Abstract
A rare case of large adrenal mass which was non-functioning is presented. It is difficult to make preoperative diagnosis in these cases as the imaging findings are non-specific. Radical excision is mandatory as preoperative malignancy cannot be ruled out.
Background
The differential diagnosis for large adrenal masses (more than 6 cm) includes phaeochromocytoma, adrenal cortical tumour, Cushing's syndrome and adrenal metastasis.1 Inflammatory myofibroblastic tumour (IMT) is a rare tumour of unknown aetiology, occurring at various sites such as lungs, gastrointestinal tract, orbit and genitourinary tract.2 IMTs of the adrenal are rare and only two cases of adrenal IMT have been reported in literature.3 4 Imaging features of IMT are non-specific and hence, it is a dilemma to preoperatively differentiate these types of tumour from other conditions of adrenal gland. Histopathologically, it is composed of spindle cells (myofibroblasts) with inflammatory components like lymphocytes and plasma cells. We hereby report a case of large adrenal mass which was reported on histopathology as adrenal IMT.
Case presentation
A 20-year-old boy presented with dull left flank ache. He was otherwise healthy without any endocrine symptoms. Family history was not suggestive of either multiple endocrine neoplasia syndrome, neurofibromatosis 1,Von Hippel-Lindau disease or familial pheochromocytoma/paraganglioma syndrome. Physical examination was essentially normal. His serum biochemical parameters for adrenal mass work-up were normal. The 24 h urinary catecholamines like metanephrine, normetanephrine and vanillylmandelic acid were within normal limits. Ultrasound abdomen revealed a 6 cm×6 cm hypoechoic mass occupying the left suprarenal region. Contrast enhanced CT (CECT) showed a well-defined heterogeneous hypodense lesion of size 6.8 cm × 7 cm×5.5 cm with mild non-uniform