Abstract
Left ventricular non-compaction (LVNC) is a rare disorder caused by the arrest of myocardial compaction during embryogenesis, leading to a non-compacted endocardial layer with marked trabeculations. The diagnosis is primarily based on echocardiographic demonstration of a spongy myocardium. Here, we present a young male with LVNC presenting with left heart failure and multiple left ventricular thrombi. We also review the presentation, diagnosis and management of this condition.
Background
Isolated left ventricular non-compaction (LVNC) is an extremely rare disease due to the arrest of myocardial compaction with an estimated prevalence between 0.05% and 0.24%.1 More recently, however, with improved diagnostic imaging, the frequency of this disease has dramatically risen in both children and adults.2 Morphologically, the disease is characterised by a spongy left ventricle with prominent myocardial trabeculations and deep intertrabecular recesses, leading to systolic dysfunction.3 Clinical presentation ranges from no symptoms to congestive heart failure, arrhythmias and thromboembolic events.4 Early diagnosis as in our case seems to be imperative to prevent significant morbidity and mortality.
Case presentation
A 28-year-old Hispanic man presented with increasing shortness of breath, weight gain and orthopnoea for 10 days. He denied any chest pain, sore throat or upper respiratory tract symptoms. He had an unremarkable medical history and denied alcohol and drug abuse. Family history was negative for any cardiac disease. Vital signs on admission were blood pressure 112/77 mm Hg, heart rate 100/min, respiratory rate 18/min, temperature 36.9°C and oxygen saturation of 94% on room air. Systemic examination was positive for distended neck veins, bilateral basilar rales, S3 gallop and marked pedal oedema.
Investigations
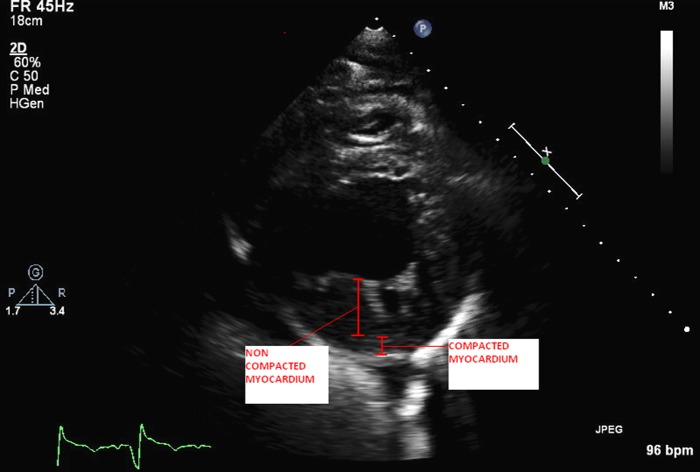
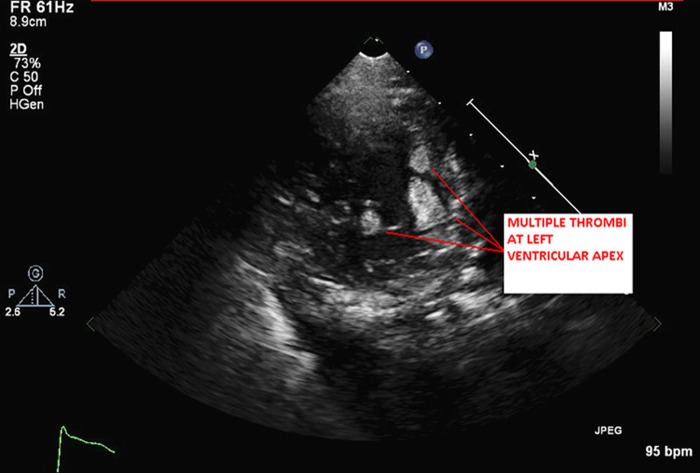
The patient's white cell count and troponin were within normal limits. Chest radiography revealed bilateral infiltrates suggestive of pulmonary oedema. Brain natriuretic peptide was elevated at 751 pg/mL. An ECG showed sinus tachycardia with left ventricular hypertrophy with secondary repolarisation abnormality and left anterior fascicular block. QRS duration was prolonged at 130 ms. Transthoracic echocardiogram (TTE) revealed an enlarged and dilated left ventricle (left ventricular end diastolic dimension of 6.5 cm) with severe global left ventricular systolic dysfunction. Left ventricular ejection fraction (LVEF) was calculated at 17% by biplane Simpson's method. There were marked trabeculations, especially in the mid to apical portions of the left ventricle consistent with non-compaction cardiomyopathy (figure 1 and video 1). Multiple left ventricular thrombi were noted at the cardiac apex within the trabeculations (figure 2 and video 2).
