Abstract
Cancers adopt diverse strategies to safeguard their survival, which often involve blinding or incapacitating the immune response, thereby gaining battleground advantage against the host. In immune responses against cancer, an important stimulatory lymphocyte receptor is NKG2D because the tumor-associated expression of its ligands promotes destruction of malignant cells. However, with advanced human cancers profound changes unfold, wherein NKG2D and its ligands are targeted or exploited for immune evasion and suppression. This negative imprinting on the immune system may be accompanied by another functional state wherein cancer cells co-opt expression of NKG2D to complement the presence of its ligands for self-stimulation of tumor growth and presumably malignant progression. This review emphasizes these conflicting functional dynamics at the immunity – cancer biology interface in humans, within an overview of the immunobiology of NKG2D and mechanisms underlying the regulation of its ligands in cancer, with reference to instructive clinical observations and translational approaches.
The human NKG2D receptor is normally expressed on NK cells, CD8 T cells, γδ T cells, and some autoreactive and immunosuppressive CD4 T cells (1, 2). Its significance springs from the biology of its ligands, which are absent or poorly expressed on most cells but are induced in infections and malignancies by viral transactivation, and mechanisms associated with cellular stress responses and tumorigenic states, respectively (3, 4). These attributes have given rise to the concept of “induced self” serving to alert the immune system to pathological threats. Typically, engagement of NKG2D elicits protective cytolytic and cytokine responses by activation, either directly or via costimulation, of NK cells and CD8 T cells (5, 6). However, depending on cytokine environments together with conditions of anomalous ligand induction, NKG2D can also initiate and/or exacerbate autoimmune disease inflammatory processes such as in rheumatoid arthritis and celiac disease (5, 7).
NKG2D forms homodimers that are assembled as hexameric complexes with four chains of the DAP10 signaling adaptor (6, 8). Distinct from mice, there is no shortened human NKG2D isoform that can also bind DAP12. Upon ligation of NKG2D, cytoplasmic tail tyrosine-phosphorylated DAP10 recruits PI3K or the growth factor receptor-bound protein 2 (GRB2)/VAV1 signaling intermediate, thereby activating protein kinase B (PKB/AKT), and ERK and JNK in MAPK cascades (8). These branched signaling pathways ultimately trigger lymphocyte cytotoxic granule polarization and degranulation, cytokine production, proliferation, and survival. Stable surface expression of NKG2D is dependent on its association with DAP10, which is mediated by oppositely charged amino acid residues in their transmembrane domains.
NKG2D expression can be induced by several cytokines (IL-2, IL-7 and IL-15) with IL-15 being biologically most significant (7, 9). High-dose IL-15 can license NKG2D–DAP10 for direct activation of NK cell and T cell cytotoxic functions, which play a detrimental role in certain T cell-mediated pathologies such as in celiac disease (7). Moreover, IL-15 and TNF-α induce NKG2D receptors on CD28− CD4 T cells, thus enabling costimulation of TCR-dependent autoreactivity against rheumatoid arthritis synoviocytes displaying inappropriately induced NKG2D ligands (5). Inhibitors of NKG2D expression include IL-21 and TGF-β, which regulates provision of DAP10 (10, 11). Macrophage migration inhibitory factor (MIF) is another negative regulator of NKG2D that is also produced by cancer cells (12). Finally, NKG2D is subject to ligand-induced down-modulation, which is especially significant in cancer settings and will be discussed further below.
Diversity and limited tissue distribution of NKG2D ligands
NKG2D ligands are distant relatives of MHC class I polypeptides but without their attributes linked to antigen presentation. There is a multitude of structurally diverse ligands including the closely related MHC class I-related chains A and B (MICA and MICB) and the more distant family of six UL16-binding proteins (ULBP1-6) (13). As with MHC class I, all NKG2D ligands have a distal α1α2 receptor binding platform domain. MICA and MICB also have the membrane-proximal α3 domain and are transmembrane-anchored. All ULBP ligands lack the α3 domain. ULBP1-3 and ULBP6 have GPI instead of transmembrane anchors. In outer cell membranes, GPI-anchored ULBP proteins are clustered in lipid raft microdomains, which may increase avidity of lower affinity ligands for NKG2D (14). Reflecting the evolutionary divergence of NKG2D ligands, the crystal structure of MICA shows a profoundly altered MHC class I fold with only a remnant of a shallow peptide binding groove and restructured α1α2 platform and α3 domain interfaces that preclude binding of β2m (5). MICA and MICB are represented by numerous alleles, which differ in binding affinities for NKG2D (5, 15).
The ability of NKG2D to interact with diverse ligands seems perplexing. In a complex crystal structure, the saddle-shaped NKG2D homodimer sits astride the α1α2 platform domain of MICA, with each NKG2D monomer contacting either one of the subdomains. The majority of the interaction energy resides in two NKG2D binding site tyrosines that make dominant contacts at each interface in the absence of conformational plasticity (16). Complementary information from an NKG2D–ULBP3 complex structure suggests that binding of different ligands involves common interfaces and overlapping sets of amino acid side-chain interactions, thus permitting conservation of general shape complementarities and binding energies (17).
