Abstract
Visceral larva migrans (VLM) is a systemic manifestation of migration of second stage larvae of nematodes through the tissue of human viscera. It is not uncommon but is underdiagnosed in developing countries. The liver is the most common organ to be involved due to its portal venous blood supply. The imaging findings are subtle and differentiation from hepatocellular carcinoma (HCC), metastases, cystic mesenchymal hamartoma and granulomatous diseases is difficult. This case report highlights the imaging features of hepatic lesions of VLM along with clinical and laboratory data which help in clinching the diagnosis.
Background
Dogs and cats are definitive hosts for Toxocara, while humans are paratenic hosts. In humans, the ingested larvae hatch in the intestine, penetrate the intestinal wall, flow through the portal vein and reach the liver, lungs, orbit and brain. Some larvae move slowly in the liver (visceral larva migrans) or become encapsulated and remain in that state with no further growth for an indefinite period. VLM is due to migration of second stage larvae of nematodes through human viscera.
Case presentation
A 16-year-old girl from a rural background presented with intermittent abdominal pain, malaise, loss of appetite and restlessness for 3 months. The pain was not associated with food intake. There was no vomiting, fever, black stools, bleeding per rectum, passage of worms in stools or urticaria. She was a vegetarian. There were no pets at home but plenty of stray dogs in neighbourhood. The patient was slightly pale and had hepatosplenomegaly, (liver span being 17 cm and spleen being 15 cm) but no significant lymphadenopathy.
Investigations
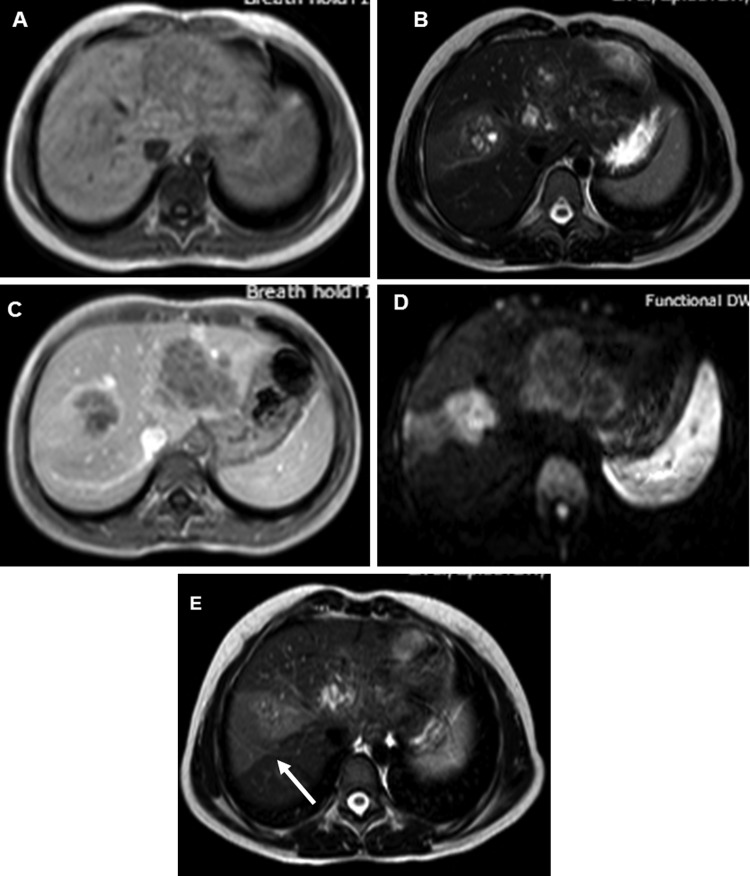
Haematological examination revealed mild microcytic hypochromic anaemia (Hb=10.9 g/dL) and eosinophilia (blood eosinophil count being 16%, absolute eosinophil count 1360/mm3). Serum alkaline phosphatase was raised (193 IU/L), all other liver function parameters being normal. Renal functions and coagulation profile were normal. Serum α-fetoprotein was within normal limits (1.26 