic M1-associated chemokine and cytokine outputs in M2-prone FVB macrophages. RON did not appear to impact the TLR4-MyD88-dependent signaling axis, as indicated by the lack of effect on p38 MAPK phosphorylation, phosphorylation of the p65 subunit of NF-κB or the lack of impact on early TLR4-induced NF-κB-regulated genes (Figures 2 and 3, and data not shown). MSP induced p38 MAPK phosphorylation at early time points; however, the sustained activation of p38 MAPK was TLR4 dependent. This finding was confirmed using RON-KD macrophages, where the early activation of p38 MAPK by RON was abrogated. This observation supports the finding that RON and TLR4 independently act to induce p38 MAPK phosphorylation. A recent study similarly failed to detect an effect of RON on p38 MAPK phosphorylation in peritoneal macrophages pretreated with MSP.64 However, these conclusions appear to conflict with other studies using tissue-derived macrophages, or a macrophage cell line stimulated with LPS, where the authors observed an impact of RON on the NF-κB pathway.64, 65, 66 This discrepancy may arise from these studies using alveolar macrophages or Kupffer cells, whose response to TLR4 and/or RON may diverge from that of peritoneal macrophages used in our study. It is also possible that the preparations of LPS used in these studies contained impurities, such as bacterial-derived TLR2 ligands, which may initiate distinct signaling networks.67
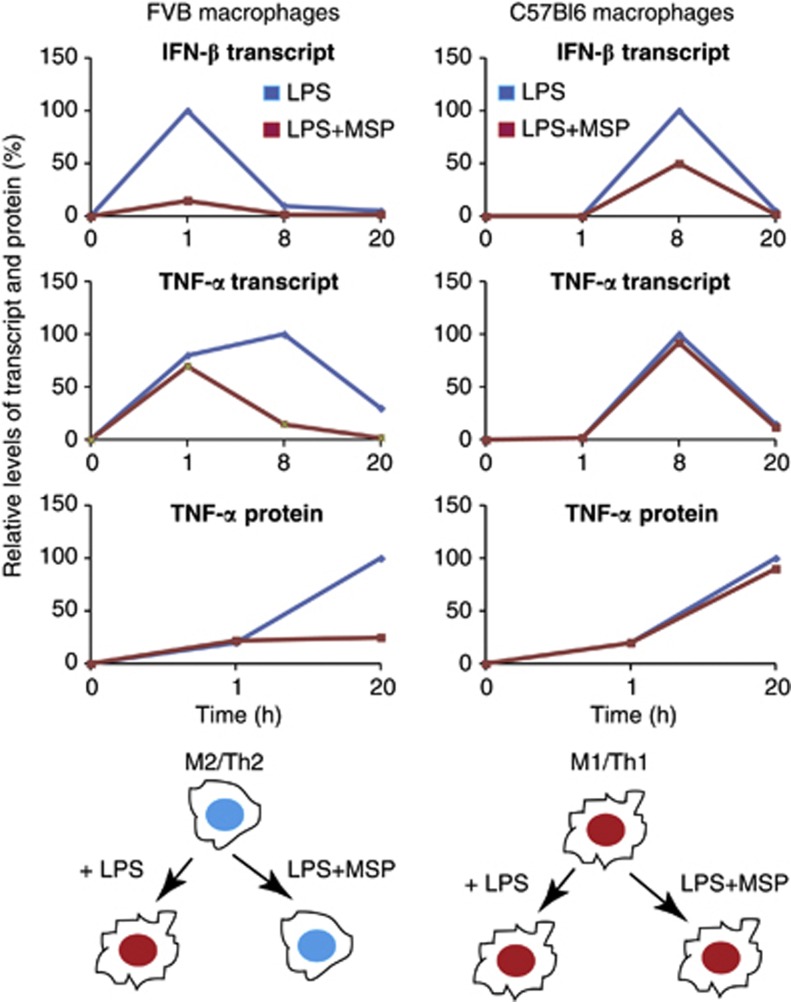
Importantly, we have identified a previously unknown link between RON signaling and the attenuation of TLR4-induced IFN-β gene signature. Type-I IFNs signal through the IFN-α/β receptor and are important mediators of innate and adaptive immunity.48, 68, 69, 70 Moreover, type-I IFNs together with TNF-α are recognized as important modulators of macrophage function, particularly for their ability to polarize cells toward an M1 differentiation state.71, 72, 73, 74 The rapid kinetic repression of IFN-β by RON in FVB macrophages prompted us to explore how this might impact other TLR4-induced inflammatory mediators in this strain (Figure 6). In particular, we observed an early increase in TNF-α mRNA in FVB macrophages (1 h) treated with LPS, as compared with C57Bl6 cells (8 h). Indeed, MSP selectively repressed TNF-α mRNA and protein levels in FVB macrophages. This provided the hypothesis that TNF-α was produced indirectly through early IFN-β production. Owing to poor sensitivity, we were unable to measure IFN-β protein directly from cell supernatants (data not shown); however, we were able to confirm this mechanism using a neutralizing anti-IFN-β antibody. We therefore propose that MSP preserves an M2 differentiation program in LPS-stimulated macrophages from FVB but not from C57Bl6 mice (Figure 6, schematic). Together, these differences exemplify how genetic background can influence the RON pathway's impact on the kinetics and magnitude of TLR4 responses in macrophages.6, 75, 76 This conclusion appears consistent with the finding that IFN-β-deficient C57Bl6 macrophages show no delay in the early kinetics of TNF-α production upon LPS treatment in vitro.37 Conversely, in strains like FVB, or other Th2-predisposed backgrounds, the impact of IFN-β-deficiency may more markedly attenuate TNF-α production in response to danger-associated molecular patterns or pathogen-associated molecular patterns recognized by TLR4. Finally, although RON signaling impacted the type-I IFN pathway in response to LPS, additional effects of the RON TLR4 pathway are likely to be IFN-β independent. This is highlighted by the inability of IFN-β neutralization to affect IL-12p40 or IL-10 production. Additional mechanisms that mediate RON's impact on the TLR4 pathway, such as the augmentation of MCP-1, CSF-2 and IL-10 secretion, remain to be resolved. Interestingly, the p42/44 MAPK inhibitor PD98059 repressed CSF-2 production in RON- and TLR4-co-stimulated macrophages, possibly implicating this signaling axis in CSF-2 production in response to TLR4 stimulation (data not shown).