arterial, porto-venous and delayed-phase enhancement while no evidence of haemorrhage/calcification was seen, involving the left adrenal gland (figure 1A,B). The mass had replaced the left adrenal gland. The opposite gland was normal and there was no evidence of any other mass lesion in the abdomen.
Figure 1.
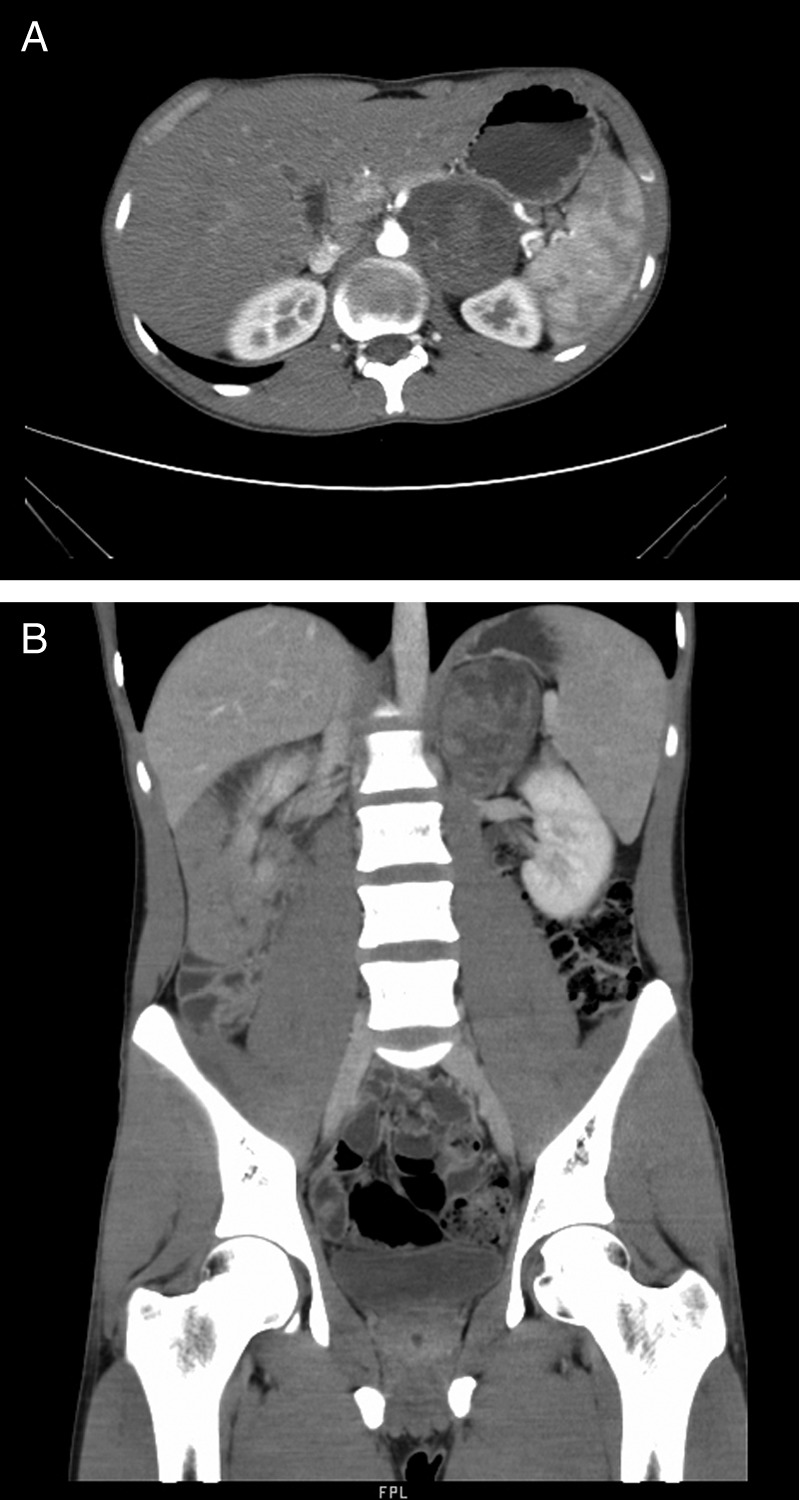
(A) Contrast enhanced CT (CECT) axial images showing a 6.8 cm×7 cm×5.5 cm well-defined heterogeneous hypodense lesion in the left adrenal gland. (B) CECT coronal images showing a 6.8 cm×7 cm×5.5 cm well-defined heterogeneous hypodense lesion in the left adrenal gland.
The patient was planned for exploration, with preoperative preparation for any intraoperative haemodynamic changes. Under general anaesthesia, in the left flank position, the transperitoneal approach through the bed of the 11th rib, exploration revealed a 7 cm×6 cm encapsulated tumour of the left adrenal gland was found displacing the left renal vein. A complete surgical extirpation of the tumour was performed. There were no changes in the haemodynamic status during and after the resection of tumour. The postoperative course was uneventful. The excised specimen was sent for histopathological examination.
On gross examination, an encapsulated greyish brown mass measuring 6.5×6 × 5 cm with cut section showing pale yellow myxoid areas and peripherally compressed adrenal gland was seen. Histopathological study showed an encapsulated tumour composed of elongated spindle-shaped cells with vesicular elongate, indented nucleus and abundant eosinophilic cytoplasm arranged in focal fascicles and in singles suspended in abundant myxoid stroma with interspread thin and thick walled blood vessels, scattered ganglion-like cells admixed with lymphocytes, plasma cells, eosinophils and few neutrophils (figure 2A,B). The features were suggestive of adrenal IMT.
Figure 2.