ng/mL). Ultrasound (US) of the abdomen showed multiple ill-defined hypoechoic lesions in the liver, slightly oval in shape along both right as well as left portal veins (figure 1). Contrast-enhanced CT scan of the abdomen showed conglomerate of cystic lesions along right as well as left portal veins with slight enhancement of the walls of cysts on portal venous phase. The lesions were most conspicuous in portal venous phase (figure 2A, B). MRI showed hepatic lesions to be hypointense on T1-weighted (T1W) images (figure 3A) and hyperintense on T2-weighted (T2W) images (figure 3B) with slight peripheral enhancement on contrast-enhanced T1W images (figure 3C). There was restricted diffusion on diffusion-weighted (DW) images with b value of 1000. The distribution of the lesions was again along the right and left portal veins. There were no peripheral lesions. In addition, there was a wedge-shaped hyperintense lesion on T2W images (figure 3D) (almost isointense on T1W images and showing subtle enhancement on contrast-enhanced MRI) in right lobe of liver. In light of lesion characteristics, and distribution along with peripheral eosinphilia, a provisional diagnosis of hepatic lesions associated with visceral larva migrans was made. The ELISA test for Toxocara canis was positive, it detected IgG class antibodies against T canis and with sensitivity and specificity >95%. The values of IgE were also high (1760 IU/L). Ultrasound-guided fine needle aspiration cytology through the lesion with special stains showed cellular smears comprising degenerated, mixed inflammatory cells predominantly consisting of eosinophils along with necrosis and fair number of Charcot-Leydon crystals. No parasites were identified in the smears. The findings were suggestive of eosinophilic abscess, thus corroborating radiological findings and a final diagnosis of visceral larva migrans with hepatic lesions was made.
Figure 1.
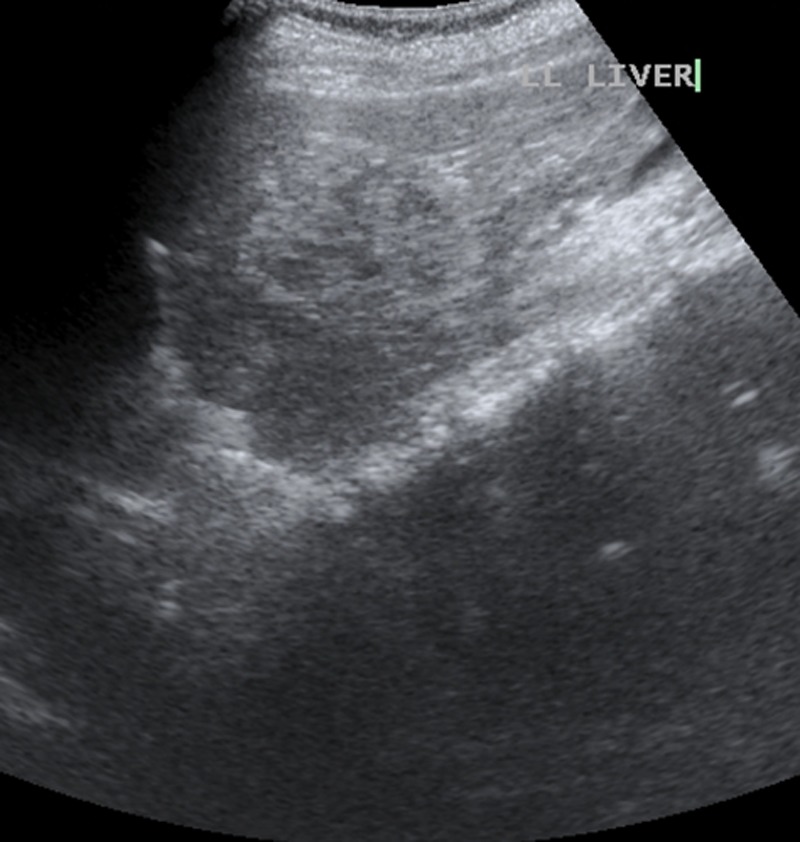
Ultrasound of the abdomen showing multiple ill-defined hypoechoic lesions in the liver, slightly oval in shape along left portal vein.
Figure 2.