Figure 6.
Overview of the impact of the RON pathway on M1 versus M2 differentiation program in the context of TLR4 signaling. Transcript and protein levels of IFN-β and TNF-α were compiled from data presented in figures, as described in the text. The IFN-β transcript level was taken from Supplementary Figure S3A (FVB) and from Supplementary Figure S5A (C57Bl6). The TNF-α transcript level was taken from Supplementary Figure S1A (FVB) and Supplementary Figure S2A for C57Bl6 mice. The intermediate time points for TNF-α protein levels in both backgrounds were analyzed (data not shown). Protein or mRNA levels at each time point are expressed as percentage of maximal expression (100%). Optimal TNF-α expression in response to LPS in macrophages from FVB mice was highly dependent on early induction of IFN-β. In contrast, M1/Th1 predisposed macrophages from C57Bl6 mice were mostly refractory to the effects of RON on TNF-α production and IFN-β. We propose that RON signaling in macrophages from FVB mice preserves M2 differentiation in the presence of TLR4 signaling, whereas C57Bl6 macrophages maintain polarization toward M1 cells in the presence of RON signaling.
In summary, we provide evidence that RON sculpts important aspects of M1/M2 macrophage differentiation in response to TLR4 stimulation, in a manner that is highly dependent on genetic background. FVB macrophages polarized to an M2 phenotype upon TLR4 stimulation in the context of RON activation. In contrast, C57/B6 macrophages maintained differentiation to an M1 state. In the M2-prone cells, TLR4 activation in the presence of MSP led to transcriptional upregulation of several genes associated with the wound-healing response, including matrix metalloproteases, other remodeling enzymes and growth factors.31, 75, 77, 78 The function of macrophage invasion during tumorigenesis has been associated with intrinsic immunosuppressive and wound-healing properties. The negative role of macrophages in tumor initiation and progression agrees with clinical studies that implicate macrophage invasion with poor clinical outcomes.28 Indeed, RON kinase deficiency substantially delayed cutaneous papilloma formation and growth in FVB mice, while having minimal effect in the apriori carcinogen-resistant C57Bl6 background. A delay in tumor initiation was also observed in RON-KD FVB mice in the MCA-induced fibrosarcoma model. These results agree with the current paradigm of immuneediting, which links with the role for type-I IFNs in mediating resistance to tumorigenesis by promoting innate and adaptive antitumor immune responses.47, 48 Using a fibrosarcoma transplant model, we were able to evaluate the contribution of innate and cellular immunity to the delay in tumor development in RON-KD mice. Depleting CD8 T cells reversed the marked reduction in tumor engraftment in RON-KD FVB mice. However, CD8 T-cell-depleted RON-KD mice were still able to restrict subcutaneous fibrosarcoma outgrowth. Therefore, although cellular immunity clearly contributed to the ‘elimination phase' during tumor engraftment, the innate immune cell response also contributed to tumor resistance in RON-KD mice. This supports the recent finding that macrophages provide critical effector functions during the cancer immunoediting process.71 Taken together, our results reveal important cross talk between the TLR4 and RON pathways and illustrate how host genetic background can impact immune cell responsiveness, which translates to susceptibility to pathogenic or carcinogenic insults. These findings strengthen the rationale for targeting the RON axis as a viable therapeutic modality, to impact oncogenic signaling in the tumor epithelial compartment, as well as to enhance innate and adaptive antitumor immunity.
