Abstract
The efficacy, tolerability, and safety of the prophylactic treatment of hepatitis C virus (HCV) after liver transplantation (LT) with peginterferon alfa-2a and ribavirin are not known. LT recipients with HCV were randomized to peginterferon alfa-2a/ribavirin treatment or observation 10 to 26 weeks post-LT. Prophylaxis patients received peginterferon alfa-2a (135 μg/week for 4 weeks and then 180 μg/week for 44 weeks) plus ribavirin (the initial dose of 400 mg/day was escalated to 1200 mg/day). Observation patients received the same regimen only upon significant HCV recurrence (histological activity index ≥ 3 and/or fibrosis score ≥ 2). The primary endpoint was the proportion of patients with histological evidence of significant HCV recurrence 120 weeks after randomization. In all, 115 patients were randomized (prophylaxis arm, n = 55; observation arm, n = 60). Sustained virological response was achieved by 12 of 54 prophylaxis patients (22.2%) and by 3 of 14 observation patients who switched to treatment (21.4%). On an intent-to-treat basis, significant HCV recurrence at 120 weeks was similar in the prophylaxis (61.8%) and observation arms (65.0%, P = 0.725). The patient and graft survival rates and the rates of biopsy-proven acute cellular rejection were similar in the 2 study arms. Approximately 70% of the treated patients in both arms had at least one dose reduction for safety reasons. The most common adverse event leading to treatment withdrawal was anemia. Because of the safety profile of peginterferon alfa-2a/ribavirin and the lack of a clear benefit in terms of HCV recurrence and patient or graft survival, this study does not support the routine use of prophylactic antiviral therapy.
End-stage liver disease due to hepatitis C virus (HCV) is the most common indication for liver transplantation (LT) in the United States and Europe.1,2 HCV recurrence post-LT is essentially universal.3 Although the natural history of HCV infection varies substantially among recipients, allograft failure secondary to HCV recurrence accounts for two-thirds of graft failures and deaths.2 LT recipients with HCV have reduced 5-year graft and patient survival rates in comparison with HCV-negative recipients.2 Thus, with a growing demand for LT and an increasing shortage of organs, there is a need to improve the strategies for managing HCV recurrence post-LT.
Although peginterferon/ribavirin antiviral therapy is common post-LT, no large, well-controlled clinical trials have determined the optimal approach to treating HCV recurrence. Reported approaches include the initiation of antiviral therapy before LT (pretransplant treatment),4 very early after LT (preemptive treatment),5 before the occurrence of significant allograft injury (prophylactic treatment), or for established HCV recurrence post-LT.6-18 Pretransplant therapy in patients with advanced liver disease is limited by reduced patient tolerability and efficacy.4,19 Potential advantages of post-LT therapy include virus eradication and the limitation of the histological effects of HCV infection on allograft survival. Preemptive treatment within 3 weeks post-LT and treatment for HCV recurrence 6 to 60 months post-LT have been investigated with peginterferon alfa-2a monotherapy.5 The sustained virological response (SVR) rates ranged from 8% to 12%, and 20% to 30% of the patients withdrew prematurely. In immunocompetent patients, the addition of ribavirin to interferon therapy significantly improves SVR, but it has been associated with increased toxicity primarily due to hemolytic anemia.20 The risk of ribavirin-induced anemia is compounded in post-LT patients by compromised renal function. Furthermore, immunocompetent patients with minimal fibrosis respond better to antiviral therapy than patients with advanced fibrosis.21 Therefore, the initiation of combination therapy with peginterferon and ribavirin after the resolution of the initial post-LT hematological and biochemical instability but before the onset of significant allograft fibrosis may improve the virological response and delay HCV recurrence.
PHOENIX is a large, randomized study designed to compare the efficacy, tolerability, and safety of an escalating-dose regimen of peginterferon alfa-2a plus ribavirin for 48 weeks in 2 situations: prophylactic initiation (before significant histological recurrence) within 26 weeks after LT and initiation upon HCV recurrence.
PATIENTS AND METHODS
Patients
Male and female patients who were 18 years old or older were eligible if they had undergone LT because of HCV infection. Patients were required (1) to have HCV RNA levels detectable by polymerase chain reaction before LT, (2) to have normal thyroid-stimulating hormone levels, and (3) to undergo LT 10 to 26 weeks before randomization. Patients with a pre-LT diagnosis of hepatocellular carcinoma were enrolled if they met the Milan criteria.22 Tacrolimus and corticosteroids were used for primary immunosuppression. If they were needed, cyclosporine, sirolimus, and mycophenolate mofetil were used according to the institution’s practice and the patient’s circumstances. Growth factor use was allowed at the investigator’s discretion. Mild acute cellular rejection (ACR) was treated with either an upward adjustment of maintenance immunosuppression (including the addition of mycophenolate mofetil) or escalating doses of corticosteroids. Exclusion criteria included coinfection with human immunodeficiency virus or hepatitis B virus, multiorgan transplantation or retransplantation, a cold ischemia time > 20 hours, evidence of ongoing or unresolved rejection, steroid-resistant rejection or the use of OKT3, the use of T cell–depleting therapies, a neutrophil count < 1500 cells/mm3, a white cell count > 20,000 × 109/L, a hemoglobin level < 10 g/dL, and a platelet count < 50,000 cells/mm3. Patients who received a split/living related liver, a liver from a non–beating-heart donor, or a liver from an HCV-positive donor were also excluded.
Study Design
This was a prospective, multicenter, open-label, randomized study. Potential patients were screened at the study center before transplantation, and patients meeting the pretransplant eligibility criteria were enrolled. At 10 to 26 weeks post-LT, enrolled patients without significant histological recurrence were randomized in a 1:1 ratio with a central, interactive, voice-activated response system to either the prophylaxis arm or the observation arm. Prophylaxis patients were treated for 48 weeks with peginterferon alfa-2a (Pegasys, Roche, Nutley, NJ) plus ribavirin (Copegus, Roche). Patients initially received peginterferon alfa-2a at a dose of 135 μg/week for 4 weeks. The dose was then increased to 180 μg/week for 44 weeks (as tolerated). The initial dose of ribavirin was 400 mg/day, and this was increased by 200 mg every 4 weeks to a target dose of 1200 mg/day for patients weighing 75 kg or more (1000 mg/day for patients weighing <75 kg). Observation patients were observed without treatment for up to 48 weeks. However, observation patients meeting the predefined endpoint of significant HCV recurrence were treated with the same antiviral regimen used for prophylaxis patients.
