Summary
The promise of personalized medicine is upon us, and in some cancers targeted therapies are rapidly becoming the mainstay of treatment for selected patients based on their molecular profile. The protein kinase BRAF is a driver oncogene in both thyroid cancer and melanoma, but whereas drugs that target BRAF and its downstream signaling pathway are effective in melanoma, they are ineffective in thyroid cancer. In this issue of Cancer Discovery, Montero-Conde and colleagues investigate why thyroid cancer is resistant to BRAF inhibitors despite the presence of BRAF mutation.
In 2002 (1) the first cancer genome re-sequencing study was published. It reported that the BRAF gene is mutated in ~7% of human cancers and it took less than a decade for this seminal discovery to impact the clinical management of human cancer. There are three RAF genes in humans (ARAF, BRAF, CRAF), and they encodes protein kinases that are components of a signaling pathway activated downstream of receptor tyrosine kinases (RTKs) and the small G-protein RAS (Fig 1A). RAF proteins activate MEK, which then activates ERK to regulate cell growth and survival (Fig 1A). BRAF mutations are common in melanoma (~50%), colorectal cancer (~15%) and papillary thyroid cancer (~40%) (http://cancer.sanger.ac.uk/cosmic/), and this discovery led to rapid increases in our understanding of the molecular mechanisms underlying tumourigenesis in those cancers, and it ignited a hunt for BRAF-targeting drug. This investment that paid off two years ago when vemurafenib (PLX4032/RG7204) was approved for treatment of BRAF mutant melanoma by the US Food and Drug Administration (FDA),receiving Canadian and European licenses a few months later.
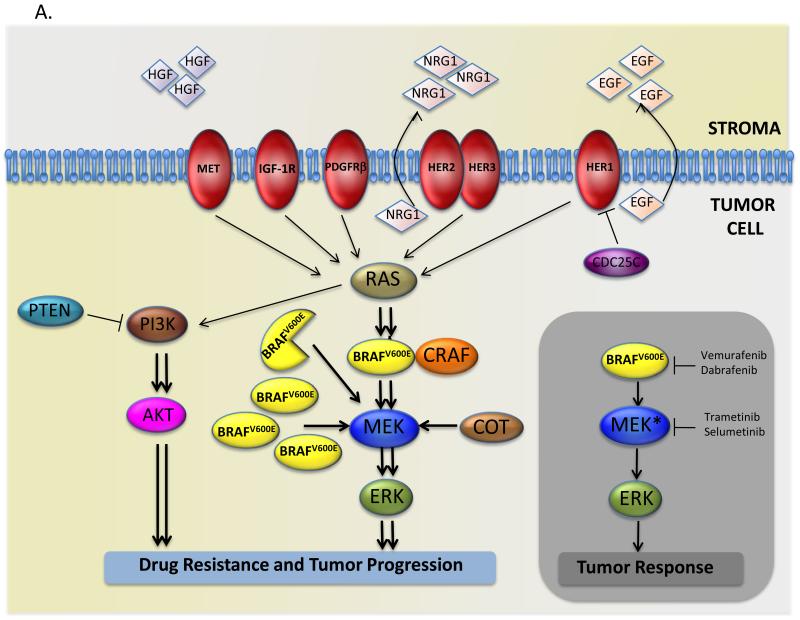
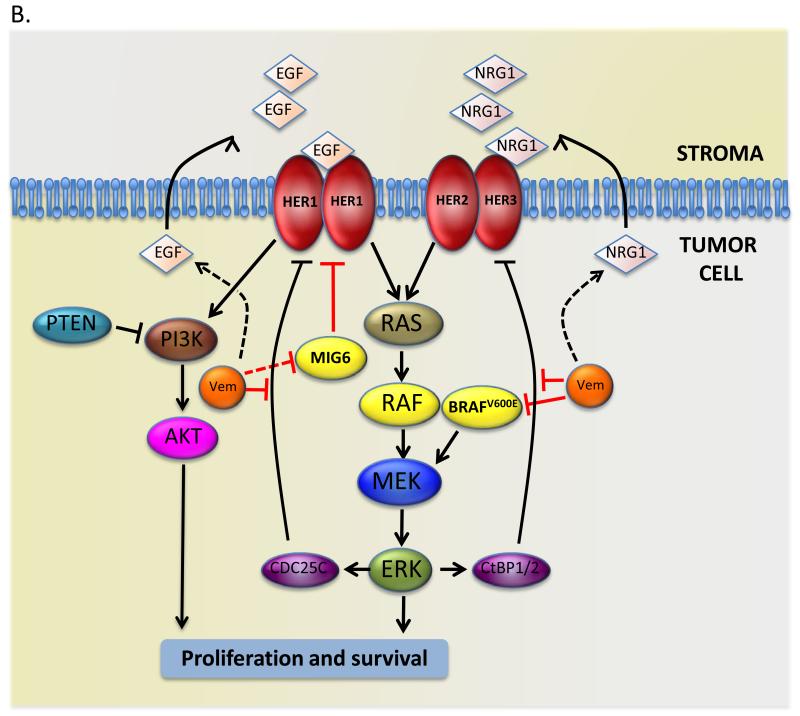
Figure 1. Mechanisms of resistance to BRAF inhibitors.