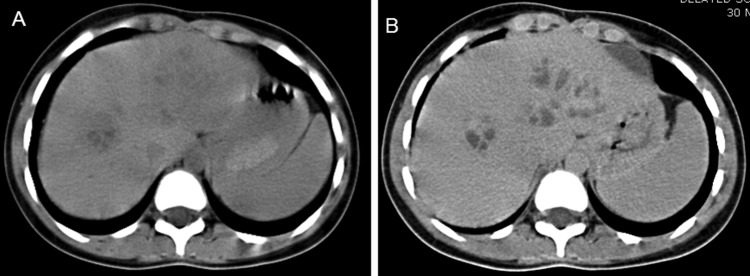
(A) CT scan of the abdomen showing conglomerate of ill-defined oval cystic lesions along right as well as left hepatic ducts. (B) Contrast-enhanced CT scan of the abdomen in portal venous phase showing slight peripheral enhancement of the walls of the lesions which are now more conspicuous.
Figure 3.
(A) T1-weighted (T1W) axial MRI image showing the lesions to be hypointense. (B) T2-weighted (T2W) axial image showing the lesions to be hyperintense. (C) T1W contrast-enhanced axial image showing slight peripheral enhancement of the lesions in portal venous phase. (D) Diffusion-weighted image with b value of 1000 showing hyperintensity of the lesions. (E) T2W axial image at slightly higher level showing wedge-shaped area of hyperintensity (arrow). The corresponding areas were hypointense on ADC map (image not available) S/O restricted diffusion.
Differential diagnosis
The radiological differential diagnoses included hepatic malignancies such as hepatocellular carcinoma, hepatoblastoma and metastases along with cystic mesenchymal hamartoma, sarcoidosis and tuberculosis. These were excluded by the clinical background and the cytological and serological findings as well as her complete response to specific therapy (vide infra).
Treatment
The patient was given 400 mg of albendazole twice a day for 3 weeks.
Outcome and follow-up
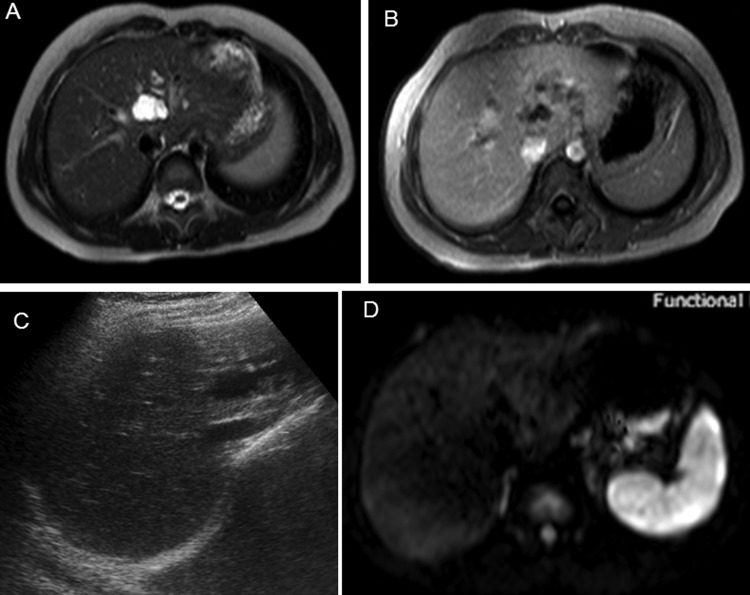
The patient was totally relieved of her symptoms after treatment. Post-treatment US and MRI showed coalescence of ill-defined lesions into one lesion with reduction of size of lesion from 68×45×44 mm pretreatment to 20×29×29 mm post-treatment. Post-treatment DW images showed no diffusion restriction (figure 4A–D). The patient is symptom free 1.5 years after treatment.
Figure 4.
(A–D) After 6 months post-treatment MRI and ultrasound-guided images showing coalescence of ill-defined lesions into one lesion whose extent is much less than pretreatment MRI. Diffusion-weighted image shows no diffusion restriction.