The study duration was 120 weeks, and all treated patients had 24 to 72 weeks of treatment-free follow-up. Assessments were performed in weeks 1, 2, 4, 8, 12, 24, 36, and 48 during the 48-week treatment/observation period and in weeks 72 and 120 in the treatment-free follow-up period.
The study was conducted in conformance with the principles of the Declaration of Helsinki and with local laws and regulations. Institutional review boards of the participating sites approved the protocol, and all participants provided written, informed consent. Roche sponsored the trial and also collected and analyzed the data.
Study Endpoints
The primary endpoint was the proportion of patients with significant histological HCV recurrence 120 weeks after randomization. Significant HCV recurrence was determined with the Batts-Ludwig system23 and was defined as a histological activity index (HAI) inflammation grade ≥ 3 and/or a fibrosis stage score ≥ 2. This degree of histological recurrence is clinically relevant and should prompt treatment initiation.24,25
Secondary endpoints included the following: the proportion of patients who experienced significant histological HCV recurrence during the 120 weeks after randomization; the HAI grades and fibrosis scores 48 and 120 weeks after randomization; the biochemical response rates (alanine aminotransferase); the virological response rates as measured by undetectable HCV RNA levels at weeks 4, 12, 24 and 48 during treatment, and after 24 weeks of treatment-free follow-up (SVR); the proportion of patients with biopsy-proven ACR, graft loss, or death (as a combined endpoint); the time from randomization to the first occurrence of biopsy-proven ACR, graft loss, or death; and the time from randomization to the first histological evidence of HCV recurrence.
Histological Analysis
Biopsy was performed at screening and 120 weeks (108-120 weeks) after randomization in all patients, and at 48 weeks (36-48 weeks) after randomization for prophylaxis patients and observation patients receiving no treatment. Biopsy was also performed upon clinical suspicion of HCV recurrence in observation patients. Biopsy samples were analyzed by a local pathologist and a single central pathologist (Dr. Lawrence Burgart) at the Mayo Central Laboratory (Rochester, MN). The central pathologist was blinded to the patient’s identity, the time point, and the treatment group. Biopsy specimens were assessed for adequacy with respect to their length and width and the number of portal tracts. Biopsy samples with no liver tissue were not read. Study analyses were based on the interpretation of the central pathologist with the Batts-Ludwig system.23 All biopsy readings at the central laboratory were performed in batches at the study’s completion.
HCV RNA and Genotype Determination
Serum HCV RNA levels were measured with the Roche HCV Quanta Sure Plus test (lower limit of detection = 10 IU/mL), and the HCV genotypes were determined with a commercially available assay (Roche Diagnostics, Branchburg, NJ) with some modifications. Both were measured at a central laboratory.
Safety Assessments
There were 5 primary safety parameters: (1) biopsy-proven rejection (moderate or severe biopsy-proven acute rejection as assessed by each center’s local pathologist), (2) depression [Beck Depression Inventory, 2nd edition (BDI-II); total score ≥ 29],26 (3) anemia (hemoglobin level < 8.5 g/dL), (4) grade 3 neutropenia (absolute neutrophil count = 0.5-0.75 × 109 cells/L) or grade 4 neutropenia (absolute neutrophil count < 0.5 × 109 cells/L), and (5) any clinically significant infections requiring treatment. Secondary safety variables included adverse events (AEs), serious adverse events (SAEs), discontinuations due to AEs and SAEs, dose adjustments related to AEs and SAEs, laboratory parameters, and vital signs.
Statistical Analysis
The study was designed to enroll 300 patients; a 17% reduction in the proportion of patients with HCV recurrence 120 weeks after randomization was assumed (α = 0.05, 80% power). Except for changes from the baseline parameters, the primary efficacy endpoints and all secondary efficacy endpoints were analyzed with the intent-to-treat (ITT) population (all randomized patients). A predefined per protocol (PP) population was also used to assess efficacy endpoints. Patients were not included in the PP population if they met any of the following criteria: (1) a baseline biopsy sample with an HAI grade > 3 or a fibrosis score ≥ 2, (2) no liver tissue in the biopsy specimen sent to the central pathologist, and (3) major protocol violations (a pre-LT diagnosis of hepatocellular carcinoma outside the Milan criteria, ongoing acute allograft rejection, or a history of immunologically mediated or autoimmune disease). The safety population included all randomized patients who received at least 1 dose of the study medication in the prophylaxis arm and had at least 1 postbaseline safety assessment and all observation patients who had at least 1 postbaseline safety assessment.
For primary and secondary efficacy analyses, the difference between the study arms was assessed with the Cochran-Mantel-Haenszel general association test. Missing data were treated as treatment failure (ie, a patient was presumed to have experienced HCV recurrence if biopsy data were missing or was presumed to be a nonresponder if HCV RNA data were missing). The Kaplan-Meier product limit method was used to estimate time-to-event variables, and the log-rank test was used to compare time-to-event distributions between study arms.
RESULTS
Study Patients and Analysis Populations
This study was conducted between October 2004 and October 2008 at 24 US study centers; enrollment was ended early because of slow enrollment. Although 236 patients were screened before LT, 121 patients were excluded (48 patients did not meet the eligibility criteria, 45 patients did not receive a transplant before the end of enrollment, 11 patients died before transplantation, and 17 patients did not participate for other reasons). In all, 115 patients were included in the study: 55 prophylaxis patients and 60 observation patients (the ITT population; Fig. 1). One prophylaxis patient withdrew consent before any study medication was received. Fourteen observation patients were switched to treatment upon HCV recurrence. The PP population included 47 prophylaxis patients, 54 observation patients, and 13 observation patients who were switched to treatment. In all, 31 prophylaxis patients, 36 untreated observation patients, and 7 observation patients who switched to treatment completed the 48 week treatment/observation period. Unless stated otherwise, the results for the ITT and PP populations were similar. In general, the baseline demographic and disease characteristics were similarly distributed in the study arms (Table 1).
