Abstract
Modification of allogeneic dendritic cells (DCs) through drug treatment results in DCs with in-vitro hallmarks of tolerogenicity. Despite these observations, using murine MHC-mismatched skin and heart transplant models, donor-derived drug-modified DCs not only failed to induce tolerance but accelerated graft rejection. The latter was inhibited by recipient injection with anti-CD8 antibody, which removed both CD8+ T cells and CD8+ DCs. The discrepancy between in vitro and in vivo data could be explained, partly, by the presentation of drug-modified donor DC MHC-alloantigens by recipient antigen presenting cells (APCs) and activation of recipient T cells with indirect allospecificity, leading to the induction of alloantibodies. Furthermore, allogeneic MHC molecules expressed by drug treated DCs were rapidly processed and presented in peptide form by recipient APCs in vivo within hours of DC injection. Using T cell receptor-transgenic T cells, antigen presentation of injected OVA-pulsed DCs was detectable for ≤3 days whilst indirect presentation of MHC alloantigen by recipient APCs led to activation of T cells within 14 hours and was partially inhibited by reducing the numbers of CD8+ DCs in vivo. In support of this observation when mice lacking CD8+ DCs were pretreated with drug-modified DCs prior to transplantation, skin graft rejection kinetics were similar to non-DC treated controls. Interestingly, when the same mice were treated with anti-CD40L blockade plus drug-modified-DCs skin graft survival was prolonged, suggesting endogenous DCs were responsible for T cell priming. Altogether, these findings highlight the risks and limitations of negative vaccination using alloantigen bearing “tolerogenic” DCs.
Keywords: Dendritic Cell, tolerance, re-processing, transplantation
Introduction
In transplantation immunosuppressive drugs have reduced the incidence of acute rejection, but the average half life of a cadaveric kidney transplant remains approximately 10 years due to chronic organ rejection [reviewed in (1)]. Transplantation research therefore aims to define which strategy can be employed to induce immunosuppressant free, indefinite allograft survival, including the injection of ex-vivo expanded regulatory T cells (Tregs) [reviewed in (2, 3)] and the use of in vitro modified tolerogenic dendritic cells (DCs) [reviewed in (4)].
Several lines of evidence suggest that immature DCs or in vitro modified DCs may be useful tools in promoting tolerance. Different agents have been used to interfere with DC differentiation, migration, antigen uptake and processing, and DC activation [reviewed in (5, 6)]. For example, in vitro treatment of murine derived DCs with either dexamethasone (Dex) or 1,25-dihydroxy vitamin D3 (D3), has been shown to impair DC phenotype and function (7). D3-treatment of DCs also inhibits IL-12 production by down-regulation of NF-κB signalling (8). In vitro treatment of bone-marrow-derived DCs (BM-DCs) with Dex has been shown to inhibit the proliferation of alloreactive T cells, preventing Th1 type skewing of responses (9, 10) and promoting the generation of IL-10-producing regulatory cells (11, 12). Furthermore, Dex and D3 have been demonstrated to have synergistic effects (13).
In man, the same drugs have shown a similar effect in vitro, with Dex-DCs secreting higher levels of IL-10 and having a reduced allo-stimulatory capacity for both naïve and memory T cells (9, 14-18). Additionally, down-regulation of NF-kB activation after treatment with D3 has been shown (19, 20). Human monocyte-derived DCs treated with a combination of Dex and D3 produce IL-10, upregulate ILT-4 and become more resistant to LPS maturation than either drug treatment alone (21). Furthermore they elicit lower proliferative responses from CD4+CD25− T cells and can induce Tregs (21).
In vivo adoptive transfer of murine Dex treated BM-DCs results in prolonged allograft survival in some strain combinations (10, 22). Recently, prolongation of heart allografts survival has been shown using D3-modified DCs (23). Furthermore the combination of Dex and D3-treatment of DCs ameliorate the development of colitis in an adoptive transfer model (24). Other studies using tolerogenic DCs have demonstrated a range of outcomes, for example prolongations of allograft survival from just a few days to greater than 100 days depending on the animal model used (25-31). Most importantly, additional therapy such as CTLA4-Ig or anti-CD40 ligand antibody (MR1) combined with tolerogenic DCs treatment, greatly improves graft survival outcomes (32, 33).
In a rat kidney allograft model we have been successful in achieving tolerance through the adoptive transfer of Dex-treated DCs derived from F1-rats (allowing concurrent presentation of allogeneic MHC molecules both via the direct and indirect pathways) combined with low dose CTLA4-Ig and short-term cyclosporine. This treatment induced indefinite allograft survival mediated through Treg expansion by Dex-DCs dependent IL-2 production (34). In contrast with our data, in the study of De Paz et al, also in the rat system, the injection of immature DCs with anti-lymphocyte serum (ALS) did not increase the effect observed with ALS alone (26), further demonstrating the variability of the effect of tolerogenic DCs treatment.
From the aforementioned in vivo data it appears that the success of negative vaccination with DCs, in achieving transplantation tolerance, may depend on many factors such as the species used, the strain combination, the time of injection, the capacity of DCs to migrate to specific sites, and the additional therapies applied. Another relevant parameter for the in vivo effect of DCs is the way that DCs are generated in vitro. Yamano et al demonstrated that while DCs generated from BM in the presence of Flt3L induced prolonged acceptance of skin allograft in the presence of MR1, CTLA-4Ig and anti-NK1.1 antibody, DCs generated in vitro with GM-CSF did not affect the time of skin rejection (35). In contrast, Divito et al have demonstrated that donor BM-derived D3-DCs or immature DCs internalised as apoptotic cells and presented by host APC can delay heart transplant rejection (23). Evidence for MHC molecules, expressed by intact apoptotic cells, being rapidly processed and presented as peptides in vivo following DC transfer has been previously shown (36). However the consequences for the immune response of the recipient was either tolerance (37) or priming (38, 39). In support of the latter statement, recently in a rat model of islet transplantation, de Kort et al have shown that injection of dexamethasone treated DCs induced antibody-mediated accelerated graft rejection (40).
We demonstrate in this study that alloantigen expressing tolerogenic BM-derived DCs, shown to induce T cell hypo-responsiveness and the expansion of FoxP3+ T cells in vitro, once injected in vivo die rapidly resulting in alloantigen presentation by recipient APC including recipient CD8+DCs. This leads to sensitisation and activation of recipient T cells through the indirect pathway leading to graft rejection. However prolongation of graft survival was achieved when tolerogenic DCs were injected in the presence of anti-CD40L antibody in the absence of CD8+ DCs.
Taken together it appears that donor-derived tolerogenic DCs are linked to an increased risk of priming the recipient immune system to the donor graft by processing and presentation of donor antigens by recipient DCs (CD8+ DCs). In addition the data presented here underline the caution that should be taken in extrapolating in vitro data that do not necessarily reflect the in vivo situation when planning future clinical interventions.
Materials and Methods
Mice
CBA/Ca (H-2k), C57BL/6 ((B6) H-2b), BALB/c (H-2d), C57BL/6 x BALB/c (B6xB/c) F1 (H-2bxd), C57BL/6 x DBA-2 (B6D2F1) F1 (H-2bxd) mice were purchased from Harlan Olac Ltd (Bicester, UK). DO11.10 Rag−/−, TCR75 Rag−/− and B6Kd (kind gifts from Pat Bucy), Baft3−/− mice, (kind gift from Kenneth Murphy, Washington University School of Medicine) (41). H-2Kbm1 (Kbm1) (kind gift from Dr Sandra Diebold, King’s College London). All experimental procedures were performed on sex-matched mice between 6-12 weeks of age, in accordance with the Home Office Animals Scientific Procedures Act (1986).
Cell culture Medium
Cell cultures were performed in RPMI 1640 medium (Sigma, Poole, UK) supplemented with 100IU/ml penicillin, 100μg/ml streptomycin, 2mM L-glutamine, 0.01M Hepes, 50μM 2β-mercaptoethanol (Invitrogen, Paisley, UK), and 10% heat-inactivated foetal calf serum (FCS) (SERAQ, Sussex, UK). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Purification of CD4+ T cells
A single-cell suspension was obtained by passing spleens or pooled lymph nodes through a 70μm cell strainer (BD Pharmingen, California, USA). Erythrocytes were lysed using ACK buffer (0.15M NH4Cl/ 1mM KHCO3/ 0.1mM Na2-EDTA) and CD4+ cells were isolated by negative selection using a CD4 Dynabeads isolation kit (Dynal, Wirral, UK). The purity of the selected CD4+ population was >90% as assessed by flow cytometry.
Generation of dendritic cells from BM
DCs were generated as previously described (42) with some modifications. BM cells were passed through a 70μm cell strainer to obtain a single-cell suspension. Erythrocytes were lysed as above. BM cells were incubated for 30 mins, at 4°C, with supernatants from the following hybridoma cultures: YTS 191 (anti-CD4; American Type Culture Collection (ATCC), Manassas, Virginia, USA), M5/114 (anti-Class II, ATCC), RA3-3A1 (anti-B220, ATCC) and YTS 169 (anti-CD8, ATCC). The cells were washed twice in RPMI then incubated with pan-anti rat-Ig Dynabeads® for 30 min (4°C) followed by magnetic separation. After washing, the BM cells were seeded in a 24-well plate at 1×106 cells/well in 1.5ml of Complete Medium supplemented with 20ng/ml of murine recombinant granulocyte/macrophage colony-stimulating factor (GM-CSF) (kind gift from GlaxoSmithKline R&D, UK). On days 3 and 5 of culture, plates were swirled gently, the medium containing small non-adherent cells removed and fresh GM-CSF-containing Complete Medium added. To some wells Dex (10−6M) and D3 (10−7M) (Sigma) were added. Three different stimuli were used independently to induce DC maturation, either 100ng/ml LPS (E. coli 026:B6, Sigma), or 20ng/mL TNFα (FirstLink, UK), or 50μg/mL α-CD40 antibody (FGK45 hybridoma, kindly provided by A. Rolink). Maturation stimuli were added to cell cultures on day 6 for the final 24hrs. Purity of DCs was greater than 90% as measured by CD11c surface expression on flow cytometry analysis (data not shown).