Figure 1.
Parasternal short-axis view at the level of the mid left ventricle showing the compacted layer of myocardium and the non-compacted layer of myocardium with trabeculations and intertrabecular recesses by transthoracic echocardiography.
Figure 2.
Parasternal short-axis view at the left ventricular apex showing multiple thrombi within the deep intertrabecular recesses.
Parasternal short axis view at the level of the mid left ventricle showing the compacted layer of myocardium and the non-compacted layer of myocardium with trabeculations and intertrabecular recesses by transthoracic echocardiography.
Parasternal short axis view at the left ventricular apex showing multiple thrombi within the deep intertrabecular recesses.
Differential diagnosis
Myocarditis was ruled out on the basis of normal troponin levels and characteristic echocardiographic findings. Ischaemic cardiomyopathy was unlikely based on his age, global cardiomyopathy and characteristic features of LV non-compaction in the TTE. Subsequently, he underwent coronary angiography at a different time, which revealed the absence of angiographically significant coronary artery disease.
Treatment
He was started on lisinopril 5 mg daily and furosemide 40 mg daily for the management of heart failure and intravenous heparin anticoagulation for his left ventricular thrombi and was subsequently transitioned to oral warfarin. The patient showed clinical improvement in his heart failure and was discharged on the eighth day of hospital admission. Low-dose metoprolol was started on the day of discharge with a plan to up titrate the dose in subsequent weeks.
Outcome and follow-up
Follow-up echocardiogram at 3 months revealed stable LVEF of 15%. Previously seen LV thrombi had resolved at this time. He still had New York Heart Association (NYHA) class III symptoms at this time. ECG showed the prolongation of QRS duration at 160 ms. Subsequently, a cardiac resynchronisation therapy defibrillator (CRT-D) device was implanted for the treatment of heart failure and primary prevention of sudden cardiac death. At present, the patient is having stable heart failure symptoms without further thromboembolic complications and malignant arrhythmias. He is maintained on oral anticoagulation with warfarin. He is referred for a heart transplantation evaluation. All first-degree relatives were advised to undergo screening echocardiogram.
Discussion
LVNC is a rare and distinct form of cardiomyopathy. In the largest series of patients with isolated LVNC, the prevalence was 0.014% in patients referred to the echocardiography laboratory.1 It is characterised by the presence of excessive and prominent trabeculations along with deep recesses that communicate with the ventricular cavity, but not the coronary circulation.2 There is a male preponderance (56–82% of total cases). The age group is highly variable, and cases have been reported in infants as well as the elderly.3
Heart failure, arrhythmias and embolic events are common presenting features of this condition. Life-threatening ventricular tachy-arrhythmias are reported in 20% of patients and they remain as a concern for sudden cardiac death, mostly in adults with advanced disease.4 Adults are known to have severe disease and higher mortality in comparison to children.5 Heart failure is the presenting feature in over two-thirds of cases with LVNC.6 LV failure is attributed to subendocardial hypoperfusion and microcirculatory dysfunction associated with this condition.6 LV systolic dysfunction, rhythm disturbances and the presence of blood in the deep intertrabecular recesses contribute to intraventricular thrombus formation.5 LVNC is sometimes known to be associated with other congenital cardiac conditions such as atrial and ventricular septal defects, congenital aortic stenosis and coarctation of the aorta.7 There are some reports of unstable conduction system and recurrent arrhythmias seen in echocardiography-documented non-compacted ventricle with normal ejection fraction.