Figure 1.
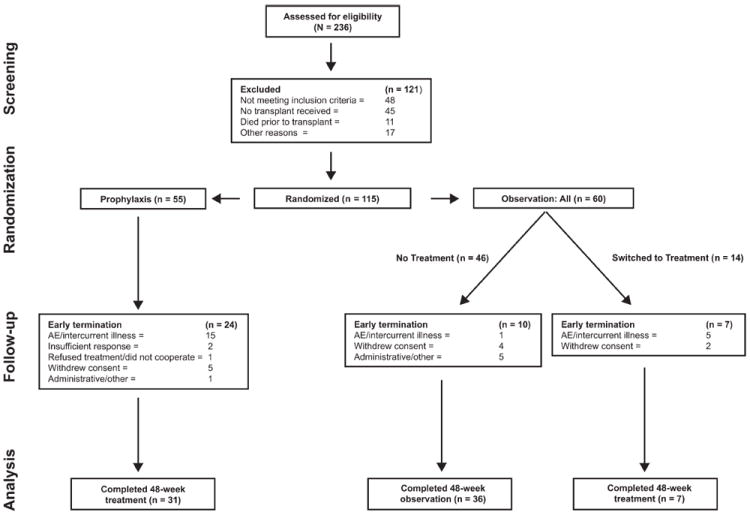
Overview of the study and its subjects. Patients were randomized 10 to 26 weeks post-LT to receive peginterferon alfa-2a/ribavirin therapy either prophylactically or upon observation of HCV recurrence. The study duration was 120 weeks: prophylaxis patients received 48 weeks of treatment and 72 weeks of follow-up, observation patients who received no treatment received 48 weeks of observation and 72 weeks of follow-up, and observation patients who switched to treatment received 0 to 48 weeks of observation followed by 48 weeks of treatment and 24 to 72 weeks of follow-up.
TABLE 1.
Patient Demographics and Clinical Characteristics at the Baseline (the ITT Population)
| Prophylaxis Arm (n = 55) | Observation Arm: All Patients (n = 60) | |
|---|---|---|
| Male sex, n (%) | 45 (81.8) | 48 (80.0) |
| Race, n (%) | ||
| White | 46 (83.6) | 50 (83.3) |
| Black | 3 (5.5) | 5 (8.3) |
| Asian | 1 (1.8) | 2 (3.3) |
| Other | 5 (9.1) | 3 (5.0) |
| Recipient age (years), median (range)* | 51.0 (35-68) | 53.5 (38-66) |
| Weight (kg), median (range)† | 81.8 (44-115) | 79.1 (53-120) |
| Weight ≥ 75 kg, n (%) | 33 (63.5) | 37 (62.7) |
| Days from transplantation to the baseline, median (range)‡ | 111.5 (71-235) | 121.0 (14-248) |
| HCV genotype, n (%) | ||
| Type 1 | 43 (78.2) | 48 (81.4) |
| Not type 1 | 12 (21.8) | 11 (18.6) |
| Unknown | 0 | 1 |
| Pretransplant HCV RNA (log10 IU/mL), median (range)§ | 4.8 (0.4-6.4) | 5.3 (0.4-6.8) |
| Baseline HCV RNA (log10 IU/mL), median (range) | 6.8 (4.6-8.0) | 6.9 (4.0-8.0)∣ |
| Baseline HCV RNA > 800,000 IU/mL, n (%) | 43 (78.2) | 48 (81.4)∣ |
| Alanine aminotransferase (U/L), median (range) | 66.0 (18-754) | 76.0 (17-401) |
| Aspartate aminotransferase (U/L), median (range) | 46.0 (15-736) | 54.0 (15-447) |
| Cold ischemia time (hours), median (range) | 7.0 (0-17) | 6.9 (0-18) |
| Identical donor/recipient histocompatibility (ABO typing), n (%) | 52 (94.5) | 57 (95.0) |
The donor age was not collected.
Prophylaxis arm, n = 52; observation arm, n = 59.
The baseline is defined as the first day of treatment for the prophylaxis arm and as the day of randomization for the observation arm (prophylaxis arm, n = 54).
Prophylaxis arm, n = 24; observation arm, n = 21.
n = 59 (for 1 patient, baseline HCV RNA levels were missing, but the patient had positive HCV RNA titers in weeks 12 and 48).
Primary Endpoint: Significant Histological HCV Recurrence
In the ITT analysis, 34 prophylaxis patients (61.8%) and 39 observation patients (65.0%) were classified as having significant HCV recurrence at 120 weeks (P = 0.725; Table 2). The majority of these patients were classified as having HCV recurrence because week 120 biopsy results were missing (25 of 34 prophylaxis patients with HCV recurrence and 32 of 39 observation patients with HCV recurrence). In the PP population, an analysis of treatment completers showed that fewer prophylaxis patients experienced HCV recurrence (16.0% versus 40.5%, P = 0.041) when any available postbaseline biopsy data were used rather than only data falling within the week 120 window (Table 2). Table 2 also shows histological recurrence in genotype 1 and non–genotype 1 patients. The times to first HCV recurrence after randomization were similar in the 2 study arms (P = 0.178; Fig. 2).
TABLE 2.
HCV Recurrence 120 Weeks After Randomization (the ITT and PP Populations)
| ITT Population at Week 120
|
PP Treatment Completers Within 120 Weeks*
|
|||
|---|---|---|---|---|
| Prophylaxis Arm (n = 55) | Observation Arm (n = 60) | Prophylaxis Arm (n = 25) | Observation Arm (n = 37) | |
| Patients, n (%)† | 34 (61.8) | 39 (65.0) | 4 (16.0) | 15 (40.5) |
| 95% confidence interval (%) | 49.0-74.7 | 52.9-77.1 | 1.6-30.4 | 24.7-56.4 |
| Difference (prophylaxis – observation) (%) | −3.2 | −24.5 | ||
| 95% confidence interval for difference (%) | −20.8 to 14.4 | −45.9 to −3.2 | ||
| P value‡ | 0.725 | 0.041 | ||
| Genotype, n/X (%)§ | ||||
| Type 1 | 25/43 (58.1) | 31/48 (64.6) | 3/21 (14.3) | 13/30 (43.3) |
| Not type 1 | 9/12 (75.0) | 7/11 (63.6) | 1/4 (25.0) | 2/6 (33.3) |
NOTE: HCV recurrence was determined with the Batts-Ludwig system and was defined as an HAI inflammation grade ≥ 3 and/or a fibrosis score ≥ 2.
