Abstract
Cellular quiescence is a state of reversible cell cycle arrest and has more recently been shown to be a blockade to differentiation and to correlate with resistance to cancer chemotherapeutics and other xenobiotics; features that are common to adult stem cells and possibly tumor stem cells. The biphasic kinetics of mammary regeneration, coupled to its cyclic endocrine control suggest that mammary stem cells most likely divide during a narrow window of the regenerative cycle and return to a state of quiescence. This would enable them to retain their proliferative capacity, resist differentiation signals and preserve their prolonged life span. There is accumulating evidence that mammary stem cells and other adult stem cells utilize quiescence for this purpose, however the degree to which tumor stem cells do so is largely unknown. The retained proliferative capacity of mammary stem cells likely enables them to accumulate and harbor mutations that lead to breast cancer initiation. However it is currently unclear if these causative lesions lead to defective or deranged quiescence in mammary stem cells. Evidence of such effects could potentially lead to the development of diagnostic systems that monitor mammary stem cell quiescence or activation. Such systems may be useful for the evaluation of patients who are at significant risk of breast cancer. Additionally quiescence has been postulated to contribute to therapeutic resistance and tumor recurrence. This review aims to evaluate what is known about the mechanisms governing cellular quiescence and the role of tumor stem cell quiescence in breast cancer recurrence.
Keywords: Stem cell, Mammary stem cell, Quiescence, Hedgehog, Notch, Breast cancer, Therapeutic resistance
Introduction
Throughout female reproductive life, the lactogenic portion of the mammary gland undergoes periodic biphasic regenerative cycles of proliferation and differentiation. The ability to do so relies upon establishment and maintenance of a regenerative cellular hierarchy that is initiated by mitotic division of mammary stem cells [1]. Each division results in elaboration of a bipotent mammary progenitor that directs the expansion and differentiation necessary for physiologic function [2, 3], and a selfrenewing daughter stem cell that preserves regenerative capacity for subsequent cycles. Therefore, preserved regenerative stasis and execution of physiologic function represent opposing demands that are thought to be satisfied through a cell-fate decision to either preserve or forfeit self renewing capacity following mitotic division of a mammary stem cell. This decision is likely to be executed through non-random partitioning of cell fate determinants during asymmetric mitosis [4, 5], resulting in a committed mammary progenitor that exits the mammary stem cell niche and forfeits self-renewing capacity. In this model, the self-renewing daughter cell remains closely associated with the mammary stem cell niche and becomes quiescent. Doing so enables the mammary stem cell to preserve replicative capacity while simultaneously evading telomeric erosion and resisting differentiation [6, 7], suggesting that the ability to become quiescent is tightly linked to self-renewing capacity. Here we discuss the potential role of cellular quiescence in preservation of self-renewal by mammary stem cells and other adult stem cell populations. Genetic pathways and mechanisms governing entry into and maintenance of quiescence will be discussed and specific attention will be paid to the potential contribution of cellular quiescence to the features of breast cancerinitiating cells, and their implications for therapeutic resistance, disease recurrence and metastasis. The goal of this review will be to synthesize from diverse studies a model for these contributions that can be tested in both normal mammary regenerative stasis and in breast cancer initiation, progression and drug resistance.
An important difference between the mammary gland and other epithelial structures such as skin or small intestine is the kinetics underlying cellular stasis and the degree of cellular amplification associated with each regenerative cycle. Tissues such as skin and intestine utilize monophasic regenerative cycles in which the rate of new cell generation approximates the rate of cell death and shedding resulting in an epithelial structure that varies little in size or cellularity over the course of a regenerative cycle [8, 9]. Additionally the linear spatial relationship between stem cells and terminally differentiated cells in these tissues suggests that the transient amplifying capacity of committed progenitors is lost as cells physically progress towards the apical surface of the tissue. Together these observations suggest that there is only a very modest stage of cellular amplification carried out by committed progenitors. This in turn requires that stem cells within these tissues proliferate at a rate similar to the rate at which committed progenitors exit the cell cycle and commit to differentiation. Consistent with this model is the finding that cells within the basal epidermis of skin or in intestinal crypts consistently express markers of cell proliferation such as Ki-67 [10, 11]. These cells also express detectable levels of the catalytic subunit of telomerase, TERT [12, 13], suggesting that active telomeric maintenance may contribute to their prolonged life span. Alternatively TERT expression may stimulate proliferation in these cells as has been demonstrated in the stem cell compartment of the murine hair follicle [14]. In contrast to these monophasic regenerative cycles, the regenerative cycle of the mammary gland is a biphasic sequence of cellular proliferation and differentiation [15] that results in significant expansion and contraction of the epithelia. The degree of expansion, particularly during pregnancy is consistent with a prolonged and vigorous transient amplification stage that is most likely driven by bipotent progenitors. Within the context of the cyclic endocrine control of the mammary regenerative cycle, these kinetics predict that mammary stem cells are likely to divide only within a narrow window of the mammary regenerative cycle before returning to a quiescent state that ensures initiation of future regenerative cycles. Consistent with this prediction is the observation that, in the resting mammary gland, there are low-to-undetectable levels of Ki-67 and hTERT [16]. Additionally experimental evidence indicating that cellular fractions enriched in mammary stem cells are also highly enriched with cells capable of long-term label retention supports the hypothesis that mammary stem cells may utilize quiescence for preservation of self-renewal [17, 18]. These studies support the assertion that distinctions between monophasic and biphasic regenerative cycles reflect differences in mitotic activity of adult stem cells or in the ratio of time spent in quiescence to time spent in the cell cycle. They do not imply that adult stem cells associated with monophasic regenerative cycles proliferate continuously or are incapable of entering a quiescent state. To the contrary, there is accumulating evidence that adult stem cells from diverse tissues utilize cellular quiescence as a way to preserve self-renewing capacity [19–21] and it will be of significant interest to determine if the mechanisms governing quiescence in other adult stem cell systems overlap with those governing quiescence in mammary stem cells.
