Abstract
An elderly woman presented with extensive bruising and a haemorrhagic stroke. Initial investigations revealed an abnormal clotting screen with a prolonged activated partial thromboplastin time. Further investigations revealed this to be due to antibodies that the patient had developed against clotting factor VIII also known as acquired haemophilia A.
Background
This case discusses a relatively common presentation, a haemorrhagic stroke caused by a rare condition, acquired haemophilia A (AHA). It highlights the importance of recognising this condition as a delay in diagnosis can lead to significant morbidity and mortality.
Case presentation
A 72-year-old woman presented to the accident and emergency department with a sudden onset of left-sided weakness which persisted for more than 12 h. Her medical history included Parkinson's disease, Sjorgen's syndrome, hypothyroidism and type II diabetes mellitus. There was no previous surgical history and she was not taking any anticoagulant medication. On examination, the patient was alert and orientated to time, place and person. She was haemodynamically stable with a blood pressure of 130/70, heart rate of 80 and her ECG showed sinus rhythm. Neurological examination revealed a dense left-sided hemiparesis (upper limb power 1/5, lower limb power 3/5). She was also found to have extensive bruising over her left shoulder, arm and chest wall extending to her abdomen which was attributed to a fall due to her weakness.
Investigations
Her full blood count and clotting profile are shown below and revealed a raised activated partial thromboplastin time (APTT). A CT scan of her brain showed evidence of an intracranial haemorrhage in the right thalamus region.
Haemoglobin: 13.5 g/dL (11.6–16.5)
White cell count: 11.5 109/L (3.9–11.1)
Platelet count: 259 109/L (150–450)
Prothrombin time (PT): 11.7 s (9.8–12.7)
APTT: 53 s (24.4–35.1)
APTT ratio: 1.75 (0.8–1.2)
Differential diagnosis
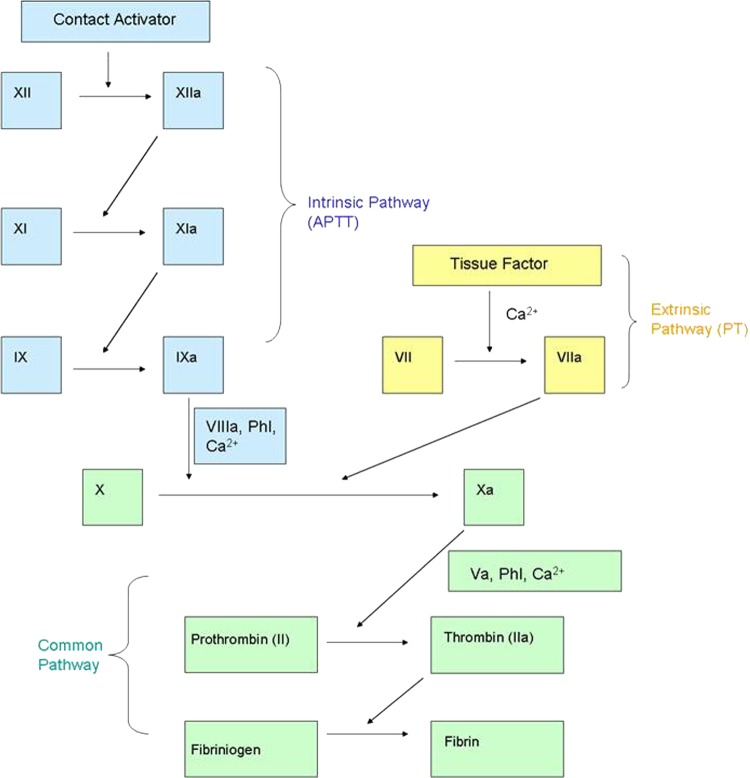
An isolated finding of a raised APTT suggested an underlying coagulation defect as a cause of the haemorrhagic stroke and pointed towards a problem with the ‘intrinsic’ pathway of the coagulation cascade (figure 1). The separation of the clotting cascade into ‘intrinsic’ and ‘extrinsic’ pathways has little relevance to what actually is thought to occur in vivo, but they remain useful concepts when interpreting APTT and PT results. Figure 1 describes the clotting factors involved in each of these pathways.
Figure 1.
The ‘intrinsic’ and ‘extrinsic’ parts of the clotting cascade. Activated partial thromboplastin time (APTT): contact activator, for example, kaolin or micronised silica is added to the test sample which produces factor XIIa, this activates the other clotting factors in the cascade. The APTT provides information on the ‘intrinsic’ pathway. Prothrombin time (PT): thromboplastin which contains tissue factor is added to the patient's plasma which leads to the activation of factor VII. The PT provides information on the ‘extrinsic’ pathway.
The potential causes of a raised APTT in this patient were as follows:
Deficiencies in clotting factors XII, XI, IX, VIII (intrinsic pathway) X V, II (common pathway) or von Willebrand 's disease by causing low factor VIII levels.
The presence of coagulation inhibitors (either antiphospholipid antibodies or antibodies against the clotting factors listed above).
Heparin use.