Flow Cytometry
Fluorochrome-conjugated (fluorescein isothiocyanate, FITC; phycoerythrin, PE; Cy-Chrome, Cy; allophycocyanin, APC) mAbs against the following mouse cell-surface antigens, CD80 and CD86, MHC class I and II, CD40, CD11c, CD8, CD4, Thy1.1, KJ126, CD90.1, CD25 and CD69 were purchased from eBioscience (San Diego, California, USA) with their relevant isotype controls. For flow cytometry analysis, 1×105 cells were labelled with Fluorochrome-conjugated mAbs for 20 min (4°C), washed twice in FACS Buffer (PBS/ 2% FCS/ 0.1% EDTA), and analyzed on a FACSCalibur™, using Cell Quest™ software (Becton Dickinson, Mountain View, CA). FoxP3 staining was performed using a murine FoxP3 kit according to the manufacturer’s protocol (eBioscience). Subsequent analysis was performed with FlowJo software (TreeStar Inc. Ashland, Oregan, USA)
Cytokine Production
Day 6 immature and DexD3-BALB/c DCs were co-cultured with a CD40L expressing murine L-cell line for 18 hours and supernatants harvested. Control supernatants were from non-stimulated cells (20). Cytokine production was assayed on 50μl of culture supernatant using a double antibody sandwich enzyme-linked immunosorbant assay (ELISA) using purified mAbs paired with a biotinylated mAb. Murine IL-12p40/p70 and IL-10 capture and biotinlyated antibodies were obtained from BD Pharmingen.
Proliferation assays and FoxP3 expression
Sequential dilutions of irradiated DCs (either drug treated or not) were added to CD4+ T cells (2.5×104) in complete media. The assay was carried out in 96-well round-bottomed plates, with a total volume/well of 250 μl. CD4+ T cells alone were used as controls. On day two of culture, cells were pulsed with 1μCi/well 3H thymidine (Amersham Pharmacia, UK). Proliferation was measured by 3H thymidine incorporation after twenty hours by liquid scintillation counting using a Beta plate counter. DCs treated with or without DexD3 in the presence or absence of LPS were co-cultured with CFSE labelled (1μM per 107 cell, Invitrogen) CD4+ T cells or CD4+ T cells depleted of CD25+ cells (CD4+CD25−) derived from B6 mice, at a ratio of 1:10 for 5 days. IL-2 (200U/ml) was added at the beginning of the co-culture. On day 5, T cells were harvested and analysed for expression of CD4, CD25 and FoxP3 by flow cytometry, and also analysed for CFSE dilution by flow cytometry.
In vitro and in vivo rechallenge experiments
In vitro
MLRs, comprising BALB/c DCs (± drug treatment) and CD4+ T cells isolated from CBA/Ca mice, were performed in 48-well plates. On day five the T cells were recovered, and were rested for two days. These cells were then re-cultured with irradiated allogeneic BALB/c DC stimulators. Proliferation was measured after three days by 3H thymidine incorporation.
In vivo
B6 mice were challenged with 2×106 DexD3+LPS-BALB/c DCs by intravenous tail vein injection (iv). 10 days later, CD4+ T cells were isolated from the spleen of recipient mice and re-challenged in vitro with DCs derived from BALB/c, CBA/Ca or B6Kd mice. Proliferation was measured on day 3, 5 and 7 of culture using 3H thymidine incorporation.
Skin and heart transplantation
Skin grafting was performed according to the technique described by Billingham and Medawar (43) with some modifications. Full-thickness donor tail skin (0.5-1 × 0.5-1 cm) was grafted on the right lateral flank of recipients. The graft site was covered by a Elastoplast plaster that was removed on day 7. The grafts were observed daily and considered rejected when no viable skin remained. Intra-abdominal heterotopic heart transplantation (either B6D2F1 or BALB/c grafts) was performed in B6 mice as previously described (44, 45). Heart allograft survival was assessed by direct abdominal palpation, where rejection was defined by complete cessation of cardiac impulses. To test the effect of DCs on graft survival, recipient B6 mice were injected intravenously (iv) with different types of donor-derived (B6Kd) DCs or Kd pulsed recipient DC 6 days prior to the graft. Mice were treated with or without 250μg of anti-CD8 antibody (YTS169, ATCC) on day -1 and day 1 of the transplant via intraperitonial (ip) injection.
In some experiments recipient animals were treated with 20μg of CTLA4-Ig (Abatacept, kind gift from Dr. Wendy Rowan, GSK, Stevenage, UK) at day -5 pre-transplant via ip injection, or received 500μgs of MR1 (BioXCell, NH, USA) on days -7, -4 and days 0.
Analysis of DCs processing in vivo
B6, Batf3−/− or (B6 x B/c) F1 mice were injected with 2×106 BALB/c DCs either drug treated or not (Day 0). Some DCs were pulsed with 5μgs/ml of OVA peptide for 2 hours prior to injection. At various time points mice received 2×106 CFSE labelled CD4+ T cells purified from TCR75 Rag−/− or DO11.10 Rag−/− mice via i.v injection. Some mice received anti-CD8 treatment or Polyinosinic-polycytidylic acid (dsRNA) [poly I:C] to decrease the number of recipient DCs or inhibit their function. Spleen and LNs cells were harvested and in some experiments CD4+ T cells were isolated (as above). Thy1.1, KJ126 antibodies were used to distinguish CD4+ T cells from TCR75 Rag−/− and D011.10 Rag−/− mice respectively. CD69 expression was measured by flow cytometry using a specific antibody.
In some experiments, animals were treated with 20μg CTLA4-Ig via ip injection one day after DC injection.
Alloantibody detection
B6Kd DCs (2×106) were injected i.v into naïve mice and sera collected to measure alloantibody production after 7, 14, 21 and 28 days. B6 splenocytes (used as negative control) and BALB/c (Kd) splenocytes (as target cells) were incubated with PBS/2% BSA/5% goat serum containing rat Abs against CD16/32 (0.5 μg/106 cells; BD Biosciences) for 20 min on ice. Hamster anti-mouse CD3-PE (BD Biosciences) was then added directly to the cells to enable subsequent gating on CD3+ T cells. After further incubation for 20 min on ice, cells were washed twice in staining buffer. Serum was added (final dilution 1/10) to appropriate wells and incubated as before. Cells were washed twice before the addition of goat anti-mouse IgG FITC (1/200; Sigma-Aldrich, Dorset, U.K.) for 20 min on ice. Cells were washed twice, and analyzed on a BD Biosciences FACSCalibur running CellQuest software (BD Biosciences).
MTT cell survival assays
DCs (1×105) were added to each well of a 96 well plate in 250 ls of complete media. Each DC type was tested in triplicate. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed following manufactures instructions (Invitrogen, UK) after 24 and 48 hours at 37°C/5% CO2. Statistical comparisons for MTT experiments were performed using paired Student’s t-tests.
Statistical analysis
Statistical comparisons for experiments assessing, in vitro proliferation were performed using unpaired two-tailed Student’s t-tests. Mean survival time of skin and heart allografts was assessed by logrank test. Data shown is mean ± standard deviation (SD) or standard error (SEM) as indicated. Statistical significance was expressed as follows; p<0.001***, p<0.01**, p<0.05*.
Results
DexD3-DCs have an immature phenotype, are resistant to maturation and have an impaired capacity to stimulate antigen-specific T cells in vitro
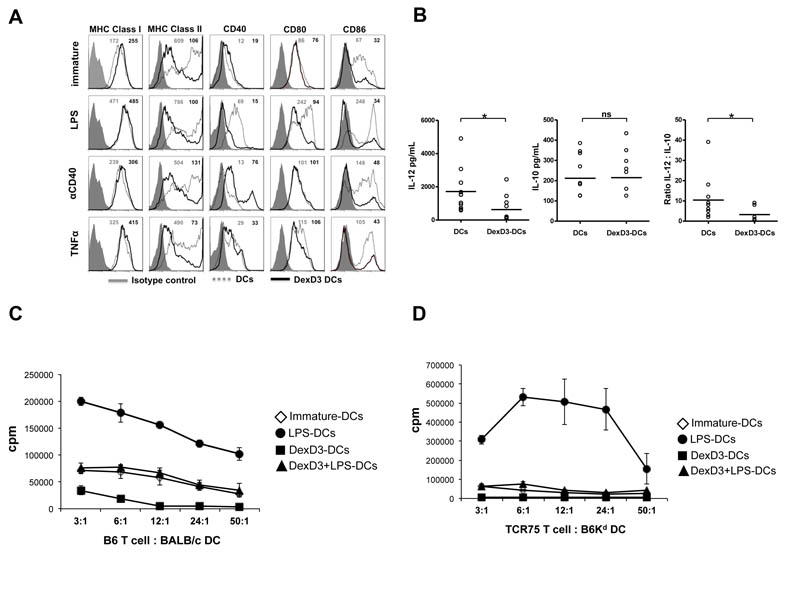
The doses of 10−6M Dex and 10−7M D3 were used in combination to generate tolerogenic DCs, from BM, as previously published (21). As shown in Figure 1A, DexD3-DCs had an immature phenotype, expressed lower levels of CD40, CD80 and CD86 compared to untreated immature DCs and were resistant to maturation as no significant increase in maturation markers was observed after LPS, TNFα or anti-CD40 antibody treatments for most of the markers.
Figure 1. DexD3-DCs are refractory to maturation and are inefficient APCs.
BALB/c-DCs were grown in the presence or absence of Dex and D3 (DexD3-DCs) for 7 days. LPS, TNFα or anti-CD40 antibody were added for the last 24 hours of culture. Control drug treated DCs received no maturation stimuli.
(A) DCs were stained for the expression of MHC class I, class II, CD40, CD80 and CD86 molecules using specific antibodies and analysed by flow cytometry. Isotype controls are shown as grey histograms. Untreated DexD3 treated DCs are shown as grey dashed lines whilst DexD3-DCs are shown as solid black lines. The MFI of both untreated (grey) and treated (black) is shown in the top right hand side of each panel. One representative experiment of 13 (LPS) and 3 (TNFα and anti-CD40 antibody) is shown.
(B) Immature and DexD3-DCs were co-cultured for 18 hours, alone or with a CD40L-expressing cell line, supernatants from these co-cultures were used to detect production of IL-10 and IL-12p40/p70 by ELISA (*p<0.05).
(C) DCs derived from BALB/c, treated or not with DexD3 and LPS [(LPS-matured DCs (closed circles), immature DCs (open diamonds), DexD3+LPS-DCs (closed triangles) and DexD3-DCs (closed squares)] were co-cultured with 1×104 CD4+ T cells isolated from B6 mice at different ratios. T cell proliferation was measured after 72 hours following addition of 3H thymidine for the last 16 hours of culture. Proliferation is expressed as counts per minute (cpm) ± SD. One representative experiment is shown out of 3 performed.