The multiplicity and diversity of NKG2D ligands apparently reflects evolutionary pressure to maintain redundancy. One explanation is based on an evolutionary arms race with viruses encoding immunoevasins, which have the capacity to obstruct the functionality of different sets of NKG2D ligands (13, 18). Another concept emanates from the function of the ligands as sensors of cellular damage cues. Raulet and colleagues proposed that NKG2D ligands may convey composite specifications, analogous to barcodes, on the surfaces of unhealthy cells, with variables including presence and relative abundancies of diverse ligands and differences in post-translational modifications (4). However, NKG2D ligands are not absent from all normal cells but presumably are insufficient, or ineffectively disposed, to stimulate an immune response when present. Some NKG2D ligand mRNAs are quite ubiquitous, which is inconsequential presumably because of their negative regulation by microRNAs (19). Nevertheless, physiologically relevant examples for NKG2D ligand expression under normal conditions exist, including the expression of MICA/B in intestinal mucosa, thymic epithelium, and the placental syncytiotrophoblast (5).
Expression and regulation of NKG2D ligands in cancer
Expression of NKG2D ligands is pronounced in malignancies, underscoring their role in promoting tumor immune surveillance. In the most comprehensive study of hematopoietic malignancies, Hilpert and colleagues documented heterogeneous surface expression of at least one NKG2D ligand type in about 75% of altogether 205 leukemia patients (20). With solid tumors, a survey of widely scattered data suggests that MICA/B predominate alongside at least one ULBP family ligand in most epithelial cancers including head and neck, lung, breast, ovarian, cervical, prostate, gastrointestinal, pancreatic, hepatocellular, and renal cell cancer, as well as in malignant glioma, neuroblastoma, and melanoma (1, 5).
Tumor expression of NKG2D ligands is linked to generic mechanisms coupled to cellular stress, proliferation, and unfolded protein responses, as well as to signaling intermediates and checkpoint anomalies associated with oncogenic states (3, 4). Within this framework, detail knowledge of ligand regulation is fragmented. In addition to transcriptional activation, protein biosynthesis can modify ligand expression (4). Moreover, different mechanisms may activate transcription of the same ligand gene, thus expanding the range of responses to cellular damage cues.
The regulation of the genes for MICA and MICB shares similarities with heat-shock protein 70 (HSP70) genes (21). These genes commonly have a promoter region heat-shock response element (HSE) for conditional binding of heat shock factor 1 (HSF1), a transcriptional activator that is responsive to homeostatic changes caused by heat shock and oxidative stress. Promoter regions of MICA/B also activate proliferation-induced transcription (21). With epithelial tumor lines and presumably cancer cells as well, epigenetic derepression of promoter accessibility, exemplified by pharmacological histone deacetylase (HDAC) inhibition, plays an essential role in the transcriptional activation of NKG2D ligand genes (3). In accord with their regulation by elementary cellular processes, relevant transcription factors in addition to HSF1 are ubiquitous. They include members of the SP zinc finger transcription factor family and the CCAAT box factor (CBF) complex, which variably interact with 5′-end flanking sequences at least of MICA/B and ULBP1 (3). The activator protein-1 (AP-1; Fos/Jun), which has diverse roles in inflammatory responses as well as a tumor promoter or suppressor, has been implicated in the regulation of a mouse NKG2D ligand gene (22). In humans, AP-2α suppresses ULBP1 transcription by interfering with SP complex binding site occupancy (3). These findings reflect the patchwork state of research with independent findings in humans and mice that are most often not mutually applicable. Along this line, E2F transcription factors involved in cell cycle entry have been implicated in mouse ligand transcriptional activation but there is no corresponding evidence in humans (4). Conversely, the p53 tumor suppressor has been implicated in transcriptional activation of ULBP1 and ULBP2 but not of mouse NKG2D ligand genes (4, 23). Taken together, knowledge of promoter regions and transcription factors relevant to tumor-associated expression is limited for most NKG2D ligand genes. As of yet, reprogramming events associated with malignant transformation do not appear to have a principal role in their transcriptional activation.
However, NKG2D ligands can be post-transcriptionally regulated by factors associated with oncogenic states. The BCR–ABL tyrosine kinase gene fusion is associated with MICA induction in chronic myeloid leukemia (CML) (24). Moreover, chronic activation of the RAS GTPase in response to extracellular signals can lead to upregulation of ULBP1-3 and MICA via stimulation of MAP kinase and PI3K signaling cascades and, ultimately, by eukaryotic translation initiation factor 4E (eIF4E)-mediated increases of protein synthesis rates (4). An explanation for the near universal presence of NKG2D ligands in cancer cells was provided by Gasser and colleagues, who associated their expression with genotoxic stress and the activation of a DNA damage response owing to double-stranded DNA breaks or stalled replication forks (25). This pathway involves the ataxia telangiectasia mutated (ATM) or ATM and Rad3-related (ATR) protein kinases, which activate checkpoint kinases that ultimately effect cell cycle arrest, DNA repair, or apoptosis. Accordingly, DNA damaging agents and replication inhibitors can induce expression of ULBP1-3 and mouse NKG2D ligands (4, 25). The mechanistic coupling of ligand induction to this cellular response is in essence not understood (4).