The aetiology of LVNC is largely unknown. The most widely believed theory is the disturbance in compaction of myocardium during embryogenesis, which occurs between 5 and 10 weeks of gestation.8 LVNC is often familial with generally autosomal dominant inheritance, but with variable penetration.5 At least nine genes have been reported in patients with LVNC including genes encoding LIM domain binding protein 3 (LDB3), α-dystrobrenin (DTNA), tafazzin (TAZ), lamin A/C (LMNA), β-myosin heavy chain (MYH7), α-cardiac actin (ACTC), cardiac troponin T (TNNT2), SCN5A and tropomyosin 1 (TPM1).9 There is some evidence of an acquired form of LVNC in adulthood, as a compensatory response to an impaired myocardium10 Such cases have been reported in conjunction with other diseases most notably, neuromuscular dystrophy.8
TTE is the most common modality of diagnosis. Other tests used for diagnosis include transoesophageal echocardiography, cardiac MRI, contrast ventriculography and CT. There is no universally accepted definition of LVNC at present. There are several echocardiographic criteria proposed for the diagnosis of LVNC (box 1). The echocardiographic criterion proposed by Jenni et al5 is the most commonly accepted criterion. It requires the absence of any coexisting cardiac anomalies, presence of numerous and excessively prominent trabeculations and deep intertrabecular recesses which are supplied by intraventricular blood on colour flow Doppler. It also requires the presence of a two-layer structure, with a thin compacted layer (C) and a thick non-compacted layer (N) measured in end systole at the parasternal short-axis views, where LVNC is defined by a ratio of N/C >2.1 MRI has been increasingly used to describe the morphological appearance of the myocardium. Jacquier et al used the trabeculated LV mass of >20% of the global LV mass as the definition of LVNC. Using the non-compacted to compacted ratio of >2.3 in diastole by MRI, Peterson et al4 found sensitivity and specificity of 86% and 99% respectively, in the diagnosis of LVNC. However, it is important to note that LVNC can often be over diagnosed based on hypertrabeculation alone in a patient with normal LV systolic function. Such cases should not be labelled as LVNC as the risk of progression of these cases to LV impairment remains low. Comprehensive clinical assessment, multimodality imaging, systematic screening of first-degree relatives and a genetic assessment can be helpful in such cases.
Box 1. Different echocardiographic criteria for the diagnosis of left ventricular non-compaction.
Jenni et al.5
All three criteria required are for diagnosis.
A thickened left ventricular wall consisted of two layers: a thin compacted outer myocardial layer and a thick inner non-compacted myocardial layer with numerous prominent trabeculations and deep recesses with a maximum ratio of non-compacted to compacted myocardium >2:1 at end-systole in the parasternal short-axis view.
Colour Doppler evidence of flow within the deep intertrabecular recesses.
Prominent trabecular meshwork in the left ventricular apex or mid-ventricular segments of the inferior and lateral wall.
Chin et al.2
The presence of X/Y ≤0.5, where X is the distance from the epicardial surface to the trough of the trabecular recess and Y is the distance from the epicardial surface to peak of trabeculation. This criterion is applied to trabeculae at the left ventricular apex on subxiphoid or apical four-chamber views at end-diastole.
Stöllberger et al.11
More than three trabeculations protruding from the left ventricular wall, apically to the papillary muscles, visible in a single image plane.
Intertrabecular spaces perfused from the ventricular cavity, visualised on colour Doppler imaging.
Histologically, the findings are non-specific, with areas of fibrosis interspersed with normal myocytes.7 Electrocardiographic abnormalities are seen in the majority of patients with LVNC, and range from left ventricular hypertrophy and repolarisation changes to inverted T waves, non-specific ST segment changes, AV block and intraventricular conduction abnormalities.6
Management of LVNC is mainly focused on control of symptoms and prevention of complications. Standard treatment of systolic and diastolic heart failure is recommended for patients with LVNC and heart failure. Cardiac failure is medically managed with β-blockers, diuretics and ACE inhibitors. Carvedilol, in particular, has been shown to improve left ventricular function, mass and neurohormonal dysfunction12 Because deep intertrabecular recesses with sluggish blood flow increase the risk of thrombus formation, some authorities recommend anticoagulation in patients with impaired systolic function (LVEF <40%) for the prevention of thromboembolism, although robust data are lacking to support this strategy.8 Regression of LVNC has also been reported following appropriate treatment13 14 Implantable defibrillators are recommended for the primary prevention of sudden cardiac death in patients with severe LV systolic dysfunction. Biventricular pacing is recommended in patients with severe LV systolic dysfunction and symptomatic heart failure.7 In refractory cases with dilated cardiomyopathy, cardiac transplantation is an option for treatment.