(D) DCs derived from B6Kd mice, treated or not with DexD3 and LPS [(LPS-matured DCs (closed circles), immature DCs (open diamonds), DexD3+LPS-DCs (closed triangles) and DexD3-DCs (closed squares)] were co-cultured with 1×104 CD4+ T cells isolated from TCR75 Rag−/− mice at different ratios. T cell proliferation was measured after 72 hours following addition of 3H thymidine for the last 16 hours of culture. Proliferation is expressed as counts per minute (cpm) ± SD. One representative experiment is shown out of 3 performed.
Immature and DexD3-DCs were stimulated with CD40L-expressing murine L-cells for 18 hours after which supernatants were collected and tested for the presence of IL-12 (p40/p70) and IL-10. As shown in Figure 1B, IL-12 (left panel) production by DexD3-DCs in response to CD40 ligation was decreased compared to untreated DCs (mean±SD IL-12 production by unstimulated DCs 199.6±159.3 vs CD40L activation 1716±1298; and unstimulated DexD3-DCs 43.7±75.8 vs CD40L activation 625.6±759.6 pg/mL), while IL-10 levels (middle panel) were very similar in the two groups of DCs (mean±SD IL-10 production by unstimulated DCs 0.0±0.0 vs CD40L activation 211.7±102.4; and unstimulated DexD3-DCs 12.1±40.1 vs CD40L activation 214.9±108.9 pg/mL). Expressing the production of both IL-12 and IL-10 as a ratio showed that drug-treated DCs had an IL-10 skewed cytokine response compared to untreated DCs (right panel).
The ‘tolerogenic’ nature of drug-modified DCs was further analysed in a mixed lymphocyte reaction. Allogeneic CD4+ T cells from B6 mice proliferated following co-culture with both immature and LPS-matured BALB/c DCs, the latter being the most potent stimulus. In comparison, co-culture with DexD3-DCs or LPS-treated Dex D3-DCs (DexD3+LPS-DCs) resulted in reduced T cell proliferation (Figure 1C). Indeed, DexD3+LPS-DCs induced the same level of proliferation as immature non-drug treated DCs, confirming their attenuated stimulatory function in vitro. The reduced ability to stimulate T cells was further confirmed using T cells derived from TCR transgenic mice, TCR75 Rag−/−, specific for Kd and restricted by H2b (Figure 1D).
To rule out the possibility that the lower T cell proliferation induced by drug-treated DCs compared to mature DCs was not due to the reduced viability of DCs, an MTT cell death assay was performed. As shown in Supplementary Figure 1, over 48 hours of culture, immature, DexD3-DCs and DexD3+LPS-DCs had an equivalent or improved cell viability compared to mature-DCs. Taken together these data confirm that the reduced stimulatory capacity of drug-modified DCs in vitro was not due to loss of cell viability.
CD4+ T cells co-cultured with allogeneic DexD3-DCs in vitro are hypo-responsive to alloantigen re-challenge and FoxP3+ T cells are expanded
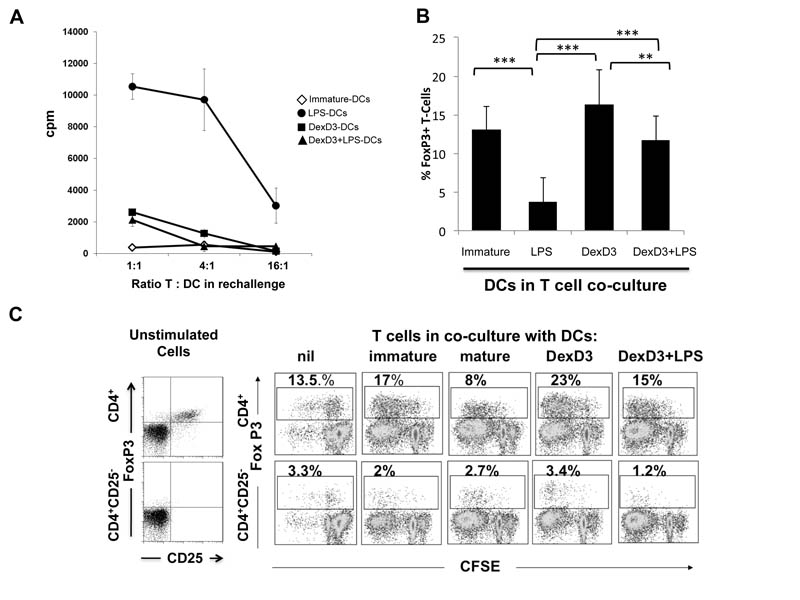
Previously it has been shown by us, and others, that drug-treated DCs induce hyporesponsiveness in vitro (14, 18, 20, 21, 46, 47). To test whether DexD3-DCs can induce T cell anergy, immature, LPS-matured, DexD3-DCs and DexD3+LPS-BALB/c DCs were co-cultured for 6 days with allogeneic CD4+ T cells. These T cells were then purified from the primary cultures and re-stimulated with untreated BALB/c-DCs. As shown in Figure 2A, CD4+ T cells isolated from a primary co-culture with allogeneic immature and mature DexD3-DCs, were hypo-responsive to re-challenge with BALB/c DCs. A similar response was obtained with T cells co-cultured with immature DCs. In contrast, CD4+ T cells isolated following co-culture with LPS-matured BALB/c DCs were able to respond to a second challenge with allogeneic DCs.
Figure 2. CD4+ T cells co-cultured with allogeneic DexD3-DCs are hypo-responsive to alloantigen re-challenge in vitro and preferentially expand CD4+25+ T cells.
(A) CD4+ T cells (2 × 105) from CBA mice were co-cultured with BALB/c derived DCs treated or not with DexD3 in the presence or absence of LPS [(LPS-matured DCs (closed circles), immature DCs (open diamonds), DexD3+LPS-DCs (closed triangles) and DexD3-DCs (closed squares)]. On day 6 of culture the CD4+ T cells were purified and re-stimulated with immature BALB/c DCs at different ratios. T cell proliferation was measured after 72 hours by addition of 3H thymidine for the last 16 hours. Proliferation is expressed as counts per minute (cpm) +/− SD. One representative experiment is shown out of 5 performed.
(B) BALB/c DCs treated with or without DexD3 in the presence or absence of LPS were co-cultured with total CD4+ T cells (1×106), derived from B6 mice, at a ratio of 1:10 in the presence of rIL-2 (200U/ml). On day 5, T cells were harvested and analysed for expression of CD4, CD25 and FoxP3 by flow cytometry. Data plotted is the mean percentage of FoxP3+ T cells detected after co-culture with DCs in 4 independent experiments. *** denotes p<0.001.
(C) CFSE labelled total CD4+ T cells, or CD4+ T cells depleted of CD25+ cells (CD4+CD25−) derived from B6 mice, were cultured with BALB/c DCs preparations as described in (B). On day 5, T cells were stained for expression of CD25 and FoxP3, and also analysed for CFSE dilution by flow cytometry. Data shown is representative of 3 independent experiments and shows the expansion (CD4+ T cells, top panels) and induction (CD4+CD25− T cells, bottom panels) of Foxp3+ Tregs after 5 days of co-culture with DCs.
To address whether the hypo-responsive state of T cells cultured with DexD3-DCs was accompanied by an increase in FoxP3+ T cells as compared to T-cells co-cultured with mature DCs the percentage of FoxP3+ T cells was analysed after 6 days of co-culture. As shown in Figure 2B immature, DexD3-DCs and DexD3+LPS-DCs were all able to generate a significantly higher percentage of FoxP3+ T cells compared to mature DCs following co-culture in vitro. The highest percentage of FoxP3+ T cells was observed when T cells were co-cultured with DexD3-DCs. To investigate whether the increase in FoxP3+ T cells was due to an expansion of CD4+CD25+ Tregs or de novo generation of Tregs, CD4+CD25− T cells were co-cultured with each preparation of DC. As shown in Figure 2C the major contribution to FoxP3+ T cell generation was due to an expansion of naturally occurring Tregs and not due to the de novo induction of these cells. Altogether, these data demonstrate that DexD3-DCs induce T cell unresponsiveness in vitro that is accompanied by the expansion of FoxP3-expressing putative regulatory T cells.
Pretreatment of recipient mice with tolerogenic allogeneic donor DCs accelerates skin allograft rejection; this effect is abolished by anti-CD8 antibody treatment
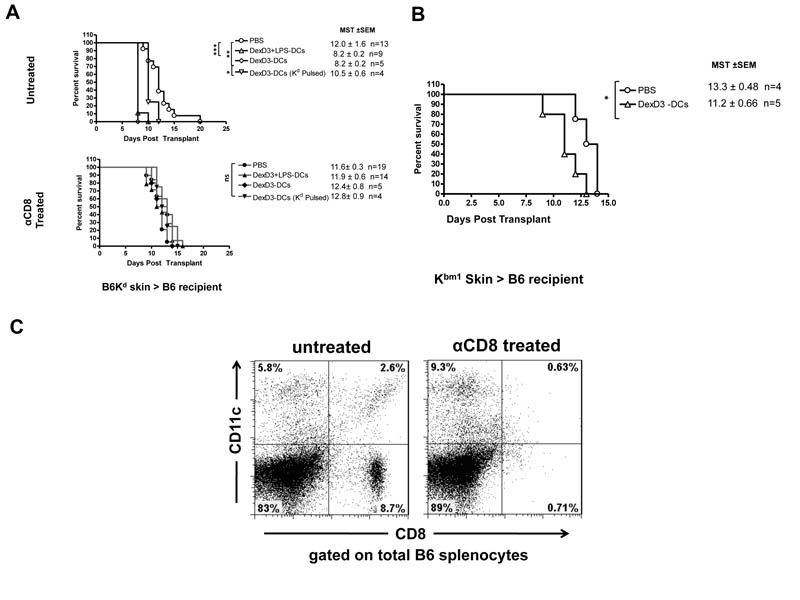
Having demonstrated the “tolerogenic” potential of DexD3-DCs in vitro, the capacity of these cells to prolong skin allograft survival in vivo was investigated. B6 recipient mice received 2×106 donor DCs (from B6Kd mice) 6 days prior to the transplant. The i.v. route of injection was selected because it has been shown to be more effective at delivering DCs to the spleen than ip inoculation and has previously been used to induce tolerance in vivo (48). Donor derived DexD3-DCs and DexD3+LPS-DCs were used in these experiments, the latter condition was used because TLR activation in combination with drug-treatment has been shown to induce IL-10 production and migratory capacity to draining LNs (9, 49). Control mice received PBS. In addition, control and DC treated animals were also treated with a CD8-depleting (anti-CD8) monoclonal antibody (d-1 and d+1 of transplant), to prevent the direct response to Kd molecules by CD8+ T cells (50). As shown in Figure 3A, skin allografts from mice that had received DexD3-DCs or DexD3+LPS-DCs were rejected significantly earlier (MST±SE, 8.2±0.2) than the skin grafts transplanted onto recipient mice that received PBS only (MST 12.0±1.6). Similar results were obtained following transplantation of Kbm1 skin allografts onto B6 recipient mice that had received DexD3-DCs, derived from donor Kbm1 mice (MST+/−SE 11.2+/−0.66 vs 13.3+/−0.48 for PBS) (Figure 3B). These results suggest that the injection of drug-treated donor DCs expressing intact donor MHC molecules primed the recipient immune system to the alloantigens, even when there are only 3 differences in the MHC molecules expressed between recipient and donor cells.