Once induced in cancer cells, NKG2D ligands MICA/B and various ULBP are negatively regulated by cytokines including TGF-β and IL-10 (1). Carcinoembryonic antigen-related adhesion molecule 1 (CEACAM1) impedes glycosylation and hence intracellular transport of NKG2D ligands (26). Of interest is a report of O-glycan modification of MICA by core2 β-1,6-N-acetylglucosaminyltransferase (C2GnT) in bladder tumor cells. Here, poly-N-acetyllactoseamine attached to MICA core2 O-glycans (N-acetylgalactosamine linked to branched N-acetylglucosamine) binds galectin-3, which impairs ligand affinity for NKG2D. This modification may explain correlations between high expression of C2GnT and poor prognosis and highly metastatic behavior of bladder cancers (27).
Regulation of NKG2D ligands by microRNAs
As with many cellular processes, immune system functions and oncogenesis are regulated by microRNAs, which assemble with partially complementary sequences in 3′-untranslated regions of target mRNAs, thus inhibiting translation or promoting degradation. Stern-Ginossar and colleagues and subsequent studies identified cellular microRNAs of various families that suppress expression of MICA and/or MICB, or ULBP2 (19, 28). Most of those microRNAs are present in steady quantities in normal human cells, consistent with their proposed role in calibrating baseline expression of NKG2D ligand mRNAs such that no lymphocyte attack ensues. The current model hence posits that the repressive capacity of the microRNAs is exceeded in pathological conditions as in malignancies when transcriptional induction of NKG2D ligand genes leads to consequential mRNA and protein expression.
Nonwithstanding the attractiveness of this model, evidence in support of the significance of microRNAs in suppressing NKG2D ligands in human cancer is scant because of insufficient or contradictory correlative data. There are no larger studies correlating clusters of relevant microRNAs in ex vivo cancer cells with absence or presence of surface NKG2D ligands. Moreover, unrelated studies of microRNA expression patterns in human cancers have yielded discordant results. A microRNA with regulatory activity for MICA/B (miR-106b) was found reduced in primary ovarian cancer and increased in colorectal cancer, gastric cancer, and hepatocellular carcinoma (29-31). Among other microRNAs specific for MICA/B, miR-17-5p and miR-373 were found highly expressed in multiple cancers of diverse tissue origins; however, these microRNAs have pleiotropic effects in tumor progression, which may influence NKG2D ligand expression (29, 32). Lastly, there is the conundrum that most cancers comprise substantial cell populations that are positive for NKG2D ligands, which may in fact benefit tumor progression as discussed further below.
Protective role of NKG2D and its ligands in tumor immunity
Mouse model experiments substantiated NKG2D-dependent rejection of ligand-bearing tumor lines by NK cells and/or cytotoxic CD8 T cells in syngeneic mice (1). Most decisively, a study of NKG2D-deficient mice revealed defective immune surveillance in experimental spontaneous malignancies. Absence of NKG2D resulted in higher incidence and increased aggressiveness of early-arising tumors. Reflecting the capacity of NKG2D to trigger NK cell and CD8 T cell cytotoxicity, its functionality was associated with negative selection of cancer cells bearing its ligands (33).
In humans, evidence for a protective role of NKG2D against cancer is limited and naturally indirect. Single-nucleotide polymorphisms (SNPs) linked to NKG2D identified natural killer complex (NKC) haplotypes that are associated with low or high NK cell cytotoxic activity and more or less risk of colorectal and aerodigestive tract cancers in life style-adjusted control and patient cohorts (34-36). A biallelic (threonine/alanine) variant at amino acid position 72 in its transmembrane segment may implicate NKG2D more directly. Threonine72 may alter binding of DAP10 thereby increasing signaling strength. This variant was significantly less frequent in a study of patients with cervical carcinoma than in the healthy controls (37). Perhaps more instructive are correlative studies of NKG2D ligand expression in cancers and clinical outcomes. In a colorectal cancer study, MICA was an independent marker of good prognosis for stage I and stage II but not later stage cancers (38). A similar relationship was observed in pancreatic cancer (39). In a breast cancer study, MICA/B and ULBP2 were associated with decreased relapse in early-stage cancer (40). These clinical data support a consensus that NKG2D and its ligands are protective at early but not advanced tumor stages, reflecting the progressive immune system failure that allows unchecked cancer growth. Consequently, tumor progression coupled to persistent expression of NKG2D ligands points to changes in functional dynamics that may increasingly favor host tumor susceptibility instead of resistance.