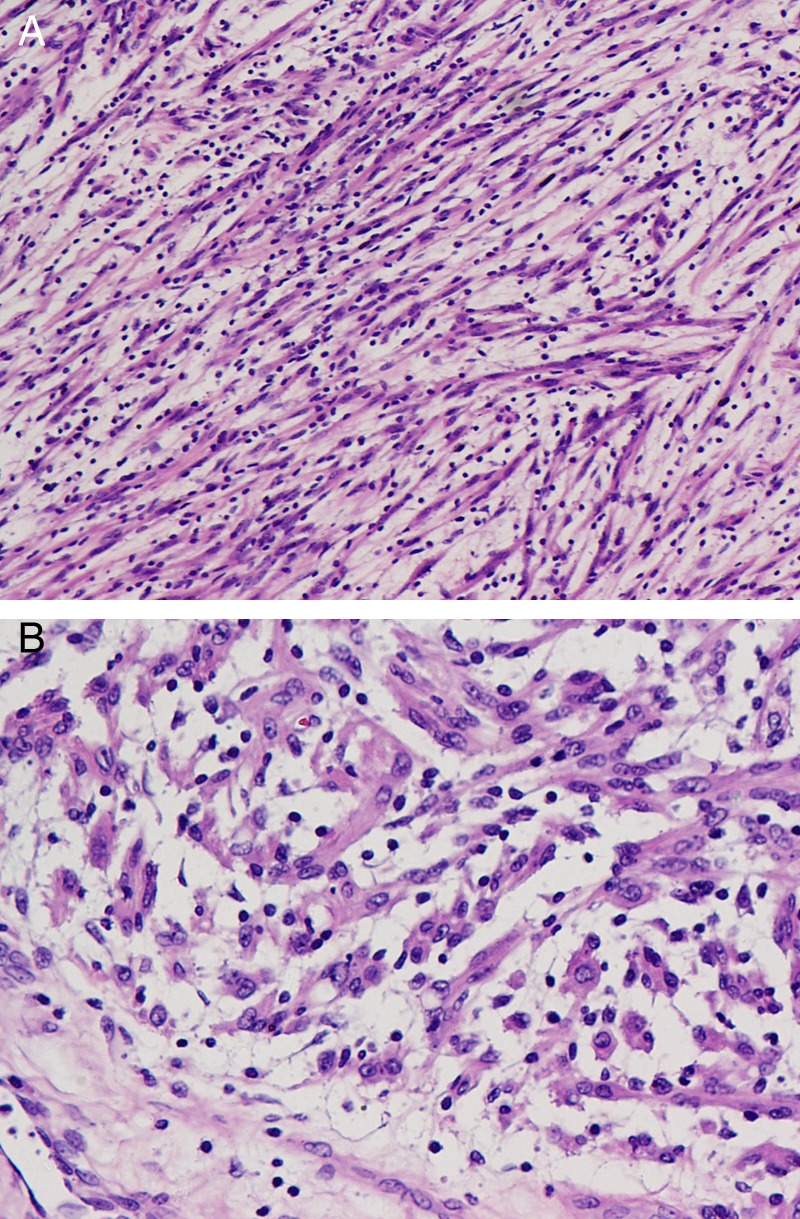
(A) Section shows elongated spindle-shaped cells suspended in abundant myxoid stroma with a sprinkling of lymphocytes. H&E, ×200 (B).Scattered ganglion-like rounded cells with enlarged nucleus seen. H&E, ×400.
Investigations
Ultrasound of the abdomen revealed a 6 cm×6 cm hypoechoic mass occupying the left suprarenal region. CECT showed a well-defined heterogeneous hypodense lesion of size 6.8 cm × 7 cm×5.5 cm with mild non-uniform arterial, porto-venous and delayed-phase enhancement and no evidence of haemorrhage/calcification was seen, involving the left adrenal gland (figure 1A,B). The mass had replaced the left adrenal gland. The opposite gland was normal and there was no evidence of any other mass lesion in the abdomen.
Differential diagnosis
Phaeochromocytoma, adrenal cortical tumour, Cushing's syndrome, adrenal metastasis.
Treatment
The patient was planned for exploration, with preoperative preparation for any intraoperative haemodynamic changes. Under general anaesthesia, in the left flank position, the transperitoneal approach through the bed of the 11th rib, exploration revealed a 7 cm×6 cm encapsulated tumour of the left adrenal gland was found displacing the left renal vein. A complete surgical extirpation of the tumour was performed. There were no changes in the haemodynamic status during and after the resection of tumour. The postoperative course was uneventful. The excised specimen was sent for histopathological examination.
Outcome and follow-up
The patient is doing well after 3 months follow-up.
Discussion
IMT is a relatively rare neoplasm. It was previously referred as plasma cell granuloma. IMT was first observed by Brunn in 1939. It was later termed inflammatory pseudotumour by Umiker and Iverson in 1954. It has long been debated regarding the origin of IMT, whether it was truly neoplastic or a postinflammatory process. IMT is usually seen in the lung, followed by the orbit.5 In the genitourinary tract, it most commonly occurs in the bladder. However, it rarely originates in the kidney, renal pelvis and ureter. Only two cases of adrenal IMT have been reported so far. Commonly seen in childhood or in adults, these tumours are considered benign. It has been described in almost any location, in both sexes and at all ages. They are usually asymptomatic; they rarely have symptoms like loss of weight, fever, decreased appetite and other vague symptoms. Similarly, our patient presented with dull flank ache without any associated symptoms.