Figure 3. DexD3-DCs pre-treatment enhances skin allograft rejection.
(A) B6 mice were given 2×106 B6Kd DCs treated or not with DexD3 in the presence or absence of LPS, or Kd-pulsed B6 DCs [DexD3-DCs Kd pulsed), via i.v. injection. Control mice received PBS. Seven days later these mice received a B6Kd skin transplant. In addition, some mice were treated with 250μgs of YTS169, an anti-CD8 depleting antibody, one day before and one day after the transplant (filled symbols). Mice were monitored daily and rejection was deemed to have occurred when no viable skin remained. The mean survival time (MST) + SEM is shown (*p<0.05 **p<0.01** p<0.001) and the number of animals per condition (n=). Data shown is representative of 2 independent experiments.
(B) B6 mice were given 2×106 DCs Kbm1 treated with DexD3 via i.v. injection. Control mice received PBS. Seven days later these mice received a Kbm1 skin transplant. Mice were monitored daily and rejection was deemed to have occurred when no viable skin remained. The mean survival time (MST) + SEM is shown and the number of animals per condition (n=5).
(C) Mice treated with two doses of 250μgs of anti-CD8 antibody are depleted of both CD8+ T cells and also CD8+ DC subsets. Data shows analysis of total splenocytes, using flow cytometry after CD8 and CD11c staining, 2 days after the last anti-CD8 treatment and is representative of 2 independent experiments.
Next we addressed whether pulsing DexD3-DCs with an allogeneic peptide derived from the Kd molecule was enough to prime the immune system to donor alloantigens. As shown in Figure 3A the time of graft rejection between mice injected with PBS or DexD3-DCs pulsed with Kd peptide was not statistically different (MST 10.5±0.6) further supporting the idea that donor DCs expressing intact alloantigens need to be processed and presented by endogenous APC to enhance graft rejection.
Interestingly, when the mice that received Kd-skins were treated with anti-CD8, to delete CD8+ T cells, all the skin transplants were rejected within a similar time frame, irrespective of whether recipient mice were injected with PBS (MST 11.6±0.3), donor derived DexD3-DCs (MST 12.4±0.8), DexD3+LPS-DCs (MST 11.9±0.6) or DexD3-DCs pulsed with Kd peptide (MST 12.8±0.9).
To understand the difference in skin graft outcomes between recipient animals injected with anti-CD8 antibody or left untreated, splenocytes from animals treated with anti-CD8 antibody were analysed two days after antibody treatment. As shown in Figure 3C, not only CD8+ T cells but also CD8+ DCs were effectively depleted by anti-CD8 antibody treatment. This result explains, the difference in graft survival time between mice treated with anti-CD8 antibody and those left untreated, since the relative contribution of the direct pathway alone in skin graft rejections may be indicated by the difference (non-significant P=0.58) between the PBS control groups. It has been previously shown that additional therapies, such as treatment with CTLA4-Ig or anti-CD40 ligand antibody, in conjunction with murine tolerogenic DCs greatly improved graft survival (27, 51). We have previously shown in a rat kidney transplant model that treatment with CTLA4-Ig was necessary to induce the indefinite survival of kidney allografts (34). We hypothesised that CTLA4-Ig inhibited presentation of alloantigens derived from “tolerogenic” DCs (24). However when we tested the effect of CTLA4-Ig treatment of recipient mice on skin allograft rejection, at an equivalent dose applied in the rat model (34), the skin graft survival times did not significantly change compared to untreated mice, although the effect of anti-CD8 antibody treatment described earlier was maintained (Supplementary Figure 2). This result suggests that CTLA4-Ig in this strain combination and at the dose used, was insufficient to prevent the activation of alloreactive T cells.
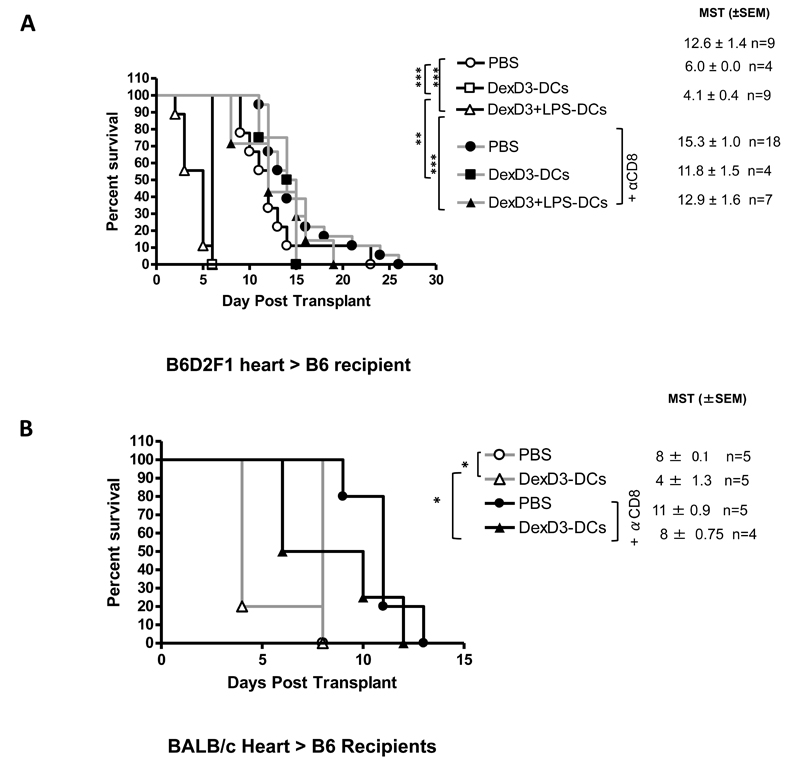
Adoptive transfer of tolerogenic allogeneic donor DCs accelerates heart allograft rejection in both semi and completely mismatched strain combination
The data obtained with mice that received skin transplants were further extended to heart allograft models. First B6D2F1 hearts were transplanted into B6 mice. As shown in Figure 4A, compared to treatment with PBS alone (PBS 12.6.0±1.4), mice that received 2×106 donor derived DexD3-DCs or DexD3+LPS-DCs (day -6), rapidly rejected the allografts (MST 6.0 ±0.0 and 4.1±0.4, respectively) with mice receiving DexD3+LPS-DCs rejecting even earlier than mice injected with DexD3-DCs. This rapid rejection is perhaps a result of the amplification of the indirect response due to constitutive indirect presentation by donor APC contained within the heart. However, when donor DC treatment of mice was combined with anti-CD8 antibody treatment, as previously observed for the skin transplants, prolongation of allograft survival was observed (MST; anti-CD8 treatment with PBS 15.3±1.0; DexD3-DCs 11.8±1.5, with DexD3+LPS-DCs 12.9±1.6). Differences in graft survival were now observed between animals treated with PBS alone or PBS in the presence of anti-CD8 antibody, suggesting that CD8+ T cells with direct allospecificity may contribute to heart allograft rejection.
Figure 4. DexD3-DCs pre-treatment enhances heart allograft rejection.
(A) B6 mice were given 2×106 B6D2F1 DCs treated with DexD3 in the presence or absence of LPS or PBS via i.v. injection, followed by a B6D2F1 heart transplant 7 days later. Some mice received 250μg anti-CD8 antibody one day before and after the transplant. Results plotted show the mean survival time of allografts ±SEM, and are pooled data from 2 independent experiments. (B) B6 mice were injected with DexD3-BALB/c DCs or PBS before receiving a heart BALB/c transplant 7 days later. Some mice received 250μg anti-CD8 antibody one day before and after the transplant. Results plotted show the mean survival time of allografts ±SEM and n= number of mice.
Most of the published work with tolerogenic DCs and their effect on heart transplant rejection have been performed in completely mismatched strain combination (23, 28, 52). To test whether by using such a strain combination a delay in graft rejection was observed, B6 mice were transplanted with heterotopic BALB/c hearts (Figure 4B). As previously shown in Figure 4A, DexD3-DCs accelerated graft rejection in the absence of anti-CD8 antibody treatment Figure 4A (MST 4 +1.3). This maybe due to indirect presentation, which is entirely dependent on recipient APCs presenting alloantigens in this combination. No differences in graft survival were observed between mice that received PBS or DexD3-DCs when CD8 cells were depleted (MST 11+0.9 and 8+0.75).
Taken together it appears that donor-derived drug-treated DCs despite their tolerogenic in vitro effects, when used in vivo not only fail to promote tolerance, but can sensitise recipient mice.