Exploitation of NKG2D ligands as tumor survival assets enabling immune evasion
NKG2D is subject to ligand-induced downmodulation by endocytosis and partial degradation. This is most evident in patients with cancers positive for MICA/B, in which large proportions of CD8 T cells and NK cells among infiltrating lymphocytes have diminished surface NKG2D. Moreover, NKG2D is systemically reduced on peripheral blood CD8 T cells and NK cells. This deficiency is caused by circulating tumor-derived soluble ligands and associated with impaired immune responses (41, 42). Shedding of soluble MICA and/or MICB and ULBP2 has been reported for most cancers and some hematopoietic malignancies. Tumor-derived exosomes may also contribute to effects initially attributed to soluble ligands alone (43). Experimental support for a critical role of soluble ligands in promoting tumor immune evasion was provided by a mouse model study comparing tumor formation by implants expressing either wild-type or a shedding-resistant form of MICB, or just the secreted soluble ligand. The results confirmed the capacity of soluble ligand to foster tumor growth, wherein impairment of NK cell responses was noted as the probable cause (44). This relationship is highlighted by beneficial effects of neutralizing anti-MICA autoantibodies that were induced as a result of anti-CTLA-4 immunotherapy in a clinical trial with melanoma patients (45). By and large, the observed negative imprints on the immune response are corroborated by clinical data, as tumor-associated or soluble NKG2D ligands are associated with parameters of disease progression and poor prognosis in pancreatic and prostate cancer (MICA/B; 39, 46), ovarian cancer (ULBP2 and ULBP4; 47), melanoma (ULBP2; 48), and multiple myeloma (MICA; 49).
Biological precedent for tumor shedding of NKG2D ligands exists in pregnancy where immunosuppressive factors must prevent rejection of the semiallogeneic fetus. Placental syncytiotrophoblast cells express NKG2D ligands including MICA/B and ULBP family members. Both shedding of soluble ligands and release of exosomes may contribute to NKG2D downmodulation on NK cells and CD8 T cells and thereby to maintenance of an immune tolerogenic environment (5).
Regulation of NKG2D ligand shedding
Shedding of MICA and presumably MICB requires domain-specific deconstruction by an accessory protein before proteolytic cleavage can ensue. On the surface of tumor lines and ex vivo cancer cells, MICA interacts with endoplasmic reticulum protein 5 (ERp5), a disulfide isomerase that typically assists in the folding of nascent proteins inside cells. ERp5 and membrane-anchored MICA form transitory mixed-disulfide complexes from which soluble MICA is released after proteolytic cleavage near the cell membrane (50). ERp5 reduces a deeply buried disulfide bond in the membrane-proximal MICA α3 domain, thereby likely generating a conformational change that enables proteolytic cleavage of MICA. This physical relationship is reflected by positive correlations between ERp5 and MICA/B on the surface of leukemic cells and soluble ligands in multiple myeloma and CLL (51, 52). Recruitment of MICA into cholesterol-enriched membrane lipid microdomains by palmitoylation of cysteine residues in its cytoplasmic tail may promote shedding although there is no confirmatory evidence in cancer cells (53). NKG2D ligands are cleaved near the cell membrane by ADAM family of cell surface disintegrins and metalloproteases, among which members 9, 10, 14, and 17 have been variously implicated (54).
Persistent NKG2D ligand expression may cause systemic immune suppression
Chronic expression of NKG2D ligands, as in advanced cancer, may have broader immunosuppressive effects. Peripheral blood NK cells from patients with colorectal or cervical cancer lack functional natural cytotoxicity receptors (NCRs) NKp30 and/or NKp46, which has been associated with soluble MICA or diminished NKG2D (42, 55). Moreover, loss or diminished expression of the CD3ζ signaling adaptor is well documented in T cells and NK cells from patients with cancer (56). CD3ζ is associated with CD16 (FcγRIII), and is essential for NKp30/46 signaling and TCR complex expression and function. Experimentally, sustained ligand stimulation of signaling-competent NKG2D on activated human CD8 T cells or NK cells results in loss of CD3ζ concomitant with the gradual decrease of surface NKG2D (57). Mechanistically, these seemingly unrelated events are linked in a causal chain wherein NKG2D signaling initiates FasL/Fas receptor-mediated activation of caspases-3/7 resulting in CD3ζcleavage and subsequent degradation (57). Hence, cancer expression of NKG2D ligands may lead to impairment of multiple CD3ζ-dependent receptor functions, thus promoting far-reaching lymphocyte tolerization.