Methods
Animals
RON kinase-deficient FVB and C57Bl620 mice were obtained under license from University of Cincinnati, Ohio, and were bred and maintained at Genentech, Inc., under specific pathogen-free conditions. C57Bl6 or FVB (wild-type) mice were obtained from the Jackson Laboratory. All studies were conducted with 6- to 10-week-old animals in accordance with the Guidance for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and approved by Genentech Institutional Animal Care and Use Committee.
Reagents and antibodies
The following reagents were obtained from the indicated sources: macrophage serum-free medium (Invitrogen, Carlsbad, CA, USA), recombinant human MSP (R&D Systems, Minneapolis, MN, USA), ultrapure LPS-EB from Escherichia coli 0111:B4 strain (Invitrogen) endotoxin-free PBS (Invitrogen). Antibodies for Western blot against phosphorylated p42/44 ERK, AKT, p38 and STAT3 (Cell Signaling Technology, Beverly, MA, USA) and β-actin (Sigma, St Louis, MO, USA). All fluorescent secondary antibodies were from Rockland Immunochemicals (Gilbertsville, PA, USA). Anti-F4/80 (clone BM8), anti-CD45 (clone 104) and anti-CD11b (clone M1/70) were used to confirm macrophage purity, and in combination with anti-RON (clone Phage 4) to evaluate RON surface expression. Immune populations were analyzed using a FACScan or LSR II (Becton Dickinson, Franklin Lakes, NJ, USA) using 7AAD to exclude dead cells.
Cells
Quiescent peritoneal macrophages were isolated by peritoneal lavage using 10 ml of macrophage serum-free medium, as previously described.79 For each experiment, peritoneal macrophages of each genetic background were pooled from 20–25 mice. Cells were immediately washed in serum-free media and were plated in six-well plates at a density of 2 × 106 cells per well. Cells were allowed to adhere for 4 h and non-adherent cells were removed by washing with macrophage serum-free medium twice. Macrophage purity was routinely evaluated at greater than 85% by flow cytometry (data not shown).
RNA extraction and microarray analysis
Total macrophage RNA was made using a Qiagen RNA-plus RNA extraction kit (Qiagen, Valencia, CA, USA). Genomic DNA was removed using a DNA elimination kit from Ambion (Invitrogen). Quantity and quality of total RNA samples were determined using a ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE) and Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA), respectively. The method for preparation of Cy-dye-labeled cRNA and array hybridization was provided by Agilent Technologies. In brief, total RNA sample was converted to double-stranded cDNA and then to Cy-dye-labeled cRNA using an Agilent's Quick Amp Labeling Kit. The labeled cRNA was purified using the RNeasy mini kit (Qiagen, San Diego, CA, USA). cRNA yield and Cy-dye incorporation were determined using the ND-1000 spectrophotometer (Thermo Scientific). An amount of 750 ng of the labeled cRNA was fragmented and hybridized to the Agilent's Whole Mouse Genome 4 × 44K arrays as described in the manufacturer's hybridization kit. All samples were labeled with Cy5 and hybridized against Cy3-labeled universal mouse reference (Stratagene, La Jolla, CA, USA). Following hybridization, the arrays were washed, dried and scanned on Agilent's DNA microarray scanner. Agilent's Feature Extraction software 9.5 was used to analyze acquired array images.