T cells with indirect allospecificty are primed in vivo by injected alloantigen bearing DCs
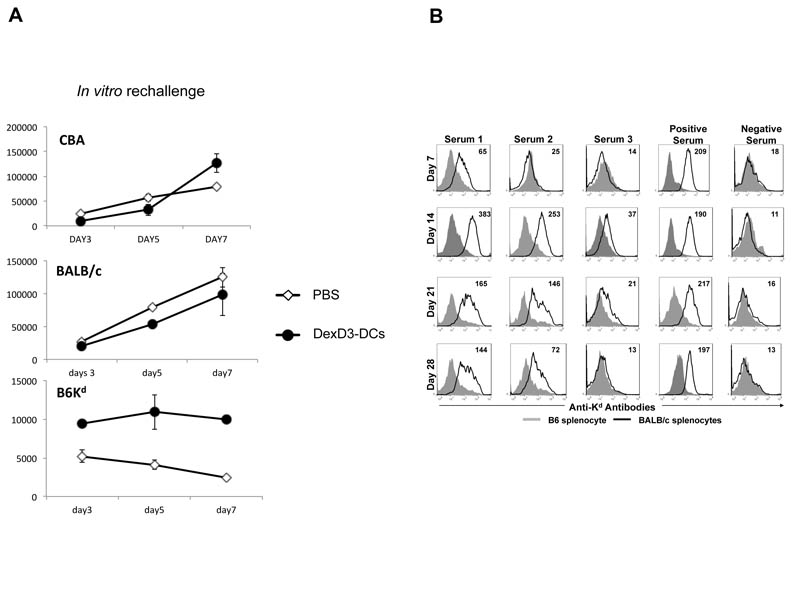
To directly prove that alloantigens derived from “tolerogenic” DCs can be processed and presented by endogenous APC and can activate recipient T cells with indirect allospecificity, the following experiments were performed. B6 mice were injected with PBS or DexD3-DCs from BALB/c mice. Ten days later CD4+ T cells were isolated from these mice and challenged in vitro with DCs derived from BALB/c (direct response), B6Kd (indirect response) or CBA/Ca (third party response) mice for 3, 5 and 7 days (Figure 5A). This time course allowed the primary (peaking on days 5 and 7) and secondary (peaking on day 3) T cell responses to be visualised. We observed that CD4+ T cells isolated from mice previously challenged with DexD3-DCs respond to an in vitro re-challenge to BALB/c-DCs optimally at day 7, at the same time as mice injected with PBS only, suggesting a primary direct response to alloantigens presented by BALB/c-DCs. The response to CBA/Ca-DCs (3rd party alloantigens) was again at day 7 and identical to the response of mice injected with PBS. However, we did observe a secondary response, that started at day 3 and was maintained up to day 7, to B6Kd-DCs by T cells isolated from recipients mice injected with Dex-DCs which was, although low, significantly greater than the PBS treated controls.
Figure 5. Demonstration of in vivo priming of T cell responses with indirect allospecificity induced by DexD3-DC.
(A) B6 mice received DexD3-BALB/c DCs or PBS (2×106). Ten days later, 2×104 CD4+ T cells isolated from recipient mice were stimulated with 1× 104 DCs from BALB/c, B6Kd or CBA/Ca mice. Proliferation was measured on days 3, 5 and 7 of culture by addition of 3H thymidine for the last 16 hours. Proliferation is expressed as counts per minute (cpm) ± SD (* and + denote p < 0.05 c.f PBS and mature DCs respectively). One representative experiment is shown out of 3 performed. (B) B6 mice were injected with B6Kd DCs (2×106) treated with DexD3 and sera collected after 7, 14, 21 and 30 days. Antibody production was evaluated by flow cytometric analysis using BALB/c splenocytes as target cells (black line) and B6 splenocytes as control ones (shaded histograms). The MFI of Ab response to BALB/c splenocytes (black) is shown in the top right hand side of each panel. Experiment represents one of two experiments.
To further support the idea that injection of DCs leads to priming of T cells with indirect alloresponse, antibody production specific for Kd was measured in B6 mice that were injected with DexD3 B6Kd DCs (the same treatment as in Figure 5A). As shown in Figure 5B antibodies specific for H2d were found at significant level in the sera of three mice that received B6Kd DCs with the peak of production between 14 and 21 days. Early production (between day 7 and 14) of IgM was observed in two out of three mice (data not shown).
These observations further confirm the observation that treating mice with donor “tolerogenic” DCs can prime the T cell response to the alloantigen indirectly presented by self-restricted MHC.
DCs injected intravenously are rapidly processed and alloantigen presented by recipient APCs
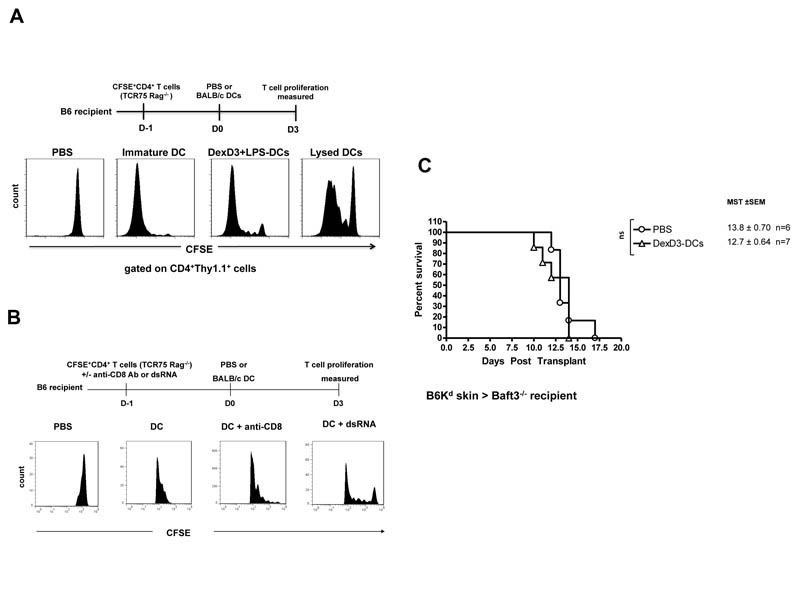
To visualise the extent and the kinetics of processing and presentation of DCs expressing alloantigens, CFSE labelled CD4+ T cells isolated from TCR transgenic mice (TCR75 Rag−/− mice, Thy1.1+) were adoptively transferred into B6 (H-2b) recipient mice. Twenty fours hours later BALB/c (H-2Kd) immature or DexD3+LPS-DCs were injected. Control mice received either PBS or lysed BALB/c-DCs. Proliferation of Thy1.1+ TCR75 cells was observed 3 days later in B6 mice given DexD3+LPS-DCs. As shown in Figure 6A, proliferation of Thy1.1+ TCR75 cells was also observed in B6 recipient mice that had received lysed BALB/c-DCs and no proliferation was seen in mice treated with PBS. As the TCR of the adoptively transferred CD4+ T cells only recognises Kd-antigen when presented by H-2Ab we can conclude that the injected BALB/c-DCs were ‘processed’ in vivo and the alloantigen (Kd) presented by the recipients APCs.
Figure 6. Allogeneic “tolerogenic” DCs injected in vivo provide alloantigens for T cells with indirect allospecificity.
(A) B6 mice received 2×106 CFSE labelled CD4+ T cells isolated from TCR75 Rag−/− mice. 24 hours later these mice received 2×106 of immature, DexD3+LPS-BALB/c DCs, lysed BALB/c DCs or PBS. 3 days later CD4+ T cells were isolated from spleen and LNs and stained with anti-Thy1.1 antibody to identify the adoptively transferred T cells. Cells were analysed by flow cytometry. Data represents the expression of Thy1.1 and CFSE on CD4+ T cells. One representative experiment is shown out of 4 performed.
(B) The same protocol as above with the additional treatment of B6 mice either with 500μgs of anti-CD8 antibody or 100ngs of dsRNA the day prior to DC injection.
(C) Baft3−/− mice were given 2×106 B6Kd DCs treated with DexD3 via i.v. injection. Control mice received PBS. Seven days later mice were transplanted with skins derived from B6Kd mice. Mice were monitored daily and rejection was deemed to have occurred when no viable skin remained. The mean survival time (MST) + SEM is shown and the number of animals per condition (n). Data shown is representative of 2 independent experiments.
To confirm directly that anti-CD8 antibody removed a fraction of the CD8+ DCs and by doing so decreased the presentation of alloantigens, mice were injected with anti-CD8 antibody the day before receiving allogeneic DC and at the same time as TCR75 injection. The results presented in Figure 6B in which treatment of the mice with anti-CD8 antibody led to a decreased proliferation of TCR75 cells further support the contribution of CD8+DCs in processing and presenting alloantigens released by injected DCs. The partial inhibition of TCR75 proliferation was further confirmed by treating recipient mice with Polyinosinic:polycytidylic acid (dsRNA) [poly I:C]. dsRNA not only inhibits the capture and processing of antigens by DCs but also reduces the CD8α+ DC population (53-56).
To further prove that CD8+ DCs are the major endogenous DC subset involved in the processing and presentation of the injected donor derived DCs, Baft3−/− mice were used. These mice lack CD8+ DCs, the subpopulation of DCs with the capacity to cross-present exogenous antigen (41). Baft3−/− mice were injected with DexD3-DCs prior to transplantation, as shown in Figure 6C Kd-skin grafts were rejected at the same time as mice that received PBS only and in a similar manner to B6 mice treated with anti-CD8 antibody (Figure 3A). This data suggests that in the absence of CD8+ DCs impaired processing/presentation of donor antigens expressed by injected DCs occurs. This hypothesis was confirmed by adoptively transferring CFSE labelled TCR75 T cells into Baft3−/− mice followed by BALB/c-DCs injection. Decreased proliferation of the TCR75 cells was observed in these animals as compared to wild type controls (from 32% to 1% cell division, data not shown), further supporting the data presented in Figure 6B.
Lastly, treatment of wild type animals with CTLA4-Ig did not alter the response of TCR75 T cells to ‘processed’ alloantigens (Supplementary Figure 3). This result is in line with the absence of a tolerogenic effect of CTLA4-Ig observed in the skin allograft experiments described above (Supplementary Figure 2).