Several definitive features of mammary stem cells make them plausible sites for breast cancer initiation. Preserved replicative capacity and resistance to differentiation in mammary stem cells or mammary epithelia that acquire these traits via mutation, may enable them to accumulate and harbor mutations over a prolonged life span, implying that mammary stem cells may be the site of breast cancer initiation [15, 18, 22]. Additionally, the developmental potency of mammary stem cells has been postulated to underlie the cellular heterogeneity observed in human breast cancers [22, 23]. While it is inherently obvious that cellular quiescence preserves replicative capacity, there is accumulating evidence that it also confers resistance to a variety of differentiation signals. Consistent with this are studies indicating that quiescence is sufficient to prevent MyoD1-induced muscle differentiation in a human dermal fibroblast culture [6]. This and other similar studies [7, 24, 25] suggest that, beyond preservation of replicative capacity, quiescence may serve as a blockade to differentiation thereby maintaining developmental potency. It further suggests that the responsiveness of bipotent mammary progenitors to mitogens, morphogens and differentiating signals may be due to the fact that they are not quiescent [26].
Prospective cell sorting strategies have lead to the identification of distinct populations of cells from breast tumors [23] and breast cancer cell lines [27–29] that are uniquely tumorigenic and able to self-renew. These features have lead to the designation of these cells as “tumor stem cells.” Presently it is unknown whether tumor stem cells are able to utilize cellular quiescence for preservation of selfrenewing capacity or whether the degree to which the genetic and microenvironmental signals that govern establishment and maintenance of quiescence in mammary stem cells are deranged in breast tumor stem cells. If breast cancer initiation is a condition of unregulated or poorly regulated self-renewal, it is intriguing to consider the possibility that oncogenic or anti-tumor suppressive lesions may disrupt mammary stem cell quiescence. Similarly, given the established link between tumorigenicity and selfrenewing capacity it is tempting to speculate that tumorigenic populations within a breast tumor may be able to exit and reenter the cell cycle and that this ability underlies their resistance to therapeutic strategies that target proliferating cells. Finally it is important to consider the underlying paradox of a model in which defective or disrupted mammary stem cell quiescence contributes to breast cancer initiation but the ability of a subset of tumorigenic cells to become quiescent underlies therapeutic resistance and disease recurrence. One model that might account for this paradox is that oncogenic or anti-tumor suppressive lesions subvert the periodicity of elaboration of committed progenitors by disrupting the maintenance of quiescence but do not completely block cells from entering the quiescent state. In this model tumor stem cells may still utilize quiescence after the elaboration of a tumor-associated progenitor cell thereby preserving their own replicative capacity and maintaining resistance to cytotoxic therapy.
Genetic Pathways and Cellular Mechanisms Governing Establishment and Maintenance of Quiescence
Cellular quiescence is a state of proliferative arrest that is distinguished from differentiation or senescence by the fact that it is reversible. Earliest descriptions came from classical studies indicating a period of mitogen responsiveness in early G1 that is distinct from a period of mitogen insensitivity later in G1 and that these periods were separated by a restriction point [30]. In these studies, fibroblasts that were deprived of mitogens prior to the restriction point took significantly longer to reach S-phase upon mitogen stimulation than those that were deprived of mitogens after the restriction point. The implication of these studies was that mitogen deprivation prior to restriction was sufficient to cause quiescence and that re-entry into the cell cycle was time-consuming and likely to involve multiple steps including the licensing of the origin of replication [31, 32]. These studies also indicated that mitogen stimulation after the restriction point did not shorten the time to S-phase progression indicating that once cells had passed the restriction point they were committed to S-phase progression and completion of the cell cycle independent of further mitogenic stimulation [30]. Extensive research to define the molecular mechanisms underlying the restriction point collectively demonstrated that phosphorylation of the retinoblastoma gene product, Rb and subsequently the other two members of the pocket protein family of tumor suppressors, p107 and p130 represented the restriction point that separated sensitivity and insensitivity to mitogenic stimulation [33]. Genetic analysis of Rb-deficient mouse embryo fibroblasts (MEFs) showed that loss of Rb was sufficient for cell cycle re-entry by quiescent mouse embryo fibroblasts [34]. Interestingly conditional ablation of Rb overcame quiescence significantly more efficiently than germline deletion of Rb. This difference was later shown to be the result of functional redundancy between Rb and p107 and suggested that increased p107 expression in the germline Rb-deficient MEFs accounted for this difference. The connection between this observation and stem cell quiescence was demonstrated conclusively by a recent report indicating that genetic ablation of Rb, p107 and p130 resulted in the loss of quiescence in long-term hematopoietic stem cells [35]. This study also indicated that restoration of a single member of the pocket protein family, p107 was sufficient to restore quiescence. Together along with studies indicating that p130 selectively represses E2F target genes in quiescent cells via an interaction with E2F4 that results in the recruitment of the DREAM complex [36] these findings indicate that all three members of the pocket protein family are likely to play an important role in enforcing a state of quiescence.