Discussion
Visceral larva migrans (VLM) was first described by Beaver et al in 1952. The term VLM is used to describe migration of second stage larvae of nematodes through the tissue of human viscera.1 T canis and Toxocara cati are most important causes of VLM.2 Others like Baylisascaris procyonis, Cappilaria hepatica, Ascaris suum and some Ankylostoma species have also been implicated. In humans, the larvae are deposited in the liver, lungs, eye, heart and brain and may cause hepatomegaly, endophthalmitis and neurological disturbances.3
The clinical triad of toxocariasis is unexplained eosinophilia, liver or lung nodules on imaging studies and a history of eating animal liver.4 Dog hair and soil also contain infective stage eggs, and infection can occur when eggs containing fully developed larvae are swallowed. Our patient appears to have contracted the infection either via stray dogs of the neighbourhood or infected soil, as there is no history of eating meat. After hatching in the intestine, the larvae penetrate the intestinal wall, flow through the portal vein and reach the liver, lungs, orbit and brain. Some larvae move slowly in the liver (VLM) or become encapsulated and remain in that state with no further growth for an indefinite period.3 Our patient was not imaged at intervals and hence the migratory nature could not be documented. There are various non-specific symptoms of VLM including general malaise, cough and liver function disorders.5 Our patient also had malaise.
The chronic production of parasite antigens, continuous stimulation of host immune system and concomitant production of eosinophils can cause systemic complications; liver being the most common site for these lesions because of its portal drainage.6
The mechanism of liver infiltration is believed to be an allergic response to the larva.7 The lesions are most conspicuous in portal venous phase and tend to be in the periphery of the liver and along the portal vein branches. Eosinophil count and percentage in the peripheral blood are considerably higher in the patient group with hepatic lesions than in the group without hepatic lesions. However, eosinophil count and percentage do not correlate with the number of hepatic lesions on CT.8
Portal vein branches traversed the centre of larger lesions. Because larvae of T canis are distributed by portal blood flow, these larger lesions may be attributed to occlusion or alteration of the microvascular portal blood supply in addition to eosinophilic inflammation itself.9 A wedge-shaped hyperintensity on T2W images in our patient might represent focal venous congestion due to occlusion of portal venules by larvae. On treatment, the larvae might have died, occlusion relieved and the area of presumed venous congestion also disappeared.
These lesions are caused by arrested immature worms during migration and thus contain worms.10 The resultant focal lesion is an abscess or granuloma, a marked eosinophilic infiltrates on pathology, also called eosinophilic abscess or granuloma.11 No worms were detected in the hepatic lesion of our patient. Many of the excretory–secretory proteins from the juvenile stages of the worms constitute a family of at least six highly antigenic mucins associated with the cuticular surface. Secreted mucins temporarily coat the surface of the worm and are shed into the host periodically. This shedding behaviour represents an attempt on the part of the parasite to confuse the host's immune system, leaving behind it a trail of slime. The worm periodically switches its secreted antigenic identity, thus avoiding harm.12
Eosinophilia can also occur in allergic reactions, parasitic infestations, dermatoses, connective tissue disorders and neoplastic diseases.11
Echinococcosis is another common parasitic infestation which can lead to eosinophilia, but liver lesion are mostly mass forming and may show daughter cyst, hydatid sand and detached membranes (water lily sign).