At any point after the baseline.
n refers to the number of patients with histologically confirmed HCV recurrence. The percentages are based on the number of patients in each study arm.
The P values were calculated with the Cochran-Mantel-Haenszel general association test, which was used to compare the prophylaxis and observation arms. The 95% confidence intervals were calculated with normal approximation.
n refers to the number of patients with histologically confirmed HCV recurrence, and X refers to the number of randomized patients. The percentages are based on the number of patients in each genotype group. Genotype data were missing for 1 patient in the observation arm.
Figure 2.
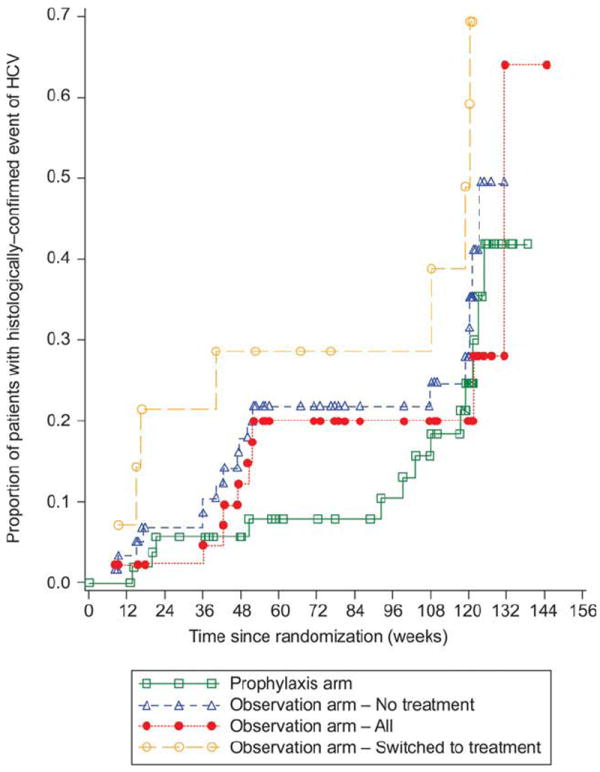
Kaplan-Meier estimates of the time to first HCV recurrence after randomization (the ITT population). P = 0.178 (log-rank test comparing survival curves between the prophylaxis and observation arms).
Severity of Liver Disease
The changes from the baseline in HAI inflammation grades and fibrosis scores are shown in Table 3. The changes in HAI grades at weeks 48 and 120 were similar in the 2 arms. The change in fibrosis score at week 48 was lower for the prophylaxis patients (P < 0.038); at week 120, the changes in fibrosis scores were similar in the 2 arms. Approximately half of the patients in each study arm had missing HAI and fibrosis data at week 120 (this included deaths and retransplants).
TABLE 3.
Changes from the Baseline in the Batts-Ludwig HAI Inflammation Grades and Fibrosis Scores Over Time (the ITT Population)
| Comparison | Prophylaxis Arm | Observation Arm | P Value* |
|---|---|---|---|
| Week 48 | |||
| n | 40 | 45 | |
| HAI grade, mean ± SD | −0.05 ± 1.26 | 0.27 ± 1.07 | 0.215 |
| Fibrosis score, mean ± SD | 0.28 ± 0.99 | 0.71 ± 0.92 | 0.038 |
| Week 120† | |||
| n | 29 | 28 | |
| HAI grade, mean ± SD | −0.03 ± 1.12 | 0.25 ± 1.17 | 0.353 |
| Fibrosis score, mean ± SD | 0.79 ± 1.37 | 0.68 ± 0.90 | 0.712 |
Based on the t test.
Approximately half of the patients in both study arms had missing HAI and fibrosis data at week 120; the causes included death and retransplantation.
The median alanine aminotransferase levels before treatment initiation were 66.0 U/L (18-754 U/L) in prophylaxis patients and 97.0 U/L (30-484 U/L) in observation patients who switched to treatment. Twenty-one prophylaxis patients (38.9%) and 6 observation patients who switched to treatment (42.9%) had a sustained biochemical response, which was defined as normal serum alanine aminotransferase levels 24 weeks after the end of treatment.
Virological Response
The virological response rates were similar for the prophylaxis patients and the observation patients who switched to treatment (Fig. 3). Fifty percent of the patients in both arms (prophylaxis, n = 27; observation, n = 7) achieved an early virological response (EVR; undetectable HCV RNA levels or a ≥2 log decline at week 12). Overall, SVR was achieved by 12 prophylaxis patients (22.2%) and 3 treated patients in the observation arm (21.4%). The SVR rates were 18.6% and 33.3% for HCV genotype 1 and non–type 1 prophylaxis patients, respectively, and 21.4% and 0% for HCV genotype 1 and non–type 1 observation patients who switched to treatment, respectively.
Figure 3.
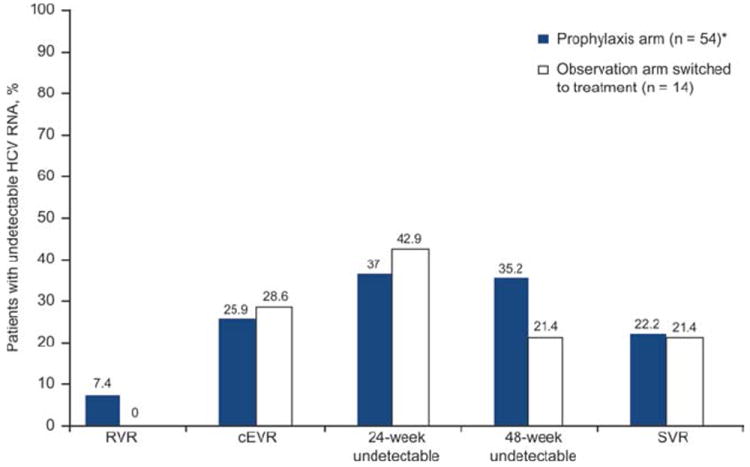
Virological responses in prophylaxis patients and in observation patients who switched to treatment (the ITT population). RVR was defined as undetectable HCV RNA after 4 weeks of treatment, cEVR was defined as undetectable HCV RNA after 12 weeks of treatment, and SVR was defined as undetectable HCV RNA 24 weeks after the end of treatment. *Before receiving any study medication, 1 prophylaxis patient withdrew consent.