While the precise function of Rb, p107 and p130 in mammary stem cells is unknown there is clear evidence that Rb-status in breast cancer is an important determinant of biologic behavior of the tumor. Rb expression is aberrant in 20–30% of breast cancers [37, 38] based upon presence of the protein in the tumor or loss of heterozygosity. Additionally several other genetic events common to breast cancer including amplification of cyclinD1 and loss of p16ink4a are known to disrupt the tumor suppressive function of Rb by promoting aberrant Rb phosphorylation. These events coupled to mutation or deletion of Rb have lead to estimates that Rb function is lost in approximately 50% of breast cancers [39]. This loss is correlated with disparate effects on breast cancer. On one hand Rb deficiency predicts an improved clinical response to cytotoxic therapeutics and this has been attributed to a greater number of cycling cells. Rb deficiency is also associated with resistance to anti-hormonal treatments and is associated with disease recurrence [40]. This latter finding in the context of a role for tumor stem cells in recurrence suggests that Rb deficiency may not be sufficient to bypass quiescence in tumor stem cells but that it promotes rapid amplification of recurrent tumors, which may account for association of Rb deficiency with short time to relapse [41]. While it is clear that Rb, p107 and p130 play important roles in regulating quiescence in other stem cell systems there is an acute need for studies aimed at elucidating the role of these proteins in control of mammary stem cell quiescence and the impact of these proteins on the ability of breast tumor stem cells to become quiescent.
Profiling Studies Identify Distinct Transcriptional Signatures of Quiescence
While the genetic events governing quiescence in mammary stem cells are unknown, recent gene expression profiling studies have identified a number of genes whose expression is correlated with either establishment or maintenance of quiescence in fibroblast systems. One study profiled the transcriptional response to three distinct sets of conditions known to induce quiescence; growth factor withdrawal, loss of adhesion and contact inhibition [6]. The results of this work indicated that distinct quiescence-inducing stimuli resulted in distinct transcriptional responses but that from these disparate profiles a common set of genes was identified as a signature of cellular quiescence. Importantly this study showed that each of these profiles was distinct from a profile of cells that were arrested in G1 via ectopic expression of p21Cip. The authors of this study interpreted this latter observation as evidence that quiescence is a distinct cellular state from that of slow or arrested progression through G1. Their study also provided evidence that the reversibility of the quiescent state is the result of active suppression of differentiation by genes associated with quiescence. This finding is consistent with the idea that quiescence preserves not only the replicative capacity of mammary stem cells but also their developmental potency. Subsequent studies indicated that one such gene is the Notch signaling effector, Hes1 [7]. Increased expression of Hes1 was noted in quiescence cells and was required for the ability of these cells to reenter the cell cycle. Remarkably, Hes1 was sufficient to prevent differentiation or cellular senescence. These findings coupled to the established role of Notch signaling in both breast cancer initiation [42] and in the regulation of cell fate decisions in mammary stem cells [43, 44] and other adult stem cell systems suggest that Hes1 may critically influence the cell fate decision to preserve selfrenewing capacity. Here again the expression of Hes1 and its ability to preserve self-renewal in mammary stem cells and breast tumor stem cells has not been evaluated. However, given the role of Notch signaling in breast cancer initiation several testable hypotheses emerge.
A second profiling study compared the gene expression profiles of serum stimulated and serum deprived fibroblasts and made several important observations [45]. This analysis indicated that serum deprivation activates a set of early response genes referred to as Serum Deprivation Early Response Gene (SDERGs). This study also showed that SDERGs are repressed in many human cancers compared to corresponding normal tissue and that repression of SDERGs is predictive of recurrence and poor prognosis in breast cancer. Among the SDERGs identified were two putative tumor suppressors, MXI1and SALL2 which were shown to be required for serum deprivation-induced quiescence. SiRNA-mediated suppression of these genes resulted in continued transition into S-Phase under serumdeprivation conditions. MXI1 is an inhibitor of c-myc and a putative tumor suppressor [46–48]. Coupled to the role of c-myc in breast cancer and its contribution to the generation of induced pluripotent stem cells these studies suggest that its ability to disrupt c-myc activity may play a role in regulation of quiescence in mammary stem cells. While the conclusion that an overall suppression of quiescence is common in aggressive cancers is not surprising, these studies do not make any predictions about the ability of tumor stem cells, which represent a very small proportion of tumor cells to utilize quiescence as a way to evade therapeutic intervention. Still the identification of genes that directly contribute to the establishment and maintenance of quiescence will prove to be a useful tool for the evaluation of gene expression in quiescent mammary stem cells and tumor stem cells.