Gene expression quantification by reverse transcriptase-PCR
Gene expression profiles were determined using custom 96-well PCR arrays from SABiosciences (Qiagen). The following genes were included: IFN-β1, IRF1, IRF3, IRF7, STAT1, STAT2, STAT3, CXCL-10, NOS2, TNF-α, IL-12p40, IL-10, CSF-2 and GAPDH (along with three internal controls to normalize plate-to-plate variations). An amount of 2 μg total RNA was reverse transcribed using the SABiosciences RT kit (Qiagen) and expression was quantified using SYBR green Supermix using ABI 7500 (Life Technologies, Grand Island, NY, USA).
Western blot analysis
Peritoneal macrophages were treated with 100 ng ml−1 LPS or 100 ng ml−1 MSP alone or in combination, and, at different time points, cells were washed once with cold PBS and lysed for 15 min in × 1 lysis buffer (Cell Signaling Technology) containing protease and phosphatase inhibitors (Sigma-Aldrich, Valencia, CA, USA). Clarified cell lysates were resolved on a 4–16% SDS polyacrylamide gel and transferred to nitrocellulose. Membranes were blocked and probed with appropriate antibodies. Proteins were detected by fluorescence-labeled antibodies using the LI-COR scanner (LI-COR Biosciences, Lincoln, NE, USA).
Measurement of cytokines and chemokines
Cytokines and chemokines secreted in the conditioned media were quantified using the mouse Group-I 23-plex panel (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's protocol. Selected cytokines and chemokines were quantified by ELISA (R&D Systems) according to the manufacturer's protocol.
Carcinogenesis models
Skin carcinogenesis: a dorsal area of mouse skin was shaved 24 h before the application of 100 nmole DMBA dissolved in 50 μl acetone using a micropipette. After 7 days, 40 nmole 12-0-TPA (Sigma-Aldrich) was applied to each mouse using a micropipette. TPA application was continued twice a week until papillomas started appearing. The papillomas were counted every week until the end of the study. Fibrosarcoma tumor initiation: FVB (wild-type) or FVB.RON-KD mice were inoculated subcutaneously in the hind flank with 100 μg of methylcholanthrene (MCA; Sigma-Aldrich) in 0.1 ml of corn oil (Sigma-Aldrich), as previously described.80 Mice were assessed weekly for tumor development from 30 days after MCA treatment. Transplantable tumor cell model: a fibrosarcoma tumor cell line was derived from an MCA-induced sarcoma as previously described.80 Cells were suspended in 200 μl PBS and injected subcutaneously into mice. Mice were monitored twice in a week for tumor growth. For CD8 T-cell depletion experiments; 10 mg per kg of anti-CD8 (clone 2.43 were delivered by intraperitoneal injection on days −7, −4, −1, +2 and +5 during fibrosarcoma tumor cell engraftment.
Analysis of macrophage infiltration in papillomas by immunohistochemistry
Immunohistochemical analysis was performed on 5-μm-thick formalin-fixed, paraffin-embedded tissue sections mounted on glass slides. Macrophage staining was performed using anti-F4/80 (clone BM8).