An analysis of HCV recurrence by SVR in prophylaxis patients was limited by the high proportion of patients with missing biopsy data (24/54) and missing HCV RNA data (19/54). There was no significant difference in HCV recurrence between SVR patients (16.7% experienced HCV recurrence, and 66.7% did not) and non-SVR patients (17.4% experienced HCV recurrence, and 52.2% did not).
ACR
Biopsy-proven ACR occurred between treatment weeks 24 and 36 in 3 prophylaxis patients (5.6%) and between treatment weeks 3 and 10 in 3 observation patients (6.5%) who received no treatment. No prophylaxis patients had more than 1 episode, and 1 observation patient had 2 episodes. In addition, 5 prophylaxis patients and 9 observation patients had clinically presumptive ACR. There were 3 cases of chronic rejection in the prophylaxis arm (between weeks 26 and 36) and 1 case in the no-treatment observation arm (during week 2). Two of the 4 cases were confirmed by the investigator, and 3 of the 4 cases were noted to have ACR on the same or previous biopsy samples.
Safety and Tolerability: Drug Doses and AEs
The number of administered treatments and doses and the average doses of the study medications were similar in the 2 study arms (Table 4). The majority of patients completed 36 weeks or more of peginterferon alfa-2a and ribavirin therapy. The average peginterferon alfa-2a doses were 170 and 171 μg/week in the 2 treatment arms while the average ribavirin doses were 671 and 631 mg/day, respectively. Peginterferon alfa-2a doses were reduced for safety reasons in 70.4% and 64.3% of the patients in the 2 treatment arms and ribavirin doses were reduced for safety in 72.2% and 71.4% of the patients, respectively.
TABLE 4.
Extent of Exposure (the Safety Population)
| Peginterferon Alfa-2a | Prophylaxis Arm (n = 54) | Observation Arm: Switched to Treatment (n = 14) |
|---|---|---|
| Treatment duration, n (%)* | ||
| 0-14 days | 1 (1.9) | 0 |
| 15-28 days | 1 (1.9) | 0 |
| 29-56 days | 1 (1.9) | 0 |
| 57-84 days | 1 (1.9) | 0 |
| 85-168 days | 5 (9.3) | 3 (21.4) |
| 169-252 days | 7 (13.0) | 2 (14.3) |
| 253-336 days | 30 (55.6) | 4 (28.6) |
| >336 days | 8 (14.8) | 5 (35.7) |
| Average weekly dose (μg), mean ± SD | 170.0 ± 9.14 | 170.8 ± 5.80 |
| Patients with dose reduction for safety reasons, n (%)† | ||
| Total | 38 (70.4) | 9 (64.3) |
| Laboratory | 25 (46.3) | 7 (50.0) |
| AE | 24 (44.4) | 6 (42.9) |
| Ribavirin | Prophylaxis Arm (n = 54) | Observation Arm: Switched to Treatment (n = 14) |
|
| ||
| Treatment duration, n (%)* | ||
| 15-28 days | 3 (5.6) | 0 |
| 29-56 days | 1 (1.9) | 0 |
| 57-84 days | 1 (1.9) | 1 (7.1) |
| 85-168 days | 8 (14.8) | 3 (21.4) |
| 169-252 days | 7 (13.0) | 2 (14.3) |
| 253-336 days | 25 (46.3) | 3 (21.4) |
| >336 days | 9 (16.7) | 5 (35.7) |
| Average daily dose (mg), mean ± SD | 671.4 ± 238.44 | 630.6 ± 222.23 |
| Patients with dose reduction for safety reasons, n (%)† | ||
| Total | 39 (72.2) | 10 (71.4) |
| Laboratory | 19 (35.2) | 8 (57.1) |
| AE | 29 (53.7) | 5 (35.7) |
Days from the first treatment to the last treatment (ie, the date of the last treatment minus the date of the first treatment plus 1).
More than 1 reason could be reported because of multiple dose reductions per patient; when a patient had more than 1 dose reduction for the same reason, the patient was counted once for that reason.
At least 1 of the 5 primary safety events was experienced by 33 prophylaxis patients (61.1%), 16 observation patients with no treatment (34.8%), and 12 observation patients who switched to treatment (85.7%; Table 5). Treatment discontinuation due to AEs occurred in 15 prophylaxis patients (27.8%) and in 5 observation patients who switched to treatment (35.7%). The most common AE leading to treatment withdrawal was anemia (13.0% of prophylaxis patients and 21.4% of observation patients who switched to treatment).
TABLE 5.
Number of Patients with Rejection, Depression, Anemia, Neutropenia, and Clinically Significant Infection by Week 48 (the Safety Population)
| Patients Experiencing Safety Parameters Within 48 Weeks | Prophylaxis Arm (n = 54) | Observation Arm: No Treatment (n = 46) | Observation Arm: Switched to Treatment (n = 14) |
|---|---|---|---|
| Patients with at least 1 endpoint, n (%)* | 33 (61.1) | 16 (34.8) | 12 (85.7) |
| Patients with biopsy-proven rejection, n (%) | 3 (5.6) | 3 (6.5) | 0 |
| Patients with depression, n (%) | 1 (1.9) | 1 (2.2) | 1 (7.1) |
| Patients with anemia, n (%) | 13 (24.1) | 1 (2.2) | 5 (35.7) |
| Patients with grade 3 or 4 neutropenia, n (%) | 14 (25.9) | 3 (6.5) | 8 (57.1) |
| Patients with clinically significant infections requiring treatment, n (%) | 19 (35.2) | 9 (19.6) | 6 (42.9) |
NOTE: For prophylaxis patients and patients switched to the treatment, the weeks are based on the initiation of treatment. For patients who received no treatment, the weeks are based on the day of randomization.