The patient was not on heparin. To differentiate between a clotting factor deficiency or the presence of an inhibitor, a mixing study was performed next. This involved mixing the patient's blood with normal pooled plasma. If there is a clotting factor deficiency, then the APTT should correct after mixing with normal pooled plasma as this would provide the deficient clotting factor. If the APTT does not correct fully or only partially, then the patient must have an inhibitor to clotting in their plasma. These inhibitors can be antibodies directed against a particular clotting factor (most commonly factor VIII) or against phospholipids such as lupus anticoagulants. If a lupus anticoagulant is suspected, further tests can be performed to confirm this (eg, dilute Russell's viper venom time). Factor VIII inhibitors can be time dependent and temperature dependent; therefore, if a mixing study is performed and incubated at 37°C, the inhibitor will work increasingly better at neutralising the clotting factor the longer the test is run.
The results of the mixing studies, incubation studies and factor VIII assay for the patient in this case are shown below (the lupus anticoagulant screen was negative and the other clotting factor assays were normal):
Baseline APTT-53 s (normal range 24.4–35.1)
APTT 80 : 20–43 s (partial correction of APTT)
APTT 80 : 20-(after 1 h incubation) 49.3 seconds
APTT 80 : 20-(after 2 h incubation) 57.5 seconds
Factor VIII assay 19% (50–150)
(In our laboratory, the mixture studies were performed with 80% of the patient's plasma and 20% of normal plasma.)
There was only partial correction of the APTT with the 80 : 20 mix. With incubation, the APTT increased with time (suggesting a time-dependent inhibitor) and the factor assays showed a low factor VIII level at 19%. These results suggest a diagnosis of AHA (an inhibitor present in the patient's blood to factor VIII).
Outcome and follow-up
The patient was started on 60 mg (1 mg/kg) of prednisolone to eradicate the inhibitor, with a response after 5 weeks which was demonstrated by a normal APTT, normal factor VIII levels and no inhibitor detected. After 5 weeks, the dose of prednisolone was tapered down. There were no further episodes of bleeding and multidisciplinary care was given for the stroke.
Discussion
AHA is a rare condition with an incidence of 1 case/million/year that is higher in the elderly (14 cases/million/year in patient's aged over 85).1 It is caused by auto-antibodies produced against factor VIII resulting in an increased bleeding tendency. Patients usually present with a pattern of subcutaneous, mucosal or soft tissue bleeding and can experience life-threatening gastrointestinal or intracranial bleeds as in this case. There is often a delay in diagnosis and appropriate treatment, contributing to a mortality rate of 8–22%.2
AHA is associated with other diseases in up to 50% of cases most commonly conditions involving dysregulation of the immune system such as rheumatoid arthritis, systemic lupus erythematosus, polymyalgia rheumatica and Sjorgen's syndrome. There is also an association with malignancy, dermatological conditions such as pemphigus and pregnancy. These associated conditions, especially malignancy in the elderly patient, should be considered. Specialist centres should be involved from an early stage and treatment started promptly as the risk of serious bleeding remains while the inhibitor is present. As bleeding can occur after minimal trauma/contact, patients should be carefully nursed with care taken when taking blood pressure measurements and venepuncture attempts kept to a minimum. Management of these patients has two main objectives: the first is to control any ongoing bleeding with the use of bypassing agents and the second is to eradicate the inhibitor with immunosuppression.
Owing to the relatively low incidence of AHA, there is a lack of prospective, randomised controlled trials evaluating the different therapies, for both inhibitor eradication and to stop bleeding, with published data largely consisting of case reports and small cohort studies. The recent publication of data from the European Acquired Haemophilia Registry (EACH2) involving 117 centres in 13 European countries has been a significant and important addition to this evidence base. There are two bypassing agents used currently: recombinant activated factor VII (Novoseven) which directly activates factors IX and X circumventing the need for factor VIII and factor eight inhibitor bypassing agent (FEIBA) which contains activated clotting factors (II, VII, IX, X). Data from the EACH2 registry found a significantly higher rate of bleeding control with the use of these bypassing agents compared with factor VIII or desmopressin (DDAVP). Both Novoseven and FEIBA showed similar rates of bleeding control in 219 patients (91% and 93%, respectively).3
To eradicate the inhibitor, the results from the EACH2 registry showed that steroids combined with cyclophosphamide resulted in a higher rate of stable complete remissions compared with steroids alone or rituximab (an anti-CD 20 monoclonal antibody)-based regimens4 (cyclophosphamide was not given in this case because of the patient's comorbidities). Eradicating the inhibitor can take up to 5 weeks and a significant proportion of patients will relapse, patients therefore require long-term follow-up.
Learning points.
Abnormal clotting screen results should be investigated promptly as delay can lead to significant morbidity and mortality from undiagnosed bleeding disorders.
When faced with an unexpectedly high activated partial thromboplastin time and/or prothrombin time, a mixing study in the first instance will help to narrow down the differential diagnoses.
Acquired haemophilia A requires prompt recognition and treatment as the bleeding risk is high.
Footnotes
Contributors: SA is the main author of the case report text, with other authors being involved in the literature review of the case report, providing guidance on the manuscript and contributing modifications to the draft manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Collins PW, Percy CL. Advances in the understanding of acquired haemophilia A: implications for clinical practice. Br J Haematol 2010;2013:183–94 [DOI] [PubMed] [Google Scholar]
- 2.Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Am Soc Hematol Educ Program 2006;2013:432–7 [DOI] [PubMed] [Google Scholar]
- 3.Baudo F, Collins P, Huth-Kuhne A, et al. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood 2012;2013:39–46 [DOI] [PubMed] [Google Scholar]
- 4.Collins P, Baudo F, Knoebl P, et al. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012;2013:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]