Other immunosuppressive effects are associated with a small subset of normally occurring CD4 T cells with activation-independent, constitutive expression of NKG2D. These T cells lack proinflammatory cytokine and cytolytic signatures and play no discernible role in anti-microbial recall responses. Instead, they are autoreactive and biased towards an immunosuppressive IL-10- and TGF-β-dominated cytokine profile (2, 58). For reasons unknown, the NKG2D+CD4+ T cells are non-susceptible to ligand-induced NKG2D downmodulation, thus maintaining NKG2D functionality under conditions of persistent ligand exposure. Hence, in environments providing stimulation of both TCR and NKG2D, the NKG2D+CD4+ T cells proliferate and may expand in numbers relative to other T cells, thereby causing imbalances in the lymphocyte pool and imposing an immunosuppressive cytokine milieu. This homeostatic adjustment may normally serve to dampen chronic immune activation. In advanced cancer patients, however, tumor expression and shedding mainly of soluble MICA/B can drive substantial proliferative expansions of the NKG2D+CD4+ T cells. Casting light on the physiological significance of these T cells, their frequencies are inversely correlated with disease activity in SLE, suggesting that they participate in suppression of effector responses (2).
Co-option of NKG2D as a potential tumor growth factor receptor
In addition to promoting tumor immune evasion and suppression, NKG2D ligands may constitute yet another tumor survival asset. In a conceptual twist, variable proportions of ex vivo breast, ovarian, prostate, and colon cancer cells express signaling proficient NKG2D, thereby complementing the presence of its ligands for self-stimulation of tumor growth and possibly malignant progression (59). Triggering of cancer cell NKG2D by antibody crosslinking or engagement of ligands on adjacent cancer cells activates the oncogenic PI3K–AKT–mammalian target of rapamycin (mTOR) signaling axis, which is commonly hyperactive in cancer, and downstream effectors controlling protein synthesis and cell growth. In addition, as in lymphocytes, engagement of NKG2D stimulates phosphorylation of ERK and JNK in MAPK cascades (59). These kinases are activation targets of receptor tyrosine kinases such as the epidermal growth factor receptor (EGFR), which, because of mutation of aberrant expression, frequently cause excessive cancer cell proliferation and increased motility and survival (60).
Consistent with these signaling activities, above-threshold expression of NKG2D–DAP10 in ligand-bearing tumor lines increases their bioenergetic metabolism and proliferation, thus implying similarities with oncogenic growth factor receptors such as the EGFR and the insulin-like growth factor-1 receptor (IGF-1R). Thus, cancers may co-opt expression of NKG2D–DAP10 for their own benefit to promote tumorigenesis. In a preliminary assessment, this role is supported by significant correlations between percentages of ex vivo cancer cells that are positive for surface NKG2D and criteria of tumor progression (59). Hence, NKG2D itself might in fact represent the main factor underlying the typically poor clinical outcomes that have been associated with cancer cell expression of its ligands (39, 46, 47, 49). Notably, tumor cell expression of NKG2D was not detected in several experimental models of spontaneous cancers in mice (59).
Targeting of NKG2D or its ligands for cancer therapy
Therapy approaches are tailored to enhance or redirect NK cell and/or T cell-mediated cytotoxicity by utilizing agents that may increase NKG2D ligand expression or bifunctional fusion proteins including an NKG2D ligand and a single-chain antibody fragment (scFv) targeting a specific tumor cell surface marker. Among pharmaceuticals tested for upregulation of NKG2D ligands on tumor lines in vitro are demethylating agents, proteasomal inhibitors, and genotoxic drugs used for chemotherapy such as HDAC inhibitors (61). Objectives for the bifunctional fusion proteins are to target NK cells and T cells onto tumor cells and engage NKG2D. Examples are ULBP2–anti-prostate-specific membrane antigen (PSMA) scFv and ULBP2–anti-syndecan-1 (CD138) scFv (62). CD138 is highly expressed in some hematopoietic malignancies such as multiple myeloma. Other ligand fusion proteins incorporating anti-HER2 (EGFR-2) antibody fragments have been developed for preliminary testing in mice (63).
Another type of bispecific T cell and NK cell engager, thus far tested in mice only, combines the extracellular domain of NKG2D with either CD3ε or the Fc portion of IgG2a for binding to CD16 (64, 65). In an in vivo tumor model, administration of the NKG2D–CD3ε fusion protein reduced growth of tumors bearing NKG2D ligands and promoted T cell infiltration. Likewise, the NKG2D–Fc region fusion protein triggered lysis and reduced growth of ligand-positive lymphomas (64, 65).
Transduction of chimeric activating lymphocyte receptors consisting of human NKG2D, CD3ζ and either costimulatory CD28 or 4-1BB (CD137), a tumor necrosis factor receptor (TNFR) family member, represents a strategy for adoptive T cell therapy (66, 67). Finally, another approach aims at induction or administration of antibodies for neutralization of soluble NKG2D ligands. It remains to be seen how these or other therapeutic approaches impact the dynamic functionality of NKG2D and its ligands at the interface between the immune system and cancer as there may be unintended consequences.