At least 1 endpoint of biopsy-proven rejection, depression, anemia, neutropenia, or clinically significant infection.
Overall, AEs were reported in 100% of the prophylaxis patients and 97% of all observation patients. (Table 6). Treatment-related AEs were reported in 52 prophylaxis patients (96.3%) and in 13 observation patients who switched to treatment (92.9%). The most common treatment-related AEs were anemia, fatigue, headache, and neutropenia. Among treated patients, the AE frequencies were similar, except for a higher rate of anemia in prophylaxis patients. The mean and median changes in the total BDI-II scores from the baseline to postbaseline assessments were similar in the 2 study arms.
TABLE 6.
AEs (the Safety Population)
| Prophylaxis Arm (n = 54) | Observation Arm
|
|||
|---|---|---|---|---|
| All Patients (n = 60) | Patients Receiving No Treatment (n = 46) | Patients Switched to Treatment (n = 14) | ||
| AE, n (%) | 54 (100) | 58 (96.7) | 44 (95.7) | 14 (100) |
| Treatment-related event, n (%) | 52 (96.3) | 13 (21.7) | Not applicable | 13 (92.9) |
| SAE, n (%) | 25 (46.3) | 21 (35.0) | 14 (30.4) | 7 (50.0) |
| Death, n (%) | 5 | 3 | 3 | 0 |
| Most common events, n (%)* | ||||
| Anemia | 38 (70.4) | 8 (13.3) | 2 (4.3) | 6 (42.9) |
| Fatigue | 35 (64.8) | 24 (40.0) | 13 (28.3) | 11 (78.6) |
| Headache | 29 (53.7) | 15 (25.0) | 7 (15.2) | 8 (57.1) |
| Neutropenia | 24 (44.4) | 7 (11.7) | 2 (4.3) | 5 (35.7) |
| Diarrhea | 23 (42.6) | 18 (30.0) | 11 (23.9) | 7 (50.0) |
| Nausea | 23 (42.6) | 13 (21.7) | 6 (13.0) | 7 (50.0) |
| Insomnia | 18 (33.3) | 6 (10.0) | 2 (4.3) | 4 (28.6) |
| Abdominal pain | 16 (29.6) | 11 (18.3) | 7 (15.2) | 4 (28.6) |
| Depression | 14 (25.9) | 11 (18.3) | 4 (8.7) | 7 (50.0) |
| Pain | 14 (25.9) | 3 (5.0) | 2 (4.3) | 1 (7.1) |
| Vomiting | 13 (24.1) | 6 (10.0) | 3 (6.5) | 3 (21.4) |
| Dizziness | 13 (24.1) | 6 (10.0) | 2 (4.3) | 4 (28.6) |
| Pyrexia | 12 (22.2) | 5 (8.3) | 3 (6.5) | 2 (14.3) |
| Arthralgia | 10 (18.5) | 9 (15.0) | 6 (13.0) | 3 (21.4) |
| Pruritus | 10 (18.5) | 8 (13.3) | 6 (13.0) | 2 (14.3) |
| Dyspnea | 10 (18.5) | 5 (8.3) | 3 (6.5) | 2 (14.3) |
| Back pain | 9 (16.7) | 10 (16.7) | 9 (19.6) | 1 (7.1) |
| Muscle spasms | 9 (16.7) | 4 (6.7) | 2 (4.3) | 2 (14.3) |
| Thrombocytopenia | 9 (16.7) | 1 (1.7) | 0 | 1 (7.1) |
| Hypertension | 8 (14.8) | 7 (11.7) | 6 (13.0) | 1 (7.1) |
| Decreased appetite | 8 (14.8) | 4 (6.7) | 1 (2.2) | 3 (21.4) |
| Alopecia | 8 (14.8) | 3 (5.0) | 3 (6.5) | 0 |
| Myalgia | 7 (13.0) | 4 (6.7) | 0 | 4 (28.6) |
| Chills | 7 (13.0) | 3 (5.0) | 1 (2.2) | 2 (14.3) |
| Rash | 6 (11.1) | 6 (10.0) | 5 (10.9) | 1 (7.1) |
| Upper abdominal pain | 6 (11.1) | 3 (5.0) | 2 (4.3) | 1 (7.1) |
| Irritability | 6 (11.1) | 2 (3.3) | 0 | 2 (14.3) |
| Influenza-like illness | 6 (11.1) | 0 | 0 | 0 |
| Peripheral edema | 4 (7.4) | 12 (20.0) | 10 (21.7) | 2 (14.3) |
| Cough | 4 (7.4) | 7 (11.7) | 4 (8.7) | 3 (21.4) |
| Sinusitis | 1 (1.9) | 6 (10.0) | 3 (6.5) | 3 (21.4) |
AEs reported in 10% or more of patients in either treatment arm.
Five deaths were reported for the prophylaxis arm, and all but 1 death occurred more than 30 days after the discontinuation of the study treatment. One patient died of a cerebrovascular hemorrhagic stroke during treatment, and this was attributed by the investigator to preexisting hypertension. The deaths of the patients who received study treatment were considered unrelated to the study medication. All 3 patients in the observation arm who died had received no study treatment.
Hemoglobin concentrations decreased to <10.0 g/dL in 40 prophylaxis patients (74%) and in 6 observation patients who switched to treatment (43%). Neutrophil counts decreased to <0.5 × 109/L in 5 prophylaxis patients (9%) and in 2 observation patients who switched to treatment (14%). Platelet counts decreased to <50 × 109/L in 14 prophylaxis patients (26%) and in 2 observation patients who switched to treatment (14%).
In all, 38 prophylaxis patients (69%), 2 observation patients with no treatment (4%), and 9 observation patients who switched to treatment (64%) received epoetin/darbepoetin alfa. Filgrastim/pegfilgrastim was used in 28 prophylaxis patients (51%), 1 observation patient with no treatment (2%), and 5 observation patients who switched to treatment (36%). In all, 11 prophylaxis patients (20%), 3 observation patients with no treatment (7%), and 1 observation patient who switched to treatment (7%) received transfusions with packed red blood cells.