The prognosis of LVNC is poor and larger left ventricular end-systolic diameter at initial presentation, NYHA class III/IV heart failure, chronic atrial fibrillation and the presence of bundle branch block are markers of poor prognosis.1 As many as 47–60% of adults die or undergo cardiac transplantation within 4–6 years.1 A familial occurrence has been shown in as many as 18–51% of the cases,5 which is the rationale for screening echocardiogram in the first-degree relatives.
Learning points.
Despite being a rare condition, left ventricular non-compaction is being recognised more frequently than before due to increased awareness about its natural history, clinical manifestations and improved modalities of cardiac imaging.
If not recognised early, LVNC can present with fatal arrhythmias, sudden cardiac death and systemic embolism, which can lead to significant morbidity and mortality. It is important for physicians to recognise this condition in anticipation of successful treatment.
Because of strong familial occurrence, all first-degree relatives are recommended to have screening echocardiography.
Footnotes
Contributors: MRA and SG wrote the initial manuscript. MB collected the images and edited initial manuscript. MRA and RP were involved in the final revision of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Oechslin EN, Attenhofer Jost CH, Rojas JR, et al. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000;2013:493–500 [DOI] [PubMed] [Google Scholar]
- 2.Chin TK, Perloff JK, Williams RG, et al. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990;2013:507–13 [DOI] [PubMed] [Google Scholar]
- 3.Ichida F. Left ventricular noncompaction. Circ J 2009;2013:19–26 [DOI] [PubMed] [Google Scholar]
- 4.Oechslin E, Jenni R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J 2011;2013:1446–56 [DOI] [PubMed] [Google Scholar]
- 5.Suvarna JC, Deshmukh CT, Hajela SA. Left ventricular noncompaction: a cardiomyopathy often mistaken. Indian J Med Sci 2009;2013:303–7 [PubMed] [Google Scholar]
- 6.Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation 2004;2013:2965–71 [DOI] [PubMed] [Google Scholar]
- 7.Pantazis AA, Elliott PM. Left ventricular noncompaction. Curr Opin Cardiol 2009;2013:209–13 [DOI] [PubMed] [Google Scholar]
- 8.Finsterer J. Left ventricular non-compaction and its cardiac and neurologic implications. Heart Fail Rev 2010;2013:589–603 [DOI] [PubMed] [Google Scholar]
- 9.Xing Y, Ichida F, Matsuoka T, et al. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab 2006;2013:71–7 [DOI] [PubMed] [Google Scholar]
- 10.Hutchins IM, Schaefer S. Progressive left ventricular noncompaction and systolic dysfunction. Exp Clin Cardiol 2012;2013:81–3 [PMC free article] [PubMed] [Google Scholar]
- 11.Stöllberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol 2002;2013:899–902 [DOI] [PubMed] [Google Scholar]
- 12.Toyono M, Kondo C, Nakajima Y, et al. Effects of carvedilol on left ventricular function, mass and scintigraphic findings in isolated left ventricular non-compaction. Heart 2001;2013:E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stöllberger C, Keller H, Finsterer J. Disappearance of left ventricular hypertrabeculation/noncompaction after biventricular pacing in a patient with polyneuropathy. J Card Fail 2007;2013:211–14 [DOI] [PubMed] [Google Scholar]
- 14.Eurlings LWM, Pinto YM, Dennert RM, et al. Reversible isolated left ventricular non-compaction? Int J Cardiol 2009;2013:e35–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal short axis view at the level of the mid left ventricle showing the compacted layer of myocardium and the non-compacted layer of myocardium with trabeculations and intertrabecular recesses by transthoracic echocardiography.
Parasternal short axis view at the left ventricular apex showing multiple thrombi within the deep intertrabecular recesses.