VLM is more common in Korea and Japan. An interesting association is with patients having primary malignancies such as stomach cancer and hepatocellular carcinoma (HCC).13 In these societies, it is believed that eating raw meat by patients with cancer helps in better recuperation. Such practices lead to eating of raw liver and hence transmission of larvae. Such patients usually have eosinophilia. It then becomes difficult to distinguish between focal hepatic lesions due to VLM or metastases or satellite lesions of HCC. Nodules of VLM have fuzzy margins, non-spherical shape and are subtly low attenuating whereas metastases/HCCs have well-defined margins, are spherical and low attenuating. Rim enhancement at equilibrium phase is seen almost exclusively in metastases but seldom found in eosinophilic necrosis.14 Metastases are more conspicuous on the arterial phase while nodules of VLM are more conspicuous on the portal venous phase. This is explained by the fact that hypovascular hepatic lesions are most clearly delineated during the portal venous phase, when the hepatic parenchyma enhances maximally. On the equilibrium phase images, most foci disappear. This suggests that contrast material diffuses more easily into small, low-attenuating foci, thus becoming isodense in this phase. Some lesions of VLM have been seen to enhance on the hepatic arterial phase but do not show any washout on equilibrium and delayed phases and hence can be differentiated from the nodules of HCC.13 Hyperintense rim of lesions on T1W images and hyperintensity on diffusion-weighted images at b value of 1000 with associated restriction on the corresponding apparent diffusion coefficient maps suggests nodules of VLM.15 The lesions of our patient also had restricted diffusion. In contrast to VLM, hepatic metastatic tumours do not have Kupffer cells within the tumour itself. VLM showed a markedly increased number of Kupffer cells.16 So it is possible to differentiate between VLM and metastases using superparamagnetic iron oxide (SPIO)-enhanced MRI. These lesions (VLM) have reduced signal intensity on SPIO-enhanced T2-weighted images.13
Some malignant neoplasms such as lymphoma, leukaemia and carcinoma are often associated with eosinophilia.17 It is very important to exercise caution in such patients while reporting VLM. Molecular diagnostics can be very helpful in such cases.18 19 PCR and DNA sequencing techniques utilising a range of genetic markers in the nuclear and mitochondrial genomes can be useful to supplement the traditional ELISA-based immunodiagnosis and for precise corroboration of radiodiagnosis because of their high sensitivity, specificity and rapidity.20 21 Particularly two mitochondrial genes (complete ATPase 6 and partial small subunit ribosomal RNA (12S rDNA), two nuclear ribosomal genes (second internal transcribed spacer region (ITS-2) and part of the large subunit 28S region can be analysed as molecular markers for diagnosis.22 These also help in differentiating toxocara infections from other cross infections in tropical countries where traditional immunodiagnostics are not very specific.
At present, the diagnosis of toxocariasis is mostly serological. Molecular vaccines can aid in control of infection in dogs and cats. Several fragments of myosins of toxocara have proved highly antigenic and can be potentially used to develop vaccines against toxocara when used in combination with ELISA for IgG antibodies.12 An IgG-ELISA assay based on a recombinant version (rTES-30USM) of the 30 kDa toxocara excretory–secretory antigen (TES-30) has recently been developed.23 rTES-30USM demonstrated a sensitivity of 92.3% and a specificity of 89.6% compared with a sensitivity of 100% but a specificity of only 55.7% with commercial kit. The high sensitivity and specificity of new IgG-ELISA gives it an advantage of potential use in tropical countries prone to cross-reactive helminthic infections.
Future control programmes can involve molecular or DNA-based vaccines offering lifelong protection. Oral baits laced with vaccine can be ideal for stray dogs and cats.
Learning points.
Hepatic nodules due to visceral larva migrans (VLM) should be considered as one of the diagnostic possibility in cases with multiple hepatic lesions.
Eosinophilia is a useful clue but is not completely specific for VLM. Cytology/histology along with clinical correlation help exclude other causes like malignancies.
Newer generation ELISA-based serological tests form the mainstay of diagnosis although molecular assays for ribosomal and mitochondrial genes, if available, are highly sensitive and specific.
Periportal distribution of lesions and conspicuity on portal venous phase should raise the possibility of VLM.