DISCUSSION
PHOENIX compared 2 approaches to managing post-LT HCV infection: (1) treatment initiation within 6 months post-LT before significant histological injury occurs and (2) observation until histological HCV recurrence is demonstrated. Therapeutic goals included the achievement of SVR, the reduction of the severity of allograft hepatitis, and improvements in patient and graft survival while treatment-related AEs were minimized. Even though the target enrollment was not met, this study is the largest randomized controlled trial investigating the efficacy of peginterferon/ribavirin therapy post-LT. It highlights the many difficulties encountered when HCV is being treated post-LT. Previously, only 41% of patients undergoing transplantation were eligible for preemptive therapy (initiation very early post-LT).27 This was attributed to postoperative complications such as cytopenias, renal insufficiency, infections, and debilitation. Delaying treatment in our study up to 6 months post-LT did not significantly increase the number of patients who were eligible for treatment. Despite growth factor use and a dose escalation protocol, anemia and neutropenia remained significant barriers to treatment; few patients were able to tolerate the low ribavirin doses used in this treatment protocol. Thus, the first important finding of this study is that peginterferon/ribavirin antiviral therapy can benefit only a minority of LT recipients in the early postoperative period.
Overall, our results are broadly comparable with the results of a pooled analysis of 21 studies, which reported that two-thirds of patients required dose reductions and one-quarter discontinued treatment early.28 In our study, only 65% of the patients were able to complete therapy and approximately 25% and 50% of the patients had missing biopsy results at weeks 48 and 120, respectively. Although our experience was disappointing, these results are similar to those reported for a smaller randomized controlled trial in which prophylaxis treatment was initiated 6 to 60 months post-LT.5
The histological changes observed in this study deserve detailed consideration. In the ITT analysis, we observed similar rates of histological HCV recurrence at 120 weeks (primary endpoint) in the prophylaxis arm (61.8%) and the observation arm (65.0%, P = 0.725). These rates are broadly similar to previous findings.15,27 In our ITT analysis, patients with missing biopsy data (eg, due to a withdrawal of consent, loss to follow-up, death, retransplantation, or samples inadequate for interpretation) were presumed to have met the criteria for histological HCV recurrence. For more than two-thirds of the patients with HCV recurrence at 120 weeks (25 prophylaxis patients and 32 observation patients), the diagnosis was based on missing biopsy data rather than observed recurrence. Thus, the HCV recurrence rates in the ITT analysis were substantially greater than the observed recurrence rates in both study arms. A possible histological benefit of prophylaxis is suggested by one of the secondary endpoints. Compared with observation patients, prophylaxis patients had significantly lower mean HAI grades and less fibrosis progression at the end of the treatment period. However, the difference was lost by week 120 of follow-up. Furthermore, among prophylaxis and observation patients who completed their assigned treatment, the rates of histological HCV recurrence were 16.0% and 40.5%, respectively (P = 0.041). The difference in the observed proportions of patients with histological recurrence of HCV was greater than the 17% difference initially targeted as clinically important for the study. This suggests that there is a benefit to prophylactic therapy (as difficult as it is to achieve), and the lower than intended enrollment and the missing data may have led to a type II error. Overall, these results suggest that prophylactic treatment with the currently available antiviral agents is unlikely to meaningfully reduce the impact of HCV recurrence on post-LT outcomes. An earlier randomized controlled trial of peginterferon alfa initiated in the first postoperative month as prophylaxis for HCV was similarly handicapped by a low frequency of eligible recipients and also by low efficacy (SVR rate = 7%).5 Because of this, our study was initiated between postoperative weeks 10 and 26. Despite this, we also found many patients who were not eligible for, did not tolerate, or did not respond to peginterferon/ribavirin therapy in the early postoperative period. A more tolerable and effective treatment for HCV infection is needed. Until such treatments are available, a case can be made for administering peginterferon/ribavirin therapy only to recipients with histological recurrence.
Our study also measured several secondary endpoints. On an ITT basis, SVR rates were low in both study arms: 22.2% and 21.4% in the prophylactic arm and the treatment upon recurrence arm, respectively. Although a higher SVR rate of 33% was reported in a randomized controlled trial of peginterferon alfa-2a/ribavirin treatment for established HCV recurrence,6 another study reported a lower rate of only 8%.5 A review of retrospective peginterferon-based studies for HCV recurrence29 found an overall SVR rate of approximately 33%. The low SVR rate observed in our study may reflect the assumption that all patients missing HCV RNA data were nonresponders (19 of 54 prophylaxis patients and 5 of 14 treated observation patients). Because of the high probability that some of the patients with missing data achieved SVR, it is almost certain that our ITT SVR rates underestimate the actual SVR rate. In the nontransplant setting, RVR and EVR are highly predictive of SVR.30,31 Initial virological responses may predict SVR in the posttransplant setting.6 In the current study, 50% of patients achieved EVR in both arms. The lower EVR rates in comparison with the rates in previous studies could also have contributed to the relatively low SVR rates in this study.
An important concern with the use of interferon in LT recipients is the risk of acute and chronic rejection. Alloimmune hepatitis may also occur with post-LT antiviral therapy, typically after HCV RNA clearance.12,32 Although we observed late ACR, no difference was observed in the rates of ACR between prophylaxis patients and untreated observation patients; similar rates for chronic rejection were also observed.
In conclusion, the results from this study do not support the routine use of prophylactic therapy in the management of recurrent HCV infection. One of the confirmatory findings of this comparatively large randomized study is that the treatment of HCV recurrence early in the post-LT setting is difficult. Novel antiviral therapies currently in development, such as the directly acting antivirals telaprevir and boceprevir, are expected to be major therapeutic options for HCV patients. However, because these molecules increase the frequency of AEs in combination with peginterferon and ribavirin, the treatment of post-LT HCV infections in the early postoperative period will remain difficult at least in the medium term.
Supplementary Material
Acknowledgments
Third-party writing assistance for this article was furnished by Paul MacCallum, Ph.D. (Envision Scientific Solutions), and was supported by Genentech, Inc.