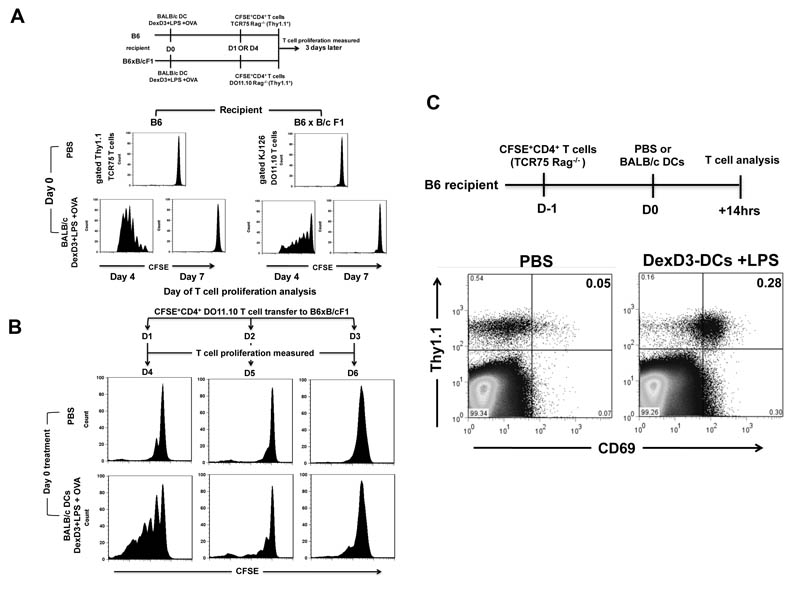
To investigate how long processed alloantigen remained available for indirect allorecognition in vivo, as well as the longevity of intact drug-treated donor DCs, the following three experiments were performed. First, DexD3+LPS-BALB/c DCs were injected into B6 recipient mice and CFSE labelled CD4+ T cells from TCR75 Rag−/− mice were adoptively transferred 1 or 4 days later. Proliferation was measured 3 days later. Second, OVA peptide pulsed drug-treated DCs were injected into (B6 x B/c) F1 recipient mice and CFSE labelled CD4+ T cells from DO11.10 Rag−/− mice were injected 1 and 4 days later. As shown in Figure 7A (left panel) good proliferation of TCR75 cells was observed when T cells were adoptively transferred 1 day after DCs(proliferation measured at day 4), but T cell responses were lost when TCR75 cells were adoptively transferred 4 days following DC challenge (proliferation measured at day 7). Similarly, lack of proliferation was observed when CD4+ T cells derived from DO11.10 Rag−/− mice were adoptively transferred 4 days after DC injection into (B6 x B/c) F1 recipient mice, despite a good response being observed if the T cells were given day 1 after the DCs injection (Figure 7A, right panel). To further define how long drug-treated DCs survive in vivo, the above experiment using OVA-pulsed drug treated DCs was repeated. However this time CD4+ T cells derived from DO11.10 Rag−/− mice were adoptively transferred 1, 2 or 3 days after the drug-treated DCs (Figure 7B). Although OVA-specific CD4+ T cell proliferation was evident when T cells were transferred 1 or 2 days after the peptide pulsed DCs this response was greatly reduced after 3 days confirming that by day 4 no donor DCs were left in vivo. No differences between drug-treated and untreated DCs were observed in these experiments, further demonstrating the similarity in viability of drug-treated and untreated DCs in vivo at least for 3 days (data not shown). Lastly, to determine how rapidly ‘processed’ alloantigen is available for presentation by endogenous APC, CD4+ T cells from TCR75 Rag−/− mice were adoptively transferred 1 day before DC challenge and T cell activation was analysed 14 hours after DC challenge by measuring CD69 up-regulation. We observed that CD69 up-regulation occurred within 14 hours on Thy1.1+ TCR75 cells in mice receiving DexD3+LPS-BALB/c DCs suggesting that the allogeneic DCs were rapidly processed after adoptively transfer (Figure 7C).
Figure 7. Tolerogenic donor DCs have a short life in vivo and are processed by recipient APC.
(A) Recipient B6 and (B6xB/c) F1 mice were injected with DexD3+LPS-BALB/c DCs (2×106) pulsed with 5μg/ml of OVA peptide. Control mice received PBS only. CD4+ T cell isolated from TCR75 Rag−/− or D011.10 Rag−/− mice were CFSE labelled and adoptively transferred (1.5×106) on day 1 or day 4 following DC challenge into B6 or F1 mice, respectively. T cell proliferation was measured 3 days after T cells injection by flow cytometry. Histograms represent proliferation of CD4+ T cells identified using Thy1.1 (TCR75 Rag−/−) or KJ126 (D011.10 Rag−/−) specific antibodies (left and right panels respectively). One representative experiment is shown out of 3 performed.
(B) Recipient F1 mice were injected with DexD3+LPS-BALB/c DCs (2×106) pulsed with 2μgs/ml of OVA peptide. Controls received just PBS. CD4+ T cell isolated from D011.10 Rag−/− mice were CFSE labelled and adoptively transferred (4×106) on day 1, 2 and 3 following DC challenge. T cell proliferation was measured 3 days later by flow cytometry. Histograms represent proliferation of CD4+ T cells identified using KJ126 specific antibodies. One representative experiment is shown out of 2 performed.
(C) B6 mice received 2×106 CD4+ T cells isolated from TCR75 Rag−/− mice. 24 hours later these mice received 2×106 DexD3+LPS-DCs. Controls received PBS only. 14 hours later CD4+ T cells were isolated from spleen and LNs and stained with Thy1.1, to identify the adoptively transferred T cells, and CD69. Cells were analysed by flow cytometry. Data represents the expression of Thy1.1 and CD69 on CD4+ T cells. One representative experiment out of 3 performed.
Donor-derived DexD3-DCs induce skin transplant survival in recipient mice lacking CD8+ DCs in combination with anti-CD40L antibody therapy
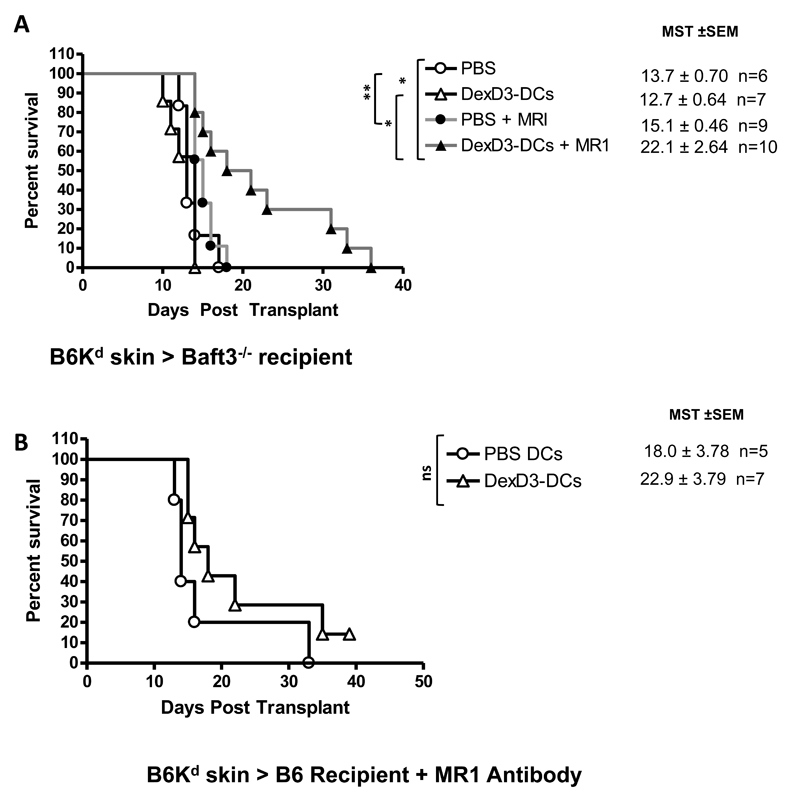
From all the experiments presented in this manuscript, we concluded that drug-treated allogenic DCs were rapidly processed and presented by recipient APCs, primarily CD8+ DCs. Removal of this DC subset inhibited the rapid priming caused by injection of drug treated donor cells. Therefore, whether drug treated tolerogenic DCs could prolong transplant survival in the presence of additional therapies in the absence of CD8+ DCs was addressed. Baft3−/− and B6 recipient mice were injected with donor derived DexD3-DCs in combination with 3 doses of anti-CD40L antibody (MR1) treatment (days -7, day -4, and day 0 at 500 μgs per injection) prior to Kd-skin transplantation. Using this protocol, prolongation of skin graft survival was observed in Baft3−/− recipients using DexD3-DCs (MST= 22.1+2.64) as compared to PBS (MST=15.1+0.46) (Figure 8A). However, when DCs are injected into B6 mice in the presence of MR1 antibody a non-significant prolongation of skin allograft survival was observed, PBS (MST= 18.0 + 3.8) vs DexD3-DCs (22.9 + 3.8) (Figure 8B).
Figure 8. Tolerogenic donor DCs induce skin transplant survival when recipient mice that lack CD8+ DCs are treated with anti-CD40L antibody therapy.
Baft3−/− (A) and B6 (B) mice received 2×106 B6Kd DCs treated with DexD3. Control mice received PBS. Seven days later mice received a B6Kd skin transplant. In addition, some mice were treated with 500μgs of MR1 4 and 7 days prior to, and on the day of transplantation (filled symbols). Mice were monitored daily and rejection was deemed to have occurred when no viable skin remained. The mean survival time (MST) + SEM is shown (*p<0.05 **p<0.01** p<0.001) and the number of animals per condition (n). Data shown represents 3 and 2 independent experiments, respectively.
Altogether the results presented in this study further support the idea that transplant survival mediated by donor-derived tolerogenic DCs can only be achieved in the absence of endogenous DCs with cross-presenting function and when recipient mice are treated with additional therapies, such as MR1.
Discussion
One strategy to promote T cell regulation in transplantation is via the in vivo induction/expansion of Tregs with modified DCs. The combination of Dex and D3 has previously been shown to be synergistic, and we adopted this drug combination as the preferred approach for exploring the therapeutic potential of tolerogenic DCs (14, 57). Our in vitro findings are in keeping with other publications that demonstrate that drug modification can maintain DCs in an immature state, resistant to LPS, CD40L or TNFα induced maturation, with reduced expression of co-stimulatory molecules and an impaired capacity to produce IL-12 (12, 20, 57, 58). We also demonstrated that the phenotype of DexD3-DCs was “tolerogenic” due to their capacity to induce T cell hyporesponsiveness and expansion of FoxP3+ T cells in vitro (12, 20, 57, 59).
However, the key and most interesting observation made in this study was that despite demonstrating ‘tolerogenic’ potential in vitro, DexD3-treated DCs did not prolong skin or heart allograft survival instead they accelerated graft rejection. We have also demonstrated that although in vivo treatment with anti-CD8 antibodies abolished this early rejection no ‘tolerance’ was established. This was further confirmed using as recipients a strain of mice that lacks DCs with the capacity to cross-present exogenous antigen (CD8+ DCs). Furthermore we observed that the injection of allogeneic DCs primed the recipient CD4+ T cells to alloantigens. Using T cells from TCR transgenic mice as a tool to measure the response to alloantigens presented indirectly we demonstrated that the life span of the injected drug-treated DCs expressing alloantigens is short lived in vivo due to efficient processing in vivo. The T cell activation by ‘processed’ alloantigens was partially inhibited by using either anti-CD8 antibody or dsRNA pre-treatment suggesting a role of recipient DCs in amplifying the immune responses to the allograft. At present we believe that CD8α+ DCs contribute to the amplification of the indirect alloresponse as the aforementioned treatments lead to a reduction in the number of these cells (Figure 3C) (53, 55). Taken together, the data presented in our study suggest that negative vaccination using drug treated donor DCs, confers a major risk of sensitisation due to processing and presentation of alloantigens by endogenous DCs leading to priming of CD4+ T cells with indirect allospecificity.