Footnotes
Contributors: SR and NJ diagnosed the case. DBD put valuable genetic pathogenesis of this case and reviewed the manuscript. RY carried out the correction and finalised manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beaver PC, Syndrer CH, Carrera GM, et al. Chronic eosinophilia due to visceral larva migrans: report of three cases. Pediatrics 1952;2013:7–9 [PubMed] [Google Scholar]
- 2.Glickman LT, Magnaval JF, Domanski LM. Visceral larva migrans in French adults: a new disease syndrome? Am J Epidemiol 1987;2013:1019–34 [DOI] [PubMed] [Google Scholar]
- 3.Baever PC, Jung RC, Cupp EW. Clinical parasitology, 9th edn. Philadelphia, PA: Lea & Febiger, 1984:320–9 [Google Scholar]
- 4.Lim JH, Lee KS. Eosinophilic infiltration in Korea: idiopathic?. Korean J Radiol 2006;2013:4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kanpen F, Buijs J, Kortbeek LM, et al. Larva migrans syndrome:toxocara, ascaris, or both? Lancet 1992;2013:550–5 [DOI] [PubMed] [Google Scholar]
- 6.Kayes SG. Human toxocariasis and the visceral larva migrans syndrome: correlative immunopathology. Chem Immunol 1997;2013:99–124 [DOI] [PubMed] [Google Scholar]
- 7.Sakai S, Shida Y, Takahashi N, et al. Pulmonary lesions associated with visceral larva migrans due to ascaris suum or toxocara canis: imaging of six cases. AJR 2006;2013:1697–702 [DOI] [PubMed] [Google Scholar]
- 8.Chang S, Lim JH, Choi D, et al. Hepatic visceral larva migrans of toxocara canis: CT and sonographic findings. AJR 2006;2013:W622–9 [DOI] [PubMed] [Google Scholar]
- 9.Lim JH, Lee WJ, Lee DH, et al. Hypereosinophilic syndrome: CT findings in patients with hepatic lobar or segmental involvement. Korean J Radiol 2000;2013:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farid Z, Kilpatrick ME, Chiodini PL. Parasitic diseases of the liver. In: Schiff L, Schiff ER, Diseases of the liver. 6th edn Philadelphia: Lippincott, 1993:1338–55 [Google Scholar]
- 11.Lee WJ, Lim HK, Lim JH, et al. Foci of eosinophil-related necrosis in the liver: imaging findings and correlation with eosinophilia. AJR 1999;2013:1255–61 [DOI] [PubMed] [Google Scholar]
- 12.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 2003;2013:265–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YK, Kim CS, Moon WS, et al. MRI findings of focal eosinophilic liver diseases. AJR 2005;2013:1541–8 [DOI] [PubMed] [Google Scholar]
- 14.Jang HJ, Lee WJ, Lee SJ, et al. Focal eosinophilic necrosis of the liver in patients with underlying gastric or colorectal cancer: CT differentiation from metastases. Korean J Radiol 2002;2013:240–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laroia ST, Rastogi A, Sarin S. Case series of visceral larva migrans in the liver: CT and MRI findings. IJCRI 2012;2013:7–12 [Google Scholar]
- 16.Cho ES, Yu JS, Kim MJ, et al. Focal eosinophilic necrosis on superparamagnetic iron oxide—enhanced MRI. AJR 2010;2013:1296–302 [DOI] [PubMed] [Google Scholar]
- 17.Wasserman SI, Goetzl EJ, Ellman L, et al. Tumor-associated eosinophilotactic factor. N Engl J Med 1974;2013:420–4 [DOI] [PubMed] [Google Scholar]
- 18.Powers T. Nematode molecular diagnostics: from bands to barcodes. Annu Rev Phytopathol 2004;2013:367–83 [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Zhou DH, Nisbet AJ, et al. Advances in molecular identification, taxonomy, genetic variation and diagnosis of Toxocara spp. Infect Genet Evol 2012;2013:1344–8 [DOI] [PubMed] [Google Scholar]
- 20.Li MW, Lin RQ, Chen HH, et al. PCR tools for the verification of the specific identity of ascaridoid nematodes from dogs and cats. Mol Cell Probes 2007;2013:349–54 [DOI] [PubMed] [Google Scholar]
- 21.Jex AR, Waeschenbach A, Littlewood DTJ, et al. The mitochondrial genome of Toxocara canis. PLoS Negl Trop Dis 2008;2013:e27–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickramasinghe S, Yatawara L, Rajapakse RP, et al. Toxocara canis and Toxocara vitulorum: molecular characterization, discrimination and phylogenetic analysis based on mitochondrial (ATP synthase subunit 6 and 12S) and nuclear ribosomal (ITS-2 and 28S) genes. Parasitol Res 2009;2013:1425–30 [DOI] [PubMed] [Google Scholar]
- 23.Norhaida A, Suharni M, Liza Sharmini AT, et al. rTES-30USM: cloning via assembly PCR, expression and evaluation of usefulness in the detection of toxocariasis. Ann Trop Med Parasitol 2008;2013:151–60 [DOI] [PubMed] [Google Scholar]