Regulation of Quiescence Versus Activation of Mammary Stem Cells by Morphogens
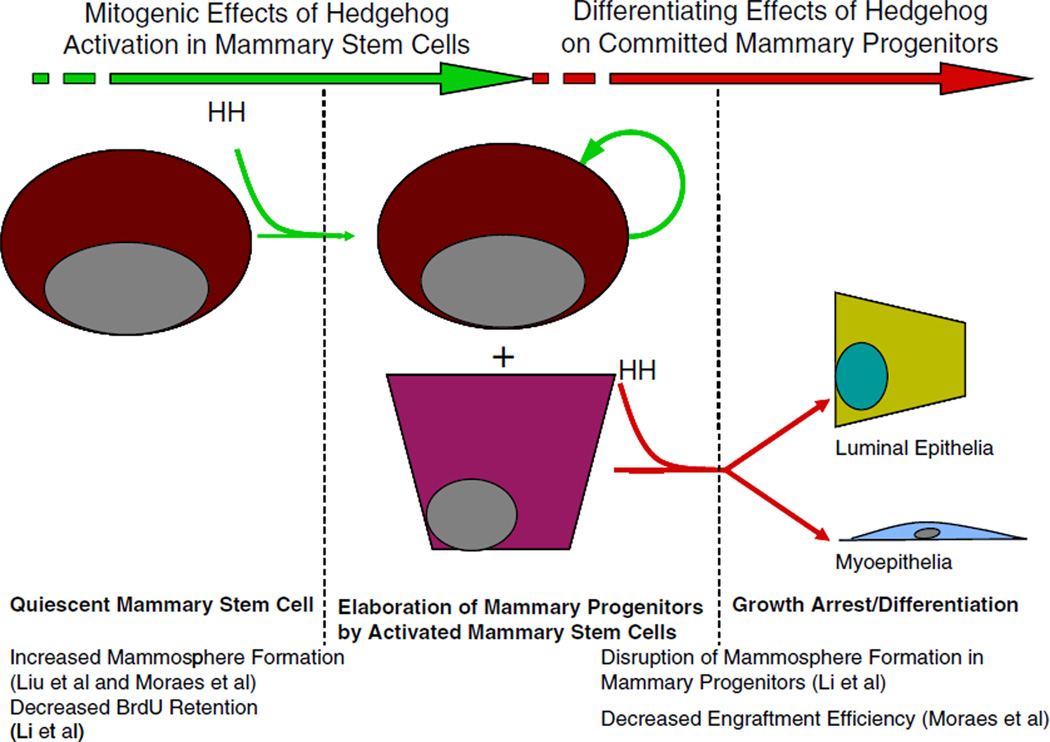
Morphogen signaling pathways such as Hedgehog, Wnt and FGF has been implicated in multiple aspects of stem cell biology and breast cancer. Three recent studies have described the effects of hedgehog activation on mammary stem cells. The first of these reports indicated that activation of hedgehog signaling had a positive effect on mammosphere formation by primary human mammary epithelial cells [49]. It also caused an increase in the number of cells per primary mammosphere. Importantly the effect of hedgehog activation on secondary mammosphere formation was more dramatic suggesting that this mitogenic response to hedgehog selectively targets cells with self-renewing capacity. In a second study hedgehog activation was achieved via ectopic expression of a constitutively active allele of Smoothened (SmoM2) under the control of the MMTV promoter [50]. In this model the investigators observed increased rates of proliferation and altered cellular differentiation that contributed to a dysplastic ductal phenotype in the mammary gland. Analysis of mammary epithelial cells from these mice and wild-type counterparts indicated a significant increase in mammosphere forming capacity relative to wild-type controls. Interestingly, transplantation of cells derived from these mammospheres into cleared fat-pads indicated that constitutive activation of Smoothened lead to a decrease in ductal outgrowths relative to wild type cells. In a third study activation of hedgehog signaling via Ptch1 heterozygosity lead to increased elaboration of mammary progenitors by mammary stem cells, increased rates of BrdU uptake and decreased rates of long-term BrdU retention in a mammary stem cell-enriched Lin−/CD24+/CD29high fraction [26]. This study suggested that constitutive hedgehog signaling in the Ptch1−/+ mouse lead to a defect in mammary stem cell quiescence. In vitro studies in this same report indicated that hedgehog activation promoted acini formation in a three dimensional matrigel culture by cells from the mammary stem cell enriched Lin−/CD24+/CD29high fraction and abolished acini formation by the mammary progenitor enriched fraction, Lin−/CD24+/CD29low. Collectively, these studies indicate that hedgehog activation was mitogenic in mammary stem cells and anti-mitogenic in mammary progenitors, which is consistent with its role as a morphogen (Fig. 1). It is intriguing to speculate that the antiproliferative effects of hedgehog on committed progenitors may account for the observed decrease in ductal outgrowth efficiency by cells derived from the MMTV-SmoM2 mammospheres. Together these three studies indicate that hedgehog activation stimulates proliferation of mammary stem cells and promotes mammosphere formation. The finding that hedgehog activation via Ptch1 heterozygosity lead to increased elaboration of committed mammary progenitors by mammary stem cells [26] coupled to the finding that hedgehog activation, via constitutive Smoothened activity diminished mammary regenerative capacity [50] is consistent with a model in which hedgehog activation results in increased asymmetric mitoses that result in one self-renewing daughter and one committed progenitor. This observation further suggests that a significant proportion of the cells in a mammosphere have forfeited self-renewal and would account for the decrease in mammary regenerating capacity observed in cells from primary mammospheres of the MMTV-SmoM2 mice. Together these studies are consistent with a model in which hedgehog activation promotes elaboration of mammary progenitors by mammary stem cells via asymmetric mitosis by driving mammary stem cells out of their quiescent state. Further studies will be necessary to determine if defective mammary stem cell quiescence has a positive or negative effect on mammary regenerating capacity in Lin−/CD24+/CD29high cells. In hematopoiesis defective quiescence has little effect on early cycle of repopulation following bone marrow ablation but ultimately leads to depletion of long-term hematopoietic stem cells and bone marrow failure [35].