Conclusion
Although best known for their protective role in tumor immune surveillance, NKG2D and its ligands are also exploited as tumor survival assets, enabling immune evasion and suppression, and quite possibly stimulation of tumor growth and malignant progression (Fig. 1). The tentative role of NKG2D as an oncoprotein still requires in vivo experimental substantiation in an animal model. Cancer biology questions of further relevance are whether NKG2D imparts cellular changes in cancer environments that are distinct or merely synergistic to those conferred by prototypic growth factor receptors, and whether its activities impact other aspects of tumorigenesis. These issues broadly reflect an increasing appreciation of a need of an integrated approach that considers tumor and host factors together in the evaluation and targeting of tumor progression.
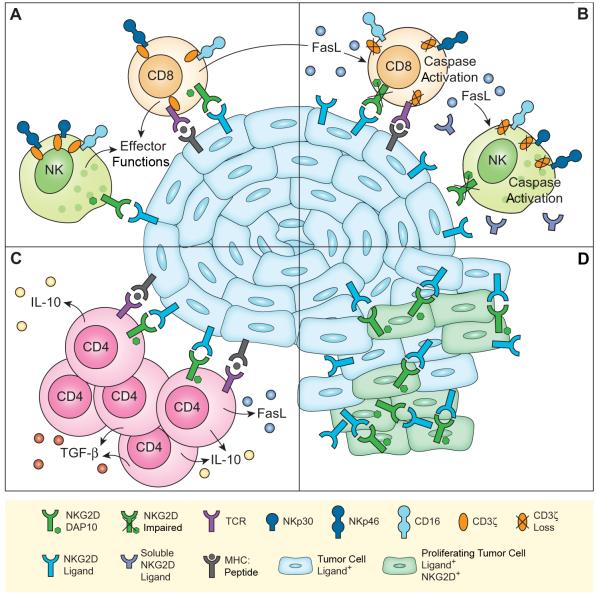
FIGURE 1.
Schematic representation of NKG2D/ligand-mediated effects in cancer settings. (A) Tumor-associated NKG2D ligands trigger effector functions of NK cells and, via costimulation, of CD8 T cells. NKG2D-mediated CD8 T cell stimulation triggers FasL release. At this stage, the TCR, CD16, and the NKp30 and NKp46 NCRs are functionally expressed in complex with CD3ζ. (B) Persistence of tumor cell membrane-bound and soluble NKG2D ligands cause NKG2D downmodulation and functional impairment. Still functional NKG2D (see A and C) initiates FasL/Fas-mediated caspase activation and resultant CD3ζ loss. As a consequence, the functional capacities of the TCR, CD16, and NKp30 and NKp46, which all signal through CD3ζ, are impaired. (C) NKG2D costimulates proliferation, and IL-10, TGFβ and FasL release by immunosuppressive NKG2D+CD4+ T cells. (D) NKG2D+ cancer cells proliferate in response to autocrine NKG2D-mediated stimulation. See text for further explanations.
Acknowledgements
We apologize for occasionally citing reviews instead of original references owing to space limitations.
Footnotes
A. E.-G. was supported by an Erwin Schrödinger Fellowship from the Austrian Science Fund (FWF; project number J3078). Work in the laboratory of V. G. and T. S. is supported by the National Institutes of Health.
References
- 1.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 2.Dai Z, Turtle CJ, Booth GC, Riddell SR, Gooley TA, Stevens AM, Spies T, Groh V. Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus. J. Exp. Med. 2009;206:793–805. doi: 10.1084/jem.20081648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of Ligands for the NKG2D Activating Receptor. Annu. Rev. Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr. Top. Microbiol. Immunol. 2006;298:121–138. doi: 10.1007/3-540-27743-9_6. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Upshaw JL, Leibson PJ. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin. Immunol. 2006;18:167–175. doi: 10.1016/j.smim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J. Immunol. 2005;174:4480–4484. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 10.Burgess SJ, Marusina AI, Pathmanathan I, Borrego F, Coligan JE. IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J. Immunol. 2006;176:1490–1497. doi: 10.4049/jimmunol.176.3.1490. [DOI] [PubMed] [Google Scholar]
- 11.Park YP, Choi SC, Kiesler P, Gil-Krzewska A, Borrego F, Weck J, Krzewski K, Coligan JE. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the gammac cytokines and TGF-beta1. Blood. 2011;118:3019–3027. doi: 10.1182/blood-2011-04-346825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, Weller M, Bucala R, Dietl J, Wischhusen J. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J. Immunol. 2008;180:7338–7348. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat. Rev. Immunol. 2007;7:737–744. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 14.Eleme K, Taner SB, Onfelt B, Collinson LM, McCann FE, Chalupny NJ, Cosman D, Hopkins C, Magee AI, Davis DM. Cell surface organization of stress-inducible proteins ULBP and MICA that stimulate human NK cells and T cells via NKG2D. J. Exp. Med. 2004;199:1005–1010. doi: 10.1084/jem.20032194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinle A, Li P, Morris DL, Groh V, Lanier LL, Strong RK, Spies T. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–287. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat. Immunol. 2001;2:443–451. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- 17.Radaev S, Rostro B, Brooks AG, Colonna M, Sun PD. Conformational plasticity revealed by the cocrystal structure of NKG2D and its class I MHC-like ligand ULBP3. Immunity. 2001;15:1039–1049. doi: 10.1016/s1074-7613(01)00241-2. [DOI] [PubMed] [Google Scholar]