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Cassady AI, Luchin A, Ostrowski MC, Hume DA. Regulation of the murine TRACP gene promoter. J Bone Miner Res. 2003;18:1901–1904. doi: 10.1359/jbmr.2003.18.10.1901. [DOI] [PubMed] [Google Scholar]
- Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- Wells CA, Ravasi T, Faulkner GJ, Carninci P, Okazaki Y, Hayashizaki Y, et al. Genetic control of the innate immune response. BMC Immunol. 2003;4:5. doi: 10.1186/1471-2172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber DA, Settleman J. Cancer: drivers and passengers. Nature. 2007;446:145–146. doi: 10.1038/446145a. [DOI] [PubMed] [Google Scholar]
- Harris TJ, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol. 2010;7:251–265. doi: 10.1038/nrclinonc.2010.41. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- Iwama A, Wang MH, Yamaguchi N, Ohno N, Okano K, Sudo T, et al. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394–3403. [PubMed] [Google Scholar]
- Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res. 2000;261:229–238. doi: 10.1006/excr.2000.5012. [DOI] [PubMed] [Google Scholar]
- Wang MH, Lee W, Luo YL, Weis MT, Yao HP. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol. 2007;213:402–411. doi: 10.1002/path.2245. [DOI] [PubMed] [Google Scholar]
- Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res. 2008;100:1–33. doi: 10.1016/S0065-230X(08)00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard EJ, Skeel AH. Enhancement of spreading, phagocytosis and chemotaxis by macrophage stimulating protein (MSP) Adv Exp Med Biol. 1979;121B:181–194. doi: 10.1007/978-1-4684-8914-9_16. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Wilson CB, Ray M, Correll PH. Macrophage-stimulating protein, the ligand for the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase, inhibits IL-12 production by primary peritoneal macrophages stimulated with IFN-gamma and lipopolysaccharide. J Immunol. 2004;172:1825–1832. doi: 10.4049/jimmunol.172.3.1825. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Correll PH. Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. J Immunol. 2002;168:853–860. doi: 10.4049/jimmunol.168.2.853. [DOI] [PubMed] [Google Scholar]
- Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Waltz SE, Eaton L, Toney-Earley K, Hess KA, Peace BE, Ihlendorf JR, et al. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest. 2001;108:567–576. doi: 10.1172/JCI11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Fisher JH, Wang MH. Activation of the RON receptor tyrosine kinase inhibits inducible nitric oxide synthase (iNOS) expression by murine peritoneal exudate macrophages: phosphatidylinositol-3 kinase is required for RON-mediated inhibition of iNOS expression. J Immunol. 1998;161:4950–4959. [PubMed] [Google Scholar]
- Sharda DR, Yu S, Ray M, Squadrito ML, De Palma M, Wynn TA, et al. Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. J Immunol. 2011;187:2181–2192. doi: 10.4049/jimmunol.1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline JN, Cowden JD, Hunninghake GW, Schutte BC, Watt JL, Wohlford-Lenane CL, et al. Variable airway responsiveness to inhaled lipopolysaccharide. Am J Respir Crit Care Med. 1999;160:297–303. doi: 10.1164/ajrccm.160.1.9808144. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, Gros P. Forward genetic dissection of innate response to infection in inbred mouse strains: selected success stories. Clin Exp Immunol. 2010;162:393–401. doi: 10.1111/j.1365-2249.2010.04249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29:243–248. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Wei X, Ni S, Correll PH. Uncoupling ligand-dependent and -independent mechanisms for mitogen-activated protein kinase activation by the murine Ron receptor tyrosine kinase. J Biol Chem. 2005;280:35098–35107. doi: 10.1074/jbc.M505737200. [DOI] [PubMed] [Google Scholar]
- Ni S, Zhao C, Feng GS, Paulson RF, Correll PH. A novel Stat3 binding motif in Gab2 mediates transformation of primary hematopoietic cells by the Stk/Ron receptor tyrosine kinase in response to Friend virus infection. Mol Cell Biol. 2007;27:3708–3715. doi: 10.1128/MCB.01838-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Jenkins KA, Mansell A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine. 2010;49:237–244. doi: 10.1016/j.cyto.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281:31119–31130. doi: 10.1074/jbc.M604958200. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Morohoshi K, Suzuki T, Matsushima K. Lipopolysaccharide-inducible gene expression profile in human monocytes. Scand J Infect Dis. 2003;35:619–627. [PubMed] [Google Scholar]
- Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Azuma YT, Matsuo Y, Nakajima H, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J Pharmacol Sci. 2011;115:105–111. doi: 10.1254/jphs.10r02cr. [DOI] [PubMed] [Google Scholar]
- Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis. 2000;59 (Suppl 1:i60–i64. doi: 10.1136/ard.59.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wilson CA, Lee SJ, Zhao X, Benveniste EN. LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood. 2005;106:3114–3122. doi: 10.1182/blood-2005-02-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll VM, Kadioglu A, Cox R, Hume DA, Denny P. Macrophages from BALB/c and CBA/Ca mice differ in their cellular responses to Streptococcus pneumoniae. J Leukoc Biol. 2010;87:735–741. doi: 10.1189/jlb.0509359. [DOI] [PubMed] [Google Scholar]
- Lipoldova M, Demant P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet. 2006;7:294–305. doi: 10.1038/nrg1832. [DOI] [PubMed] [Google Scholar]
- Demant P. Cancer susceptibility in the mouse: genetics, biology and implications for human cancer. Nat Rev Genet. 2003;4:721–734. doi: 10.1038/nrg1157. [DOI] [PubMed] [Google Scholar]
- Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- Kemp CJ. Multistep skin cancer in mice as a model to study the evolution of cancer cells. Semin Cancer Biol. 2005;15:460–473. doi: 10.1016/j.semcancer.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chan EL, Peace BE, Collins MH, Toney-Earley K, Waltz SE. Ron tyrosine kinase receptor regulates papilloma growth and malignant conversion in a murine model of skin carcinogenesis. Oncogene. 2005;24:479–488. doi: 10.1038/sj.onc.1208231. [DOI] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Havelkova H, Badalova J, Svobodova M, Vojtikova J, Kurey I, Vladimirov V, et al. Genetics of susceptibility to leishmaniasis in mice: four novel loci and functional heterogeneity of gene effects. Genes Immun. 2006;7:220–233. doi: 10.1038/sj.gene.6364290. [DOI] [PubMed] [Google Scholar]
- Quan L, Stassen AP, Ruivenkamp CA, van Wezel T, Fijneman RJ, Hutson A, et al. Most lung and colon cancer susceptibility genes are pair-wise linked in mice, humans and rats. PLoS One. 2011;6:e14727. doi: 10.1371/journal.pone.0014727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Xie MH, Yang B, Mahapatra K, Liu J, Marsters S, et al. Distinct involvement of the Gab1 and Grb2 adaptor proteins in signal transduction by the related receptor tyrosine kinases RON and MET. J Biol Chem. 2011;286:32762–32774. doi: 10.1074/jbc.M111.239384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thobe MN, Gray JK, Gurusamy D, Paluch AM, Wagh PK, Pathrose P, et al. The Ron receptor promotes prostate tumor growth in the TRAMP mouse model. Oncogene. 2011;30:4990–4998. doi: 10.1038/onc.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll PH, Iwama A, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Funct. 1997;1:69–83. doi: 10.1046/j.1365-4624.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, et al. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172:6202–6208. doi: 10.4049/jimmunol.172.10.6202. [DOI] [PubMed] [Google Scholar]
- Liu C, Gao F, Li B, Mitchel RE, Liu X, Lin J, et al. TLR4 knockout protects mice from radiation-induced thymic lymphoma by downregulation of IL6 and miR-21. Leukemia. 2011;25:1516–1519. doi: 10.1038/leu.2011.113. [DOI] [PubMed] [Google Scholar]
- Naseemuddin M, Iqbal A, Nasti TH, Ghandhi JL, Kapadia AD, Yusuf N. Cell mediated immune responses through TLR4 prevents DMBA-induced mammary carcinogenesis in mice. Int J Cancer. 2012;130:765–774. doi: 10.1002/ijc.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Yusuf N. Expression of toll-like receptors on breast tumors: taking a toll on tumor microenvironment. Int J Breast Cancer. 2012;2012:716564. doi: 10.1155/2012/716564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QP, Fruit K, Ward J, Correll PH. Negative regulation of macrophage activation in response to IFN-gamma and lipopolysaccharide by the STK/RON receptor tyrosine kinase. J Immunol. 1999;163:6606–6613. [PubMed] [Google Scholar]
- Ray M, Yu S, Sharda DR, Wilson CB, Liu Q, Kaushal N, et al. Inhibition of TLR4-induced IkappaB kinase activity by the RON receptor tyrosine kinase and its ligand, macrophage-stimulating protein. J Immunol. 2010;185:7309–7316. doi: 10.4049/jimmunol.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart WD, Kulkarni RM, Gray JK, Vasiliauskas J, Leonis MA, Waltz SE. Ron receptor regulates Kupffer cell-dependent cytokine production and hepatocyte survival following endotoxin exposure in mice. Hepatology. 2011;53:1618–1628. doi: 10.1002/hep.24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis NM, Gray JK, Gurusamy D, Fox W, Stuart WD, Huber N, et al. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock. 2010;33:197–204. doi: 10.1097/SHK.0b013e3181ae8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Type I interferon: friend or foe. J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209:1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Nathan C. Metchnikoff's Legacy in 2008. Nat Immunol. 2008;9:695–698. doi: 10.1038/ni0708-695. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Zhang X.Activation of murine macrophages Curr Protoc Immunol 2008Chapter 14Unit 14.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM.The isolation and characterization of murine macrophages Curr Protoc Immunol 2008Chapter 14Unit 14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.