Figure 1.
A two-stage model for the context-dependent effects of hedgehog signaling in the mammary regenerative hierarchy. We propose a model in which hedgehog signaling on mammary stem cells is sufficient to overcome mammary stem cell quiescence. This effect may be mediated by subverting mechanisms governing entry into or maintenance of quiescence. In this case, hedgehog activation is not directly mitogenic but would potentiate a proliferative response to mitogenic signals within the surrounding milieu. Alternatively hedgehog activation may be directly mitogenic in mammary stem cells. Following the elaboration of mammary progenitors hedgehog activation may mediate cell fate specification, cellular differentiation and growth arrest. This model may account for the observation that hedgehog activation stimulates mammosphere formation in vitro and disrupts long term BrdU retention in vivo in enriched fractions of mammary stem cells (Lin−/CD24+/CD29high). This model may also account for the observation that hedgehog activation in mammary progenitors ablates mammosphere formation and the separate observation of decreased engraftment efficiency by MECs from the MMTV-SmoM2 model. This model also raised specific questions regarding the factors within mammary stem cells and mammary progenitors that mediate this differential two stage response.
Notch signaling has been implicated in diverse aspects of stem cell renewal [51–53], breast cancer initiation [54] and breast cancer radioresistance [55]. More recently it has been linked to establishment and preservation of cellular quiescence [7, 45]. Gene expression profiling studies identified Notch3 as a gene that is upregulated in response to serum deprivation in fibroblasts [45]. Additionally Hes1 a transcriptional target of Notch signaling has recently been identified as a factor that is sufficient to maintain cellular quiescence [7]. Functional evaluation of Notch signaling in mammary stem cells also revealed increased mammary stem cell activity in the absence of the Notch signaling effector. CBF-144. In this study Lin−/CD24+/CD29high cells expressing a CBF-1-directed shRNA had a mammary repopulating frequency that was ~2.3 fold greater than identical cells infected with a control shRNA. Interestingly the Lin−/CD24+/CD29high fractions derived from CBF-1-ablated outgrowths were two fold larger than similar fractions from the control shRNA-expressing outgrowths. Consistent with this observation, ablation of CBF-1 from the Lin−/CD24+/CD29high fraction resulted in enhanced acini formation in Matrigel and a predominantly basal epithelial phenotype. Collectively these results indicate separable roles for Notch signaling in regulating the activity of mammary stem cells and for specifying luminal cell fate. While the authors of this study did not directly address the relative quiescence of Lin−/CD24+/CD29high expressing either a control or CBF-1-directed shRNA it is intriguing to consider the possibility that disruption of Notch signaling resulted in a defect in quiescence. Such a finding would be consistent with other studies linking Notch signaling to quiescence and could possibly shed light on the paradoxical link between quiescence and cancer.
Breast Tumor Stem Cells, Quiescence and Resistance to Cancer Therapeutics
Beyond their anti-tumor effects, cytotoxic cancer chemotherapeutics and ionizing radiation have many undesirable side-effects that include bone marrow suppression, hair loss, dyspepsia that is attributed to loss of the epithelial lining of the stomach, and weight loss that is attributed to ineffective nutrient absorption due to loss of the epithelial lining of the intestine. These side effects likely suggest that the therapeutics are having an effect on tissue stasis at these sites, however it is also known that after treatment, hair grows back, the epithelial lining of the gastrointestinal tract recovers and hematopoiesis recovers. These clinical observations support the assertion that adult stem cells, including mammary stem cells are likely to be resistant to these therapeutic challenges. Many adult stem cell compartments express members of the ABCG family of ATP-dependent pumps and this has been postulated as a mechanism by which stem cells efflux xenobiotics, such as cancer chemotherapeutics [56, 57]. Additionally studies have reported that adult stem cells and cancer stem cells are able to activate a remarkably robust DNA damage response [58]. While it is possible that these features are independent of or coincident with cellular quiescence it is also possible that there is a causal relationship between quiescence and these features. Therefore it is of significant interest to know if the ability to efflux xenobiotics and the enhanced response to DNA damage correlate with the state of quiescence versus activation in mammary stem cells and breast tumor stem cells.