Two papers were recently published in which the effect of murine DCs on allograft survival was investigated and the results obtained were conflicting. In the first manuscript Divito et al. presented data whereby donor derived immature DCs injected in vivo, in a completely mismatched strain combination, induced indefinite survival of heart allografts. These authors suggest that this is due to anergy induction in alloantigen-specific recipient T cells following processing and presentation of apoptotic donor DCs by endogenous APC (23). By contrast, Yamano et al. observed that skin allografts, in mice injected with donor derived DCs (obtained using similar protocol to the Divito study) were rejected at the same time, if not slightly earlier, than PBS treated mice (35). More recently, in a rat model of islet transplantation the treatment of the recipient mice with donor derived Dex-DCs led to antibody-mediated allograft rejection (40). Both the aforementioned paper, as well as the observations of Yamano et al, support our data that donor-derived ‘tolerogenic’ DCs can prime rather than induce tolerance in vivo.
For a long time apoptosis was thought to be tolerogenic whilst necrosis was immunogenic. However, now it is clear that processing and presentation of antigen from apoptotic cells can lead to immunogenicity, in particular in the presence of pro-inflammatory signals. Although early evidence of immune responses to minor histocompatibility (mH) antigens demonstrated strict MHC restriction in vitro (58, 60) a responder mouse expressing H-2b and H-2d alleles, primed in vivo with cells homozygous for H-2b and differing in mH antigens, demonstrated a strong in vitro secondary response against the minor antigens restricted not only by H-2b but also H-2d (61). Bevan hypothesised and subsequently demonstrated that this was the result of cross-primed cytotoxic T cells (62). Cross-presentation and cross-priming of anti-tumour CTL responses occurs by the processing of apoptotic cells by DCs, resulting in augmented tumour immunogenicity, highlighting the significance of this immunological pathway (63). Although cross-presentation is generally regarded as a pathway by which exogenous antigen becomes available to CD8+ T cells it has also been observed for antigen presentation on MHC class II antigens. In a corneal transplant model it was observed that recipient APCs transported antigen from the donor cornea and cross-presented this to host antigen-specific CD4+ T cells leading to activation and expansion of these cells (64).
In our study the occurrence of cross-presentation of donor alloantigens and therefore cross-priming to CD4+ T cells with indirect allospecificity is suggested and at least in part demonstrated by the reduction of TCR75 proliferation when anti-CD8 antibody and dsRNA were used, and in CD8α− DC animals. Our results are different from the data recently published by DiVito et al. and similar to the results published by Yamano et al (23, 35). Others have also published in transplant models, that injected cells that died of apoptosis prime the recipient immune system and factors such as the inflammatory state of the recipient may contribute to the balance between activation of the immune response and tolerance (39, 65). In our study due to the nature of antigen presentation and the observation that the processed alloantigen is available to responder T cells within 14 hours and for up to 4 days, there is equal likelihood that an effector response will be generated, particularly in the context of an inflammatory response induced by the transplant itself (66). As demonstrated by DiVito et al (23, 67) the processing and presentation of injected DCs may be the result of NK-mediated killing (67).
The involvement of endogenous DCs in the accelerated graft rejection observed in our study was supported by the data obtained when recipient mice were treated with anti-CD8 antibody and when the Baft3−/− mice were used. Our data suggest that the CD8+ DC subset plays the major role in the priming and activation of recipient T cells with indirect allospecificity that contribute to graft rejection. The observation that treatment with anti-CD8 antibody causes CD8+DC deletion has major implications for the large number of publications in which anti-CD8 antibody was used and thought to delete CD8+ T cells only.
Although it is clear that the removal of recipient CD8+ DCs is not enough to induce transplant tolerance when mice are treated with donor-derived tolerogenic DCs, the additional therapy with anti-CD40L antibody led to graft survival. In Yamano et al study, the injection of MR1 (together with CTLA4-Ig and anti-NK1.1 antibody) with immature DCs into wild type mice is not enough to induce tolerance (35). The difference between the two studies further support our idea that the increased survival of an allograft is a very delicate equilibrium in which many factors play an important role. It is clear from our data that only when endogenous DCs are absent together with interfering with CD40-CD40L interaction, this combination can induce graft survival. This result differs from the experiment in which CTLA4-Ig rather than MR1 was used. While this additional therapy was enough in the rat kidney allograft model (40) no improvement in transplant survival was seen using our strain combination. One possible explanation for the difference observed is the amount of CTLA-4 used (equivalent amount used in the rat model but lower than the amount used in other murine studies). However when a combination of MR1 and CTLA4-Ig [at the dose normally used in murine models, (27)] was injected during donor-derived DCs-treatment lack of skin transplant survival was also observed in wild type animals (data not shown), further confirming the need for recipient DCs removal to achieve graft survival.
An alternative to the use of donor derived-DCs to achieve tolerance in the context of autoimmunity or transplantation, is the adoptive transfer of recipient DCs rendered tolerogenic and expressing either auto or alloantigens. This approach has already entered the clinical arena. There is a safety study currently recruiting utilising autologous dendritic cell therapy for type 1 diabetes suppression, in this study autologous monocyte-derived dendritic cells are treated ex-vivo with antisense phosphorothioate-modified oligonucleotides targeting the primary transcripts of the CD40, CD80 and CD86 co-stimulatory molecules (immunoregulatory DC; iDC). The hypothesis to be tested in this study is that iDC are safe and without toxicity in established type 1 diabetic patients. (68). The closest studies in terms of modification of recipient DCs to those described here, are in locally injected DCs with DexD3-DCs injected into the knees of patients with rheumatoid arthritis (69), this is one of the first efficacy trials to see if recipient drug modified DCs can alter the course of disease locally in a systemic disorder.
Finally, another way to achieve tolerance is by targeting recipient DCs with antigen directly in vivo; e.g. in a quiescent state (70). Delivering antigen specifically to conventional DCs via DEC-205 or 33D1 lead to effective presentation by MHC class I and II molecules respectively that is followed by induction/ expansion of Tregs and/or T cell deletion (71-74). The usefulness of this concept was shown in mouse models of Type 1 diabetes where targeting of autoantigens to CD205 could prevent the onset and progression of disease (72, 75). More recently, we have demonstrated for the first time in a skin transplant model that delivering alloantigens to cDCs via 33D1 leads to indefinite transplant survival but only in the presence of anti-CD8 depleting antibody (76).
Altogether the results presented in this study suggest that cellular negative vaccination with donor-derived DCs is linked to the risk of sensitisation to donor antigens. The results have major implications in clinical strategies where donor tolerogenic DCs are to be used to induce tolerance to a graft.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by grants from the British Heart Foundation, EU RISET and MRC. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The authors acknowledge the support of the MRC Centre for Transplantation.
References
- 1.Lechler RI, George AJT. Transplantation and Rejection. In: Male D, Brostoff J, Roth DB, Roitt I, editors. Immunology. 7th ed Mosby Elsevier; Philadelphia: 2006. pp. 383–399. [Google Scholar]
- 2.Long E, Wood KJ. Regulatory T Cells in Transplantation: Transferring Mouse Studies to the Clinic. Transplantation. 2009;88:1050–1056. doi: 10.1097/TP.0b013e3181bb7913. [DOI] [PubMed] [Google Scholar]
- 3.Feng G, Chan T, Wood KJ, Bushell A. Donor reactive regulatory T cells. Curr Opin Organ Transplant. 2009;14:432–438. doi: 10.1097/MOT.0b013e32832c58f1. [DOI] [PubMed] [Google Scholar]
- 4.Silk KM, Fairchild PJ. Harnessing dendritic cells for the induction of transplantation tolerance. Curr Opin Organ Transplant. 2009;14:344–350. doi: 10.1097/MOT.0b013e32832c6a1d. [DOI] [PubMed] [Google Scholar]
- 5.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 6.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 7.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent Inhibition of Dendritic Cell Differentiation and Maturation by Vitamin D Analogs. Biochem Biophys Res Commun. 2000;270:701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD. Direct Transcriptional Regulation of RelB by 1α,25-Dihydroxyvitamin D3 and Its Analogs. J Biol Chem. 2003;278:49378–49385. doi: 10.1074/jbc.M308448200. [DOI] [PubMed] [Google Scholar]
- 9.Rea D, van Kooten C, van Meijgaarden KE, Ottenhoff TH, Melief CJ, Offringa R. Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood. 2000;95:3162–3167. [PubMed] [Google Scholar]
- 10.Roelen DL, Schuurhuis DH, van den Boogaardt DEM, Koekkoek K, van Miert PPMC, van Schip JJ, Laban S, Rea D, Melief CJM, Offringa R, Ossendorp F, Claas FHJ. Prolongation of skin graft survival by modulation of the alloimmune response with alternatively activated dendritic cells1. Transplantation. 2003;76:1608–1615. doi: 10.1097/01.TP.0000086340.30817.BA. [DOI] [PubMed] [Google Scholar]
- 11.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 12.Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000;30:1233–1242. doi: 10.1002/(SICI)1521-4141(200004)30:4<1233::AID-IMMU1233>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Xing N, Maldonado ML, Bachman LA, McKean DJ, Kumar R, Griffin MD. Distinctive dendritic cell modulation by vitamin D3 and glucocorticoid pathways. Biochem Biophys Res Commun. 2002;297:645–652. doi: 10.1016/s0006-291x(02)02262-3. [DOI] [PubMed] [Google Scholar]
- 14.Woltman AM, van der Kooij SW, de Fijter JW, van Kooten C. Maturation-resistant dendritic cells induce hyporesponsiveness in alloreactive CD45RA+ and CD45RO+ T-cell populations. Am J Transplant. 2006;6:2580–2591. doi: 10.1111/j.1600-6143.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- 15.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145:351–357. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 16.Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol. 2006;120:260–271. doi: 10.1016/j.clim.2006.04.567. [DOI] [PubMed] [Google Scholar]