- 18.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol. Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat. Immunol. 2008;9:1065–1073. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 20.Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J. Immunol. 2012;189:1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 21.Venkataraman GM, Suciu D, Groh V, Boss JM, Spies T. Promoter region architecture and transcriptional regulation of the genes for the MHC class I-related chain A and B ligands of NKG2D. J. Immunol. 2007;178:961–969. doi: 10.4049/jimmunol.178.2.961. [DOI] [PubMed] [Google Scholar]
- 22.Nausch N, Florin L, Hartenstein B, Angel P, Schorpp-Kistner M, Cerwenka A. Cutting edge: the AP-1 subunit JunB determines NK cell-mediated target cell killing by regulation of the NKG2D-ligand RAE-1epsilon. J. Immunol. 2006;176:7–11. doi: 10.4049/jimmunol.176.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011;71:5998–6009. doi: 10.1158/0008-5472.CAN-10-3211. [DOI] [PubMed] [Google Scholar]
- 24.Boissel N, Rea D, Tieng V, Dulphy N, Brun M, Cayuela JM, Rousselot P, Tamouza R, Le Bouteiller P, Mahon FX, Steinle A, Charron D, Dombret H, Toubert A. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J. Immunol. 2006;176:5108–5116. doi: 10.4049/jimmunol.176.8.5108. [DOI] [PubMed] [Google Scholar]
- 25.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Chen L, Baker K, Olszak T, Zeissig S, Huang YH, Kuo TT, Mandelboim O, Beauchemin N, Lanier LL, Blumberg RS. CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J. Exp. Med. 2011;208:2633–2640. doi: 10.1084/jem.20102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y, Hashimoto Y, Yoneyama T, Mori K, Koie T, Nakamura T, Saitoh H, Yamaya K, Funyu T, Fukuda M, Ohyama C. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011;30:3173–3185. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72:460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 29.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih Ie M, Zhang Y, Wood W, 3rd, Becker KG, Morin PJ. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, Tan TM. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100:1234–1242. doi: 10.1111/j.1349-7006.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 32.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Puré E, Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 33.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 2006;66:563–570. doi: 10.1158/0008-5472.CAN-05-2776. [DOI] [PubMed] [Google Scholar]
- 35.Furue H, Kumimoto H, Matsuo K, Suzuki T, Hasegawa Y, Shinoda M, Sugimura T, Mitsudo K, Tohnai I, Ueda M, Tajima K, Ishizaki K. Opposite impact of NKG2D genotype by lifestyle exposure to risk of aerodigestive tract cancer among Japanese. Int. J. Cancer. 2008;123:181–186. doi: 10.1002/ijc.23456. [DOI] [PubMed] [Google Scholar]
- 36.Furue H, Matsuo K, Kumimoto H, A Hiraki,, T Suzuki,, Y Yatabe,, K Komori,, Y Kanemitsu,, T Hirai,, T Kato,, M Ueda,, K Ishizaki,, K Tajima. Decreased risk of colorectal cancer with the high natural killer cell activity NKG2D genotype in Japanese. Carcinogenesis. 2008;29:316–320. doi: 10.1093/carcin/bgm260. [DOI] [PubMed] [Google Scholar]
- 37.Roszak A, Lianeri M, Jagodzinski PP. Prevalence of the NKG2D Thr72Ala polymorphism in patients with cervical carcinoma. Genet. Test Mol. Biomarkers. 2012;16:841–845. doi: 10.1089/gtmb.2011.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, Trowsdale J, Durrant LG. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin. Cancer Res. 2009;15:6993–7002. doi: 10.1158/1078-0432.CCR-09-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan X, Deng L, Chen X, Lu Y, Zhang Q, Zhang K, Hu Y, Zeng J, Sun W. Clinical significance of the immunostimulatory MHC class I chain-related molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med. Oncol. 2011;28:466–474. doi: 10.1007/s12032-010-9480-9. [DOI] [PubMed] [Google Scholar]
- 40.de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, Jafferji I, Trowsdale J, Liefers GJ, van de Velde CJ, Kuppen PJ. NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer. 2012;12:24–35. doi: 10.1186/1471-2407-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 42.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 43.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 44.Wu JD, Atteridge CL, Wang X, Seya T, Plymate SR. Obstructing shedding of the immunostimulatory MHC class I chain-related gene B prevents tumor formation. Clin. Cancer Res. 2009;15:632–640. doi: 10.1158/1078-0432.CCR-08-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA. 2006;103:9190–9195. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J. Clin. Invest. 2004;114:560–568. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi Y, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer. Cancer Immunol. Immunother. 