While many questions regarding the state of quiescence and activation of tumor stem cells have been raised here and remain largely unanswered studies using breast cancer cell lines and prospective sorting strategies have identified a correlation between quiescence within tumor-initiating populations and resistance to cancer therapeutics. In one study data are presented indicating that a CD44+/CD24−/low/ESA+ fraction that was present as a subset of cells within multiple breast cancer cell lines are uniquely able to retain BrdU over prolonged periods of time [28]. Using a series of in vitro assays, this study indicates that this fraction is able to selfrenew and reconstitute the parental cell line. Importantly treatment of parental cell lines with taxol or 5-fluorouracil (5-FU) dramatically increased the proportion of cells that were CD44+/CD24−/low/ESA+ suggesting that this fraction was preferentially resistant to these drugs. This same study also demonstrated a very high correlation between labelretaining capacity and resistance to taxol or 5-FU. These findings strongly suggest that quiescent cells are resistant to cytotoxic therapeutics. They also clearly indicate that breast cancer cell lines represent highly relevant and genetically malleable systems to dissect the connection drawn between quiescence and drug resistance. Using similar models it will be of significant interest to determine if CD44+/CD24−/low/ ESA+ cells retain their tumorigenicity and their ability to reconstitute the parental cell line following chemotherapeutic treatment as this would have a significant impact on studies aimed at elucidating the underlying causes of breast cancer recurrence.
Other sorting strategies studies using a similar cell fraction (CD44+/CD24−/low) indicate that these cells are uniquely resistant to ionizing radiation [55]. In this study CD44+/CD24−/low were propagated as mammospheres and the resulting cultures were compared to monolayer cultures of the parental cell lines following exposure to ionizing radiation. Results indicated that CD44+/CD24−/low retained their clonogenicity to significantly greater extent than other fractions. In these studies levels of reactive oxygen species were lower in CD44+/CD24−/low than in other fractions and phosphorylation of H2AX was observed in the parental monolayers but not in the CD44+/CD24−/low-enriched mammospheres. These two observations suggest that the CD44+/CD24−/low cells were either resistant to the DNA-damaging effects of ionizing radiation or were remarkably efficient at repairing damaged DNA. This latter possibility may be consistent with observations that tumor stem cells from glioma activate the DNA damage response more readily than other glioma cells and that glioma stem cells had higher basal activation of the DNA damage checkpoint than non-self renewing populations [58]. Currently it is unclear whether mammary stem cells or breast tumor stem cells have similar properties, however these studies coupled to the fact that CD44+/CD24−/low are very slow cycling cells that selectively retain BrdU suggest that quiescent cells may be less susceptible to ionizing radiation. Another study has implicated Wnt signaling in radiation resistance of mouse mammary progenitor cells. In this study mice in which Wnt β-catenin signaling is constitutively activated had considerably higher levels of mammary progenitor cells than wild-type counterparts following clinically relevant doses of radiation. In these studies the ratio of dye-effluxing side-population to non-effluxing cells increased with radiation resistance. This observation coupled to findings that the dye-effluxing side-population is enriched for longterm BrdU-retaining cells suggests that Wnt signaling was having a protective effect that correlates with quiescence. Further studies will be necessary to confirm if Wnt signaling mediates quiescence or if the expansion of the mammary regenerating population in MMTV-Wnt1a mice resulted in an expansion of quiescent mammary stem cells. Also it is interesting to note a recent study in which paracrine Wnt signaling was required to preserve the quiescence of long-term hematopoietic stem cells.
Recent clinical evidence also indicates that breast cancer stem cells are resistant to cytotoxic chemotherapy [59]. A recent study evaluated the proportion of CD44+/CD24−/low in breast tumors that received neoadjuvant chemotherapy or the EGFR/Her2 inhibitor, lapatinib in the case of Her2-positve tumors. Results indicated that following neoadjuvant chemotherapy the proportion of CD44+/CD24−/low was significantly higher than in the untreated tumor, suggesting that the cancer stem cell component of these tumors was resistant to cytotoxic therapy. Interestingly no such increase was observed in lapatinib treated cells suggesting that CD44+/CD24−/low may be sensitive to inhibition of EGFR and Her2. Again these findings coupled to the slow cycling and BrdU-retaining properties of CD44+/CD24−/low cells suggest that quiescence may provide a level of protection from the cytotoxic effects of cancer chemotherapeutics. Furthermore they strongly suggest that targeted therapeutics like lapatinib may be able to target tumor stem cells in a manner that is independent of their quiescence status.
Summary
Breast cancer recurrence continues to be a major cause of mortality and morbidity in women. While significant progress has been made towards the identification of cells that resist therapy and initiate breast cancer recurrence, the mechanisms underlying therapeutic resistance are not fully understood. Therapeutic resistance likely involves multiple strategies including xenobiotic efflux by ABCG transporters, an enhanced DNA damage response and utilization of cellular quiescence as well as others. Utilization of these strategies by non-transformed mammary stem cells and other stem cell populations suggests that therapeutic strategies aimed at killing tumor stem cells will likely have to be tailored to the underlying mutations that gave rise to the cancer. This in turn will require a more thorough mechanistic understanding of these various protective strategies. Specifically in the case of quiescence it will be critical to determine if breast cancer stem cells retain the ability to become quiescent and if so, how this is achieved in the context of activated mitogenic pathways. Currently one attractive hypothesis is that interactions between mammary stem cells or breast tumor stem cells with a specialized stem cell niche enforces cellular quiescence, however our limited knowledge of the cellular and molecular components of the niche precludes any systematic evaluation of this hypothesis. Additionally it will be critical to determine the effects of oncogenic or anti-tumor suppressive lesions on entry into and maintenance of quiescence in mammary stem cells. Addressing this will require a more detailed understanding of the molecular events governing mammary stem cell quiescence.