The PHOENIX Study Group investigators were as follows: Gary Davis, Rolland Dickson, Fredric Gordon, Steven Han, Laura Kulik, John Lake, Suthat Liangpunsakul, Mauricio Lisker-Melman, Michael Lucey, Timothy McCashland, Guy Neff, Michael Porayko, K. Rajender Reddy, David J. Reich (formerly Cosme Manzarbeitia), Thomas Schiano, Lewis Teperman, Paul Thuluvath, Hugo Vargas, and William Washburn.
This trial was funded by Roche (Nutley, NJ).
Abbreviations
- ACR
acute cellular rejection
- AE
adverse event
- BDI-II
Beck Depression Inventory, 2nd edition
- cEVR
complete early virological response
- EVR
early virological response
- HAI
histological activity index
- HCV
hepatitis C virus
- ITT
intent-to-treat
- LT
liver transplantation
- PP
per protocol
- RVR
rapid virological response
- SAE
serious adverse event
- SD
standard deviation
- SVR
sustained virological response
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Adam R, McMaster P, O’Grady JG, Castaing D, Klempnauer JL, Jamieson N, et al. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Wei Y, Eng H, Charlton MR, Persing DH, Wiesner RH, et al. Recurrent and new hepatitis C virus infection after liver transplantation. Hepatology. 1999;29:1220–1226. doi: 10.1002/hep.510290412. [DOI] [PubMed] [Google Scholar]
- 4.Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–262. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005;41:289–298. doi: 10.1002/hep.20560. [DOI] [PubMed] [Google Scholar]
- 6.Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, et al. A randomized study on peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46:1009–1017. doi: 10.1016/j.jhep.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Palau A, Fernandez A, Benlloch S, Aguilera V, Prieto M, et al. Efficacy, predictors of response, and potential risks associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2006;12:1067–1076. doi: 10.1002/lt.20737. [DOI] [PubMed] [Google Scholar]
- 8.Dumortier J, Scoazec JY, Chevallier P, Boillot O. Treatment of recurrent hepatitis C after liver transplantation: a pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol. 2004;40:669–674. doi: 10.1016/j.jhep.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Fernández I, Meneu JC, Colina F, García I, Muñoz R, Castellano G, et al. Clinical and histological efficacy of pegylated interferon and ribavirin therapy of recurrent hepatitis C after liver transplantation. Liver Transpl. 2006;12:1805–1812. doi: 10.1002/lt.20883. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee S, Gilroy RK, McCashland TM, Schafer DF. Pegylated interferon for recurrent hepatitis C in liver transplant recipients with renal failure: a prospective cohort study. Transplant Proc. 2003;35:1478–1479. doi: 10.1016/s0041-1345(03)00446-9. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Lyden E. Impact of pegylated interferon α-2B and ribavirin on hepatic fibrosis in liver transplant patients with recurrent hepatitis C: an open-label series. Liver Int. 2006;26:529–535. doi: 10.1111/j.1478-3231.2006.01261.x. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan Menon KV, Poterucha JJ, El-Amin OM, Burgart LJ, Kremers WK, Rosen CB, et al. Treatment of posttransplantation recurrence of hepatitis C with interferon and ribavirin: lessons on tolerability and efficacy. Liver Transpl. 2002;8:623–629. doi: 10.1053/jlts.2002.33968. [DOI] [PubMed] [Google Scholar]
- 13.Neff GW, Montalbano M, O’Brien CB, Nishida S, Safdar K, Bejarano PA, et al. Treatment of established recurrent hepatitis C in liver-transplant recipients with pegylated interferon-alfa-2b and ribavirin therapy. Transplantation. 2004;78:1303–1307. doi: 10.1097/01.tp.0000129811.93072.1c. [DOI] [PubMed] [Google Scholar]
- 14.Oton E, Barcena R, Moreno-Planas JM, Cuervas-Mons V, Moreno-Zamora A, Barrios C, et al. Hepatitis C recurrence after liver transplantation: viral and histologic response to full-dose PEG-interferon and ribavirin. Am J Transplant. 2006;6:2348–2355. doi: 10.1111/j.1600-6143.2006.01470.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Luna H, Khatib A, Sharma P, De Petris G, Williams JW, Ortiz J, et al. Treatment of recurrent hepatitis C infection after liver transplantation with combination of pegylated interferon α2b and ribavirin: an open-label series. Transplantation. 2004;77:190–194. doi: 10.1097/01.TP.0000100481.14514.BB. [DOI] [PubMed] [Google Scholar]
- 16.Ross AS, Bhan AK, Pascual M, Thiim M, Benedict Cosimi A, Chung RT. Pegylated interferon α-2b plus ribavirin in the treatment of post-liver transplant recurrent hepatitis C. Clin Transplant. 2004;18:166–173. doi: 10.1046/j.1399-0012.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Terrault NA. Prophylactic and preemptive therapies for hepatitis C virus-infected patients undergoing liver transplantation. Liver Transpl. 2003;9:S95–S100. doi: 10.1053/jlts.2003.50255. [DOI] [PubMed] [Google Scholar]
- 18.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426–2433. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 19.Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350–355. doi: 10.1053/jlts.2002.31748. [DOI] [PubMed] [Google Scholar]
- 20.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 21.Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388–397. doi: 10.1002/hep.23340. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 23.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Khettry U, Huang WY, Simpson MA, Pomfret EA, Pomposelli JJ, Lewis WD, et al. Patterns of recurrent hepatitis C after liver transplantation in a recent cohort of patients. Hum Pathol. 2007;38:443–452. doi: 10.1016/j.humpath.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 27.Shergill AK, Khalili M, Straley S, Bollinger K, Roberts JP, Ascher NA, Terrault NA. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005;5:118–124. doi: 10.1111/j.1600-6143.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6:1586–1599. doi: 10.1111/j.1600-6143.2006.01362.x. [DOI] [PubMed] [Google Scholar]
- 29.Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009;9:1707–1713. doi: 10.1111/j.1600-6143.2009.02702.x. [DOI] [PubMed] [Google Scholar]
- 30.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 31.Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Gonçales FL, Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Stravitz RT, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Heuman DM, et al. Effects of interferon treatment on liver histology and allograft rejection in patients with recurrent hepatitis C following liver transplantation. Liver Transpl. 2004;10:850–858. doi: 10.1002/lt.20189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