- 17.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 Affects Differentiation, Maturation, and Function of Human Monocyte-Derived Dendritic Cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 18.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 19.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 Selectively Modulates Tolerogenic Properties in Myeloid but Not Plasmacytoid Dendritic Cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 20.Buckland M, Jago CB, Fazekasova H, Scott K, Tan PH, George AJ, Lechler R, Lombardi G. Aspirin-treated human DCs up-regulate ILT-3 and induce hyporesponsiveness and regulatory activity in responder T cells. Am J Transplant. 2006;6:2046–2059. doi: 10.1111/j.1600-6143.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen AE, Gad M, Walter MR, Claesson MH. Induction of regulatory dendritic cells by dexamethasone and 1[alpha],25-Dihydroxyvitamin D3. Immunol Lett. 2004;91:63–69. doi: 10.1016/j.imlet.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic Cells Activated by Lipopolysaccharide after Dexamethasone Treatment Induce Donor-Specific Allograft Hyporesponsiveness. Transplantation. 2006;81:1451–1459. doi: 10.1097/01.tp.0000208801.51222.bd. [DOI] [PubMed] [Google Scholar]
- 23.Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, Erdos G, Larregina AT, Morelli AE. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694–2705. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders Elm P, Esben Gjerl¯ff Wedebye S, Monika G, Steen Seier P, Mogens Helweg C. Dexamethasone/1α-25-dihydroxyvitamin D3-treated dendritic cells suppress colitis in the SCID T-cell transfer model. Immunology. 2009;127:354–364. doi: 10.1111/j.1365-2567.2008.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, Hackstein H, Robbins PD, Thomson AW, Fung JJ, Qian S, Lu L. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- 26.DePaz HA, Oluwole OO, Adeyeri AO, Witkowski P, Jin MX, Hardy MA, Oluwole SF. Immature rat myeloid dendritic cells generated in low-dose granulocyte macrophage-colony stimulating factor prolong donor-specific rat cardiac allograft survival. Transplantation. 2003;75:521–528. doi: 10.1097/01.TP.0000048380.84355.4A. [DOI] [PubMed] [Google Scholar]
- 27.Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, Thomson AW. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 28.Emmer PM, van der Vlag J, Adema GJ, Hilbrands LB. Dendritic cells activated by lipopolysaccharide after dexamethasone treatment induce donor-specific allograft hyporesponsiveness. Transplantation. 2006;81:1451–1459. doi: 10.1097/01.tp.0000208801.51222.bd. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Li M, Lian D, Zheng X, Zhang ZX, Ichim TE, Xia X, Huang X, Vladau C, Suzuki M, Garcia B, Jevnikar AM, Min WP. Generation of therapeutic dendritic cells and regulatory T cells for preventing allogeneic cardiac graft rejection. Clin Immunol. 2008;127:313–321. doi: 10.1016/j.clim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Xu DL, Liu Y, Tan JM, Li B, Zhong CP, Zhang XH, Wu CQ, Tang XD. Marked prolongation of murine cardiac allograft survival using recipient immature dendritic cells loaded with donor-derived apoptotic cells. Scand J Immunol. 2004;59:536–544. doi: 10.1111/j.1365-3083.2004.01427.x. [DOI] [PubMed] [Google Scholar]
- 31.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-Conditioned Dendritic Cells Are Poor Stimulators of Allogeneic CD4+ T Cells, but Enrich for Antigen-Specific Foxp3+ T Regulatory Cells and Promote Organ Transplant Tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 32.Pearson TC, Alexander DZ, Hendrix R, Elwood ET, Linsley PS, Winn KJ, Larsen CP. CTLA4-Ig plus bone marrow induces long-term allograft survival and donor specific unresponsiveness in the murine model. Evidence for hematopoietic chimerism. Transplantation. 1996;61:997. doi: 10.1097/00007890-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Morelli AE, Hackstein H, Kaneko K, Thomson AW. Marked inhibition of transplant vascular sclerosis by in vivo-mobilized donor dendritic cells and anti-CD154 mAb. Transplantation. 2003;76:562–571. doi: 10.1097/01.TP.0000068901.11693.C3. [DOI] [PubMed] [Google Scholar]
- 34.Mirenda V, Berton I, Read J, Cook T, Smith J, Dorling A, Lechler RI. Modified dendritic cells coexpressing self and allogeneic major histocompatability complex molecules: an efficient way to induce indirect pathway regulation. J Am Soc Nephrol. 2004;15:987–997. doi: 10.1097/01.asn.0000119575.98696.1d. [DOI] [PubMed] [Google Scholar]
- 35.Yamano T, Watanabe S, Hasegawa H, Suzuki T, Abe R, Tahara H, Nitta T, Ishimaru N, Sprent J, Kishimoto H. Ex vivo-expanded DCs induce donor-specific central and peripheral tolerance and prolong the acceptance of donor skin grafts. Blood. 2011;117:2640–2648. doi: 10.1182/blood-2010-07-293860. [DOI] [PubMed] [Google Scholar]
- 36.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–4455. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 37.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman RM. Efficient Presentation of Phagocytosed Cellular Fragments on the Major Histocompatibility Complex Class II Products of Dendritic Cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman RM. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knudsen S, Schardt A, Buhl T, Boeckmann L, Schon MP, Neumann C, Haenssle HA. Enhanced T-cell activation by immature dendritic cells loaded with HSP70-expressing heat-killed melanoma cells. Exp Dermatol. 2010;19:108–116. doi: 10.1111/j.1600-0625.2009.00962.x. [DOI] [PubMed] [Google Scholar]
- 40.de Kort H, Crul C, van der Wal AM, Schlagwein N, Stax AM, Bruijn JA, van Kooten C, de Heer E. Accelerated antibody-mediated graft loss of rodent pancreatic islets after pretreatment with dexamethasone-treated immature donor dendritic cells. Transplantation. 94:903–910. doi: 10.1097/TP.0b013e31826acd01. [DOI] [PubMed] [Google Scholar]
- 41.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billingham RE, Brent L, Madawar PB. Actively aquired tolerance of foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 44.Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, Stauss HJ, Bucy RP, Lombardi G, Lechler R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsang JY, Tanriver Y, Jiang S, Leung E, Ratnasothy K, Lombardi G, Lechler R. Indefinite mouse heart allograft survival in recipient treated with CD4(+)CD25(+) regulatory T cells with indirect allospecificity and short term immunosuppression. Transpl Immunol. 2009;21:203–209. doi: 10.1016/j.trim.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood. 2002;100:1084–1087. doi: 10.1182/blood.v100.3.1084. [DOI] [PubMed] [Google Scholar]
- 47.Hackstein H, Morelli AE, Larregina AT, Ganster RW, Papworth GD, Logar AJ, Watkins SC, Falo LD, Thomson AW. Aspirin inhibits in vitro maturation and in vivo immunostimulatory function of murine myeloid dendritic cells. J Immunol. 2001;166:7053–7062. doi: 10.4049/jimmunol.166.12.7053. [DOI] [PubMed] [Google Scholar]
- 48.Creusot RJ, Yaghoubi SS, Chang P, Chia J, Contag CH, Gambhir SS, Fathman CG. Lymphoid-tissue-specific homing of bone-marrow-derived dendritic cells. Blood. 2009;113:6638–6647. doi: 10.1182/blood-2009-02-204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson AE, Swan DJ, Sayers BL, Harry RA, Patterson AM, von Delwig A, Robinson JH, Isaacs JD, Hilkens CMU. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Biol. 2009;85:243–250. doi: 10.1189/jlb.0608374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsang JY-S, Tanriver Y, Jiang S, Xue S-A, Ratnasothy K, Chen D, Stauss HJ, Bucy RP, Lombardi G, Lechler R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, Li W, Fu F, Chambers FG, Qian S, Fung JJ, Thomson AW. Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival. Transplantation. 1997;64:1808–1815. doi: 10.1097/00007890-199712270-00031. [DOI] [PubMed] [Google Scholar]
- 52.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5:255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 53.Smyth LA, Hervouet C, Hayday T, Becker PD, Ellis R, Lechler RI, Lombardi G, Klavinskis LS. Acquisition of MHC:peptide complexes by dendritic cells contributes to the generation of antiviral CD8+ T cell immunity in vivo. J Immunol. 189:2274–2282. doi: 10.4049/jimmunol.1200664. [DOI] [PubMed] [Google Scholar]
- 54.Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, Jansen ES, O’Reilly LA, Schneider P, Fasel N, Shortman K, Strasser A, Acha-Orbea H. Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by PolyIC in vivo. PLoS One. 2011;6:e20189. doi: 10.1371/journal.pone.0020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, Jansen ES, O’Reilly LA, Schneider P, Fasel N, Shortman K, Strasser A, Acha-Orbea H. Type I Interferon Drives Dendritic Cell Apoptosis via Multiple BH3-Only Proteins following Activation by PolyIC In Vivo. PLoS One. 2011;6:e20189. doi: 10.1371/journal.pone.0020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 57.Chamorro S, Garcia-Vallejo JJ, Unger WWJ, Fernandes RJ, Bruijns SCM, Laban S, Roep BO, t Hart BA, van Kooyk Y. TLR Triggering on Tolerogenic Dendritic Cells Results in TLR2 Up-Regulation and a Reduced Proinflammatory Immune Program. J Immunol. 2009;183:2984–2994. doi: 10.4049/jimmunol.0801155. [DOI] [PubMed] [Google Scholar]
- 58.Gordon RD, Simpson E, Samelson LE. In vitro cell-mediated immune responses to the male specific(H-Y) antigen in mice. J Exp Med. 1975;142:1108–1120. doi: 10.1084/jem.142.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 60.Bevan MJ. Interaction antigens detected by cytotoxic T cells with the major histocompatibility complex as modifier. Nature. 1975;256:419–421. doi: 10.1038/256419a0. [DOI] [PubMed] [Google Scholar]
- 61.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. 1976;117:2233–2238. [PubMed] [Google Scholar]
- 63.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5:255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 64.Kuffová L, Netuková M, Duncan L, Porter A, Stockinger B, Forrester JV. Cross Presentation of Antigen on MHC Class II via the Draining Lymph Node after Corneal Transplantation in Mice. J Immunol. 2008;180:1353–1361. doi: 10.4049/jimmunol.180.3.1353. [DOI] [PubMed] [Google Scholar]
- 65.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, Larsen CP. Cutting Edge: Persistent Viral Infection Prevents Tolerance Induction and Escapes Immune Control Following CD28/CD40 Blockade-Based Regimen. J Immunol. 2002;169:5387–5391. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 67.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ISaacs JD, Bell GM. Autologous Tolerogenic Dendritic Cells for Rheumatoid Arthritis (AutoDECRA) Clinical Trials.gov. 2011.
- 70.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 71.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, Buer J. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–3401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- 73.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+CD205+ Splenic Dendritic Cells Are Specialized to Induce Foxp3+ Regulatory T Cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 75.Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci U S A. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanriver Y, Ratnasothy K, Bucy RP, Lombardi G, Lechler R. Targeting MHC class I monomers to dendritic cells inhibits the indirect pathway of allorecognition and the production of IgG alloantibodies leading to long-term allograft survival. J Immunol. 184:1757–1764. doi: 10.4049/jimmunol.0902987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.