On imaging, ultrasonography shows a variable pattern of echogenicity, and the lesion has been described as hypoechogenic or hyperechogenic with ill-defined or well-defined margins.6 CECT may show homogeneity or heterogeneity and hypodensity, isodensity or hyperdensity.7 IMT of the urinary tract is extremely difficult to distinguish from malignant tumours. In this patient, the CECT of abdomen disclosed findings of heterogeneous hypodense mass in left adrenal, which were non-specific.
Grossly, IMTs may be firm, fleshy or gelatinous, with a white or tan cut surface. Calcification, haemorrhage and necrosis are identified in a minority of cases. Tumours range from 1 to 20 cm in greatest dimension, with a mean size of 6 cm. The histological differential diagnosis of IMT depends in part on the dominant pattern: myxoid/vascular, compact spindle cell or fibromatosis-like. It is characterised on the basis of electron microscopic and immunohistochemical findings.8 The WHO continues to classify IMT as a distinct borderline lesion with uncertainty as to whether it is reactive or neoplastic in nature. However, recent research suggests that IMT is probably a neoplasm with cytogenetic clonality, involvement of chromosomal region 2p23, occasional aggressive local behaviour and evidence of metastasis.6 Chromosomal translocations leading to activation of the anaplastic lymphoma kinase (ALK) tyrosine kinase and overexpression of the ALK protein can be detected in approximately 50% of IMTs, but are uncommon in older patients. Histological predictors of aggressive behaviour and metastatic potential include presence of ganglion-like cells, cellular atypia, aneuploidy and p53 overexpression.9 The present case showed scattered ganglion-like cells with enlarged nucleus (figure 2B).
The preoperative differentiation from other adrenal masses is very difficult. Though IMT will not make its place in the common differential diagnosis of adrenal masses, it should be kept in mind as one of the probability in background of indolent presentation and non-specific imaging findings. The definitive treatment is complete surgical resection of the tumour. Although recurrence of IMT arising from urinary bladder has been reported, recurrence of IMT in adrenal gland is yet not known. Still, it is advisable to keep these patients on regular follow-up.
Learning points.
Adrenal inflammatory myofibroblastic tumour is a rare neoplasm of uncertain biological potential.
Imaging findings are non-specific.
Complete surgical resection remains the mainstay of the treatment.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Belldegrun A, Hussain S, Seltzer SE, et al. The incidentally discovered adrenal mass: a therapeutic dilemma-BWH experience 1976–1983. Surg Gynecol Obstet 1986;2013:203–8 [PubMed] [Google Scholar]
- 2.Narla LD, Newman B, Spottswood SS, et al. Inflammatory pseudotumor. Radiographics 2003;2013:719–29 [DOI] [PubMed] [Google Scholar]
- 3.Luo LK, Shen HF, Zhou SY, et al. Inflammatory myofibroblastic tumor of adrenal. Chin J Pathol 2006;2013:252. [PubMed] [Google Scholar]
- 4.Wang T-Y, Chou J-W, Shih Y-S. Inflammatory myofibroblastic tumor mimicking adrenal incidentaloma. Inter Med 2011;2013:165–6 [DOI] [PubMed] [Google Scholar]
- 5.Umiker WO, Iverson L. Postinflammatory tumors of the lung; report of four cases simulating xanthoma, fibroma, or plasma cell tumor. J Thorac Surg 1954;2013:55–63 [PubMed] [Google Scholar]
- 6.Materne R, Van Beers BE, Gigot JF, et al. Inflammatory pseudotumor of the liver: MRI with mangafodipir trisodium. J Comput Assist Tomogr 1998;2013:82–4 [DOI] [PubMed] [Google Scholar]
- 7.Abehsera M, Vilgrain V, Belghiti J, et al. Inflammatory pseudotumor of the liver: radiologic–pathologic correlation. J Comput Assist Tomogr 1995;2013:80–3 [DOI] [PubMed] [Google Scholar]
- 8.Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;2013:859–72 [DOI] [PubMed] [Google Scholar]
- 9.Attili SVS, Rama Chandra C, Hemant DK, et al. Retroperitoneal inflammatory myofibroblastic tumor. World J Surg Oncol 2005;2013:66. [DOI] [PMC free article] [PubMed] [Google Scholar]