(A) Grey rectangle: mutant BRAF (BRAFV600E) hyper-activates ERK signaling and promotes tumor cell proliferation and survival, but BRAF and MEK drugs inhibit the pathway and block tumor progression. Main figure: resistance to BRAF inhibitors is mediated by several mechanisms, including expression of a truncated forms of mutant BRAF, increased expression of mutant BRAF or wild-type CRAF, acquisition of mutations in RAS or MEK, expression of MAP3K8/COT, loss of PTEN expression, or activation of the receptor tyrosine kinases PDGFRβ, IGF-1R, EGFR and HER2/HER3, or increased activation of MET through the increased secretion of HGF by the stromal compartment.
(B) EGF family receptors mediate resistance to BRAF inhibitors. In colorectal cells BRAF inhibits HER1 by inducing CDC25C, so BRAF inhibition by vemurafenib (Vem) releases the block to HER1 activation by reducing CDC25C expression. In thyroid cancer cells HER3 expression is inhibited by BRAF through the CtBP1/2 transcription repressors, so BRAF inhibition by vemurafenib (Vem) results in increased HER3 expression, and it alsoincreases NRG1 expression through unknown mechanisms. In melanoma, BRAF inhibition by vemurafenib (Vem) drives HER1 signaling by increasing EGF secretion, increasing HER1 expression and suppressing MIG6 activity through unknown mechanisms.
Vemurafenib is a potent and selective BRAF inhibitor that increases progression-free and overall survival in ~80% in melanoma patients whose tumors carry BRAF-mutations (2, 3). In its wake are several equally promising BRAF drugs, such as dabrafenib (4), and several MEK inhibitors such as trametinib (5) and selumetinib (6), which are also active in these patients. Thus, following advances in chronic myeloid leukemia, gastro-intestinal stromal tumors and lung cancer, BRAF and MEK inhibitors have made personalized medicine a reality for melanoma patients and changed the perception that this disease is essentially untreatable.
Curiously, despite the remarkable responses to BRAF/MEK inhibitors in melanoma, the response of other BRAF-driven cancers to these drugs is very disappointing. Only ~5% of BRAF-mutant colorectal patients respond to vemurafinib (7), and thyroid cancers do not respond to selumetinib (8). In this issue of Cancer Discovery, Montero-Conde and colleagues (9) investigate the reasons underlying the intrinsic resistance of BRAF-mutant thyroid cancer cells to BRAF inhibitors. They show that while BRAF and MEK inhibitors induce sustained ERK inhibition in BRAF-mutant melanoma cells, they induce only transient ERK inhibition in BRAF-mutant thyroid cancer cells and that the rapid rebound in signaling is due to increased signaling by HER3, a member of the EGF receptor family.
HER3 activation appears to be part of a generalized hyper-activation of RTKs, but it is HER3 together with the closely related HER2 that reactivates RAS-ERK signaling and allows the cells to bypass the growth-inhibitory effects of BRAF inhibition (Fig 1B). Notably, vemurafenib increases NRG1 (the HER3 ligand) production. Intriguingly, the authors also found that oncogenic BRAF normally blocks HER3 expression through the transcription repressors CtBP1/2, so BRAF inhibition increases HER3 expression (Fig 1B). Thus, they have revealed the release of a feedback loop from oncogenic BRAF to HER3 and a general call to arms by this pathway that releases the cells’ addiction to oncogenic BRAF when BRAF is inhibited.