A major gap in our understanding of the regulation of quiescence versus activation in mammary stem cells, and quite possibly breast cancer initiating cells, is the absence of knowledge regarding the mammary stem cell niche. To date the mammary stem cell niche is a largely inferred or hypothesized microenvironment composed of supporting cells and extracellular components. A generally accepted model holds that mammary stem cells reside within this three dimensional space and that the stem cell niche plays a critical role in the long-term preservation of self-renewing capacity. Based upon this model and the previously discussed role for quiescence in preservation of proliferative capacity and blockage of differentiation, it is reasonable to postulate that interactions between a mammary stem cell and its niche play a role in the establishment or maintenance of quiescence. It is also reasonable to postulate that the elaboration of a bipotent mammary progenitor by a mammary stem cell involves the physical dislocation of the progenitor from the niche and that this dislocation renders the progenitor responsive to both mitogens and differentiating signals. As the very existence of the mammary stem cell niche remains unproven it would be useful to examine the effects of genetic perturbations in mammary stem cells in situ versus those same perturbations on isolated mammary stem cells. Specifically it will be interesting to compare the response of mammary stem cells to mitogens or differentiating agents in vivo and ex vivo. Evidence that mammary stem cells are more responsive to these agents ex vivo may imply the existence of a specific mammary stem cell niche.
The ability of the bipotent progenitor to drive expansion and differentiation suggests that these cells also possess mammary regenerative activity. This point has been proven experimentally, suggesting that the precise developmental point at which developmental commitment occurs remains unknown. Still it is clear that only enriched fractions of mammary stem cells, defined as Lin−/CD24+/CD29high possess the ability to serially reconstitute mammary grand development suggesting a link between quiescence and self-renewing capacity. So while the precise moment at which developmental commitment is complete and irreversible remains undefined it is conceivable that forfeiture of long-term self-renewing capacity occurs with the loss of the ability to become quiescent. Addressing this point will require a greater understanding of the cellular and genetic mechanisms governing stem cell quiescence and it will be of interest to determine the effects of subversion of these mechanisms on breast cancer initiation, progression, therapeutic resistance and recurrence.
Abbreviations
- DREAM
DP, RB-like, E2F, and MuvB
- SDERG
Serum deprivation early response gene
- FGF
Fibroblast growth factor
- ABCG
ATP-binding cassette proteins, sub-group G
- BrdU
Bromodeoxyuridine
- EGFR
Epidermal growth factor receptor
Contributor Information
David C. Harmes, Department of Pharmacology and Toxicology, Dartmouth Medical School, 7650 Remsen, Hanover, NH 03755, USA
James DiRenzo, Email: James.DiRenzo@Dartmouth.edu, Department of Pharmacology and Toxicology, Dartmouth Medical School, 7650 Remsen, Hanover, NH 03755, USA.
References
- 1.Stingl J, Raouf A, Eirew P, Eaves CJ. Deciphering the mammary epithelial cell hierarchy. Cell Cycle. 2006;5(14):1519–1522. doi: 10.4161/cc.5.14.2983. [DOI] [PubMed] [Google Scholar]
- 2.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 3.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 4.Giebel B. Cell polarity and asymmetric cell division within human hematopoietic stem and progenitor cells. Cells Tissues Organs. 2008;188(1–2):116–126. doi: 10.1159/000112842. [DOI] [PubMed] [Google Scholar]
- 5.Lin H. Cell biology of stem cells: an enigma of asymmetry and self-renewal. J Cell Biol. 2008;180(2):257–260. doi: 10.1083/jcb.200712159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biol. 2006;4(3):e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321(5892):1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22(14):1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 10.Moll I, Zieger W, Schmelz M. Proliferative Merkel cells were not detected in human skin. Arch Dermatol Res. 1996;288(4):184–187. doi: 10.1007/BF02505222. [DOI] [PubMed] [Google Scholar]
- 11.Davenport A, Hale RJ, Hunt CR, Bigley G, McMahon RF. Expression of Ki-67 and cytokeratin 20 in hyperplastic polyps of the colorectum. J Clin Pathol. 2003;56(3):200–204. doi: 10.1136/jcp.56.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309(5738):1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 13.Yasumoto S, Kunimura C, Kikuchi K, et al. Telomerase activity in normal human epithelial cells. Oncogene. 1996;13(2):433–439. [PubMed] [Google Scholar]
- 14.Sarin KY, Cheung P, Gilison D, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436(7053):1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smalley M, Ashworth A. Stem cells and breast cancer: a field in transit. Nat Rev Cancer. 2003;3(11):832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 16.Kolquist KA, Ellisen LW, Counter CM, et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998;19(2):182–186. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- 17.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245(1):42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 18.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118(Pt 16):3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 19.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132(2):299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163(3):609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26(8):426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 23.