2009;58:641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, Steinle A, Schadendorf D, Ugurel S. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin. Cancer Res. 2009;15:5208–5215. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 49.Rebmann V, Schutt P, Brandhorst D, Opalka B, Moritz T, Nowrousian MR, Grosse-Wilde H. Soluble MICA as an independent prognostic factor for the overall survival and progression-free survival of multiple myeloma patients. Clin. Immunol. 2007;123:114–120. doi: 10.1016/j.clim.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, Strong RK, Groh V, Spies T. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 51.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, Neuberg D, Anderson KC, Carrasco DR, Dranoff G. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc. Natl. Acad. Sci. USA. 2008;105:1285–1290. doi: 10.1073/pnas.0711293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huergo-Zapico L, Gonzalez-Rodriguez AP, Contesti J, Gonzalez E, Lopez-Soto A, Fernandez-Guizan A, Acebes-Huerta A, de Los Toyos JR, Lopez-Larrea C, Groh V, Spies T, Gonzalez S. Expression of ERp5 and GRP78 on the membrane of chronic lymphocytic leukemia cells: association with soluble MICA shedding. Cancer Immunol. Immunother. 2012;61:1201–1210. doi: 10.1007/s00262-011-1195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguera-Gonzalez S, Gross CC, Fernandez-Messina L, Ashiru O, Esteso G, Hang HC, Reyburn HT, Long EO, Vales-Gomez M. Palmitoylation of MICA, a ligand for NKG2D, mediates its recruitment to membrane microdomains and promotes its shedding. Eur. J. Immunol. 2011;41:3667–3676. doi: 10.1002/eji.201141645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waldhauer I, Goehlsdorf D, Gieseke F, Weinschenk T, Wittenbrink M, Ludwig A, Stevanovic S, Rammensee HG, Steinle A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, Balderas-Pena LM, Bravo-Cuellar A, Ortiz-Lazareno PC, Daneri-Navarro A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186–193. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat. Rev. Immunol. 2004;4:675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 57.Hanaoka N, Jabri B, Dai Z, Ciszewski C, Stevens AM, Yee C, Nakakuma H, Spies T, Groh V. NKG2D initiates caspase-mediated CD3zeta degradation and lymphocyte receptor impairments associated with human cancer and autoimmune disease. J. Immunol. 2010;185:5732–5742. doi: 10.4049/jimmunol.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groh V, Smythe K, Dai Z, Spies T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat. Immunol. 2006;7:755–762. doi: 10.1038/ni1350. [DOI] [PubMed] [Google Scholar]
- 59.Benitez AC, Dai Z, Mann HH, Reeves RS, Margineantu DH, Gooley TA, Groh V, Spies T. Expression, signaling proficiency, and stimulatory function of the NKG2D lymphocyte receptor in human cancer cells. Proc. Natl. Acad. Sci. USA. 2011;108:4081–4086. doi: 10.1073/pnas.1018603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Krieg S, Ullrich E. Novel immune modulators used in hematology: impact on NK cells. Front. Immunol. 2013;3:388–397. doi: 10.3389/fimmu.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Strandmann EP, Hansen HP, Reiners KS, Schnell R, Borchmann P, Merkert S, Simhadri VR, Draube A, Reiser M, Purr I, Hallek M, Engert A. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood. 2006;107:1955–1962. doi: 10.1182/blood-2005-05-2177. [DOI] [PubMed] [Google Scholar]
- 63.Cho HM, Rosenblatt JD, Tolba K, Shin SJ, Shin DS, Calfa C, Zhang Y, Shin SU. Delivery of NKG2D ligand using an anti-HER2 antibody-NKG2D ligand fusion protein results in an enhanced innate and adaptive antitumor response. Cancer Res. 2010;70:10121–10130. doi: 10.1158/0008-5472.CAN-10-1047. [DOI] [PubMed] [Google Scholar]
- 64.Zhang T, Sentman CL. Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res. 2011;71:2066–2076. doi: 10.1158/0008-5472.CAN-10-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang B, Kracker S, Yasuda T, Casola S, Vanneman M, Homig-Holzel C, Wang Z, Derudder E, Li S, Chakraborty T, Cotter SE, Koyama S, Currie T, Freeman GJ, Kutok JL, Rodig SJ, Dranoff G, Rajewsky K. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell. 2012;148:739–751. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, Ensser A, Muller YA, Cerwenka A, Abken H, Parolini O, Ambros PF, Kovar H, Holter W. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song DG, Ye Q, Santoro S, Fang C, Best A, Powell DJ., Jr. Chimeric NKG2D CAR-Expressing T Cell-Mediated Attack of Human Ovarian Cancer Is Enhanced by Histone Deacetylase Inhibition. Hum. Gene Ther. 2013;24:295–305. doi: 10.1089/hum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]