This all sounds curiously familiar. Last year it was shown that colorectal cells are also insensitive to vemurafenib because of elevated HER1 (EGF receptor) signaling (10, 11). Here again, vemurafenib only induced transient ERK inhibition with the rebound being driven by HER1, which signaled through RAS to AKT (10, 11). In elegant experiments, it was also shown that in colorectal cells oncogenic BRAF drives expression of CDC25C, a phosphatase that negatively regulates HER1 (11). Thus, when BRAF was inhibited, CDC25C expression was lost, leading to HER1 activation. Thus, although the molecular details differ, in both thyroid and colorectal cancers BRAF inhibitors release a feedback loop that activates intrinsic resistance against themselves (Fig 1B).
Increased RTK signaling also mediates acquired resistance to BRAF inhibitors in melanoma cells. A disappointment with BRAF and MEK drugs is that despite remarkable initial responses, most melanoma patients develop resistance after a relatively short period of disease control (2-6) and even when combined, most patients relapse after just over a year (12). Furthermore, about 20% of patients present with intrinsic resistance. Resistance can be driven by amplification of the mutant BRAF gene, expression of truncated mutant protein, acquisition of mutations in RAS and MEK, or hyper-activation of the PI3-kinase/PTEN/AKT signaling pathway (Fig 1A). Another common mechanism appears to be increased RTK signaling, with the PDGF receptor, the insulin-like growth factor 1 receptor (IGF-1R), and MET all implicated (Fig 1A). More important in this context, HER1 can also drive acquired resistance (13, 14). As in thyroid cancer, the underlying mechanism appears to be a general call to arms of signaling, with increased autocrine signaling by EGF, upregulation of HER1, and downregulation of the negative signaling regulator MIG6 (13, 14).
The parallels between the different diseases is intriguing, with similar general responses driven by distinct underlying mechanisms. Some of the details in thyroid cancer still need to be worked out. It is curious that HER3-mediated reactivation of ERK does not re-suppress it’s own transcription through CtBP1/2, and it is unclear whether, as in colorectal cancer (10), CRAF rather than BRAF drives pathway reactivation. Nevertheless, the general theme that emerges is that high-content genomics and proteomics allow rapid understanding of mechanisms of resistance to targeted therapies. Genomics provides the clues, but it is the protein data that reveals the mechanisms. Critically, these studies provide biomarkers that can be used to screen patients for evidence of likely intrinsic resistance, or to monitor patients in longitudinal studies for evidence of the emergence of resistance. Critically, in all of the cases discussed above, the combination of BRAF and EGF receptor family inhibitors suppressed the growth of the resistant cells, giving hope that effective personalized treatments can be developed for patients with intrinsic or acquired resistance.
Acknowledgments
Financial Support: R. Marais: ~£3m, Cancer Research UK (2 grants); salary, PICR.
Footnotes
Conflict of Interest: As a former employee of the Institute of Cancer Research, Richard Marais participates in a “Rewards to Inventors Scheme,” that could provide financial benefit for contributions to programs that are commercialized.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 5.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalanotti F, Solit DB, Pulitzer MP, Berger M, Scott SN, Iyriboz T, et al. Phase II trial of MEK inhibitor selumetinib (AZD6244) in patients with BRAFV600E/K-mutated melanoma. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetz SD,J, Chan E, Hecht JR, O’dwyer PJ, Lee RJ, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;(suppl):28. (Abstract nr 3534) [Google Scholar]
- 8.Hayes DN, Lucas AS, Tanvetyanon T, Krzyzanowska MK, Chung CH, Murphy BA, et al. Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056–65. doi: 10.1158/1078-0432.CCR-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF mutant thyroid carcinomas. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Held MA, Langdon CG, Platt JT, Graham-Steed T, Liu Z, Chakraborty A, et al. Genotype-selective combination therapies for melanoma identified by high-throughput drug screening. Cancer Discov. 2013;3:52–67. doi: 10.1158/2159-8290.CD-12-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girotti MR, Pedersen M, Sanchez-Laorden B, Viros A, Turajlic S, Niculescu-Duvaz D, et al. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov. 2013;3:158–67. doi: 10.1158/2159-8290.CD-12-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]