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passegue E, Wagers AJ. Regulating quiescence: new insights into hematopoietic stem cell biology. Dev Cell. 2006;10(4):415–417. doi: 10.1016/j.devcel.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Walkley CR, Fero ML, Chien WM, Purton LE, McArthur GA. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2005;7(2):172–178. doi: 10.1038/ncb1214. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Singh S, Cherukuri P, et al. Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells. Stem Cells. 2008;26(5):1253–1264. doi: 10.1634/stemcells.2007-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985;82(16):5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coller HA. What’s taking so long? S-phase entry from quiescence versus proliferation. Nat Rev Mol Cell Biol. 2007;8(8):667–670. doi: 10.1038/nrm2223. [DOI] [PubMed] [Google Scholar]
- 32.Kingsbury SR, Loddo M, Fanshawe T, et al. Repression of DNA replication licensing in quiescence is independent of geminin and may define the cell cycle state of progenitor cells. Exp Cell Res. 2005;309(1):56–67. doi: 10.1016/j.yexcr.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Dyson N, Buchkovich K, Whyte P, Harlow E. Cellular proteins that are targetted by DNA tumor viruses for transformation. Princess Takamatsu Symp. 1989;20:191–198. [PubMed] [Google Scholar]
- 34.Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424(6945):223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- 35.Viatour P, Somervaille TC, Venkatasubrahmanyam S, et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell. 2008;3(4):416–428. doi: 10.1016/j.stem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litovchick L, Sadasivam S, Florens L, et al. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26(4):539–551. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen NH, Emdin SO, Cajander J, Landberg G. Deregulation of cyclin E and D1 in breast cancer is associated with inactivation of the retinoblastoma protein. Oncogene. 1997;14(3):295–304. doi: 10.1038/sj.onc.1200833. [DOI] [PubMed] [Google Scholar]
- 38.Pietilainen T, Lipponen P, Aaltomaa S, Eskelinen M, Kosma VM, Syrjanen K. Expression of retinoblastoma gene protein (Rb) in breast cancer as related to established prognostic factors and survival. Eur J Cancer. 1995;31A(3):329–333. doi: 10.1016/0959-8049(94)00463-f. [DOI] [PubMed] [Google Scholar]
- 39.Bosco EE, Knudsen ES. RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle. 2007;6(6):667–671. doi: 10.4161/cc.6.6.3988. [DOI] [PubMed] [Google Scholar]
- 40.Bosco EE, Wang Y, Xu H, et al. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J Clin Invest. 2007;117(1):218–228. doi: 10.1172/JCI28803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceccarelli C, Santini D, Chieco P, et al. Retinoblastoma (RB1) gene product expression in breast carcinoma. Correlation with Ki-67 growth fraction and biopathological profile. J Clin Pathol. 1998;51(11):818–824. doi: 10.1136/jcp.51.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang S, Ji C, Qu X, et al. A study on Notch signaling in human breast cancer. Neoplasma. 2007;54(4):304–310. [PubMed] [Google Scholar]
- 43.Buono KD, Robinson GW, Martin C, et al. The canonical Notch/ RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293(2):565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 44.Bouras T, Pal B, Vaillant F, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3(4):429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Adler AS, Segal E, Chang HY. A transcriptional program mediating entry into cellular quiescence. PLoS Genet. 2007;3(6):e91. doi: 10.1371/journal.pgen.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee TC, Ziff EB. Mxi1 is a repressor of the c-Myc promoter and reverses activation by USF. J Biol Chem. 1999;274(2):595–606. doi: 10.1074/jbc.274.2.595. [DOI] [PubMed] [Google Scholar]
- 47.Taj MM, Tawil RJ, Engstrom LD, et al. Mxi1, a Myc antagonist, suppresses proliferation of DU145 human prostate cells. Prostate. 2001;47(3):194–204. doi: 10.1002/pros.1063. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler DS, Shelly CA, Petroff CA, Dang CV. MXI1, a putative tumor suppressor gene, suppresses growth of human glioblastoma cells. Cancer Res. 1997;57(21):4905–4912. [PubMed] [Google Scholar]
- 49.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moraes RC, Zhang X, Harrington N, et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134(6):1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs E. Beauty is skin deep: the fascinating biology of the epidermis and its appendages. Harvey Lect. 1998;94:47–77. [PubMed] [Google Scholar]
- 52.Raouf A, Zhao Y, To K, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3(1):109–118. doi: 10.1016/j.stem.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Glazer RI, Wang X, Yuan H, Yin Y. Mammary stem and progenitor cell regulation. Cancer Biomark. 2007;3(4–5):171–181. doi: 10.3233/cbm-2007-34-502. [DOI] [PubMed] [Google Scholar]
- 54.Hu C, Dievart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol. 2006;168(3):973–990. doi: 10.2353/ajpath.2006.050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/ CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 56.Meissner K, Heydrich B, Jedlitschky G, et al. The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J Histochem Cytochem. 2006;54(2):215–221. doi: 10.1369/jhc.5A6750.2005. [DOI] [PubMed] [Google Scholar]
- 57.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 58.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26(17):2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]