Abstract
Acute morphine antinociception has been shown to be blocked by very low picogram doses of okadaic acid indicating that inhibition of protein phosphatase PP2A allows for increases in phosphorylation to inhibit antinociception. Comparative studies in morphine tolerant animals have not been reported. In the present study, we showed a significant increase in the total phosphatase activity in the periaqueductal gray matter (PAG) from morphine-pelleted versus placebo-pelleted mice, 72-h after pellet implantation. This supports our hypothesis that phosphatase activity is increased in tolerance as a compensatory mechanism for the increase in kinase activity during the development of tolerance. We also demonstrated that i.c.v. administration of the phosphatase inhibitor okadaic acid (3 pmol/mouse; a dose tested to be inert in placebo-pelleted mice) enhanced the level of morphine antinociceptive tolerance assessed by the tail immersion test, 72-h following pellet implantation. This was supported by the fact that the same treatment with okadaic acid blocked the increase in phosphatase activity in PAG of morphine tolerant mice indicating that selective inhibition of PP2A contributes to enhanced levels of morphine tolerance. We have previously reported that PKC or PKA inhibitors reversed morphine antinociceptive tolerance in mice. The current study shows that i.c.v administration of the PKC inhibitors bisindolylmaleimide I or Go6976 reversed the enhanced level of morphine tolerance induced by okadaic acid treatment to the same level of tolerance observed in nonokadaic acid-treated tolerant mice. However, the PKA inhibitor PKI-(14–22)-amide only partially reversed the enhancement of morphine tolerance induced by okadaic acid. Our data suggests an important role for the balance between kinases and phosphatases in modulating tolerance levels. Further studies will be directed towards a better understanding of the role of different phosphatase isoforms in morphine tolerance.
Keywords: morphine; tolerance; antinociception; okadaic acid; protein phosphatase; protein kinase C, protein kinase A
Introduction
Opioids such as morphine are the most widely used drugs for the alleviation of moderate to severe chronic pain. Systemically administered morphine produces antinociception via actions at both spinal and supra-spinal sites (Barton et al., 1980). Morphine activates descending systems within the brainstem that inhibit dorsal horn nociceptive neurons (Basbaum and Fields, 1984) as well as directly inhibiting spinal cord neurons to prevent transmission to supraspinal centers (Yaksh and Noueihed, 1985).
However, repeated use of opioids induces tolerance that results in the loss of drug effect or requires escalating doses to produce pain relief. The neurobiological mechanisms of the development of opioid tolerance are multifaceted and only partially understood. Important mechanisms involved in opioid tolerance are cellular and molecular adaptation processes like receptor uncoupling from G-protein (desensitization), opioid agonist-induced receptor internalization or opioid receptor down-regulation leading to a decrease in the number of functional binding sites (Hsu and Wong 2000; Williams et al., 2001; He et al., 2002; Freye and Latasch 2003).
The second messenger pathways activating protein kinase C (PKC) and protein kinase A (PKA) have been shown to play an integral role in the development and expression of tolerance to the inhibitory effects of opioids (Narita et al., 1996; Bernstein and Welch 1997; Smith et al., 1999). In our laboratory, we have shown that intracerebroventricular (i.c.v.) administration of PKC or PKA inhibitors not only reversed antinociceptive tolerance (Javed et al., 2004; Smith et al., 2007) but also reinstated the morphine-induced behaviors of antinociception, hypothermia and Straub tail, 72-h after implantation of a 75 mg morphine pellet, to the same extent observed following acute administration of morphine (Smith et al., 2006). These data suggest that elevations in the activity of PKC and PKA in the brain are critical to the expression of morphine tolerance.
Because blockade of protein kinases has been shown to reverse tolerance, it is likely that the opioid receptors can be dephosphorylated by phosphatases and thus returned to their non-tolerant state. We therefore hypothesized that blockade of protein phosphatases would potentiate tolerance by prolonging the phosphorylated state of proteins and/or opioid receptors. Therefore, experiments were designed in the present study to test the effects of the protein phosphatase inhibitor okadaic acid on the level of morphine antinociceptive tolerance, as well as the effects of of PKC and PKA inhibitors to reverse enhanced morphine tolerance induced by phosphatase inhibition. Okadaic acid was administered at a low picomol dose that did not affect morphine antinociception in placebo-pelleted mice.
2. Results
2.1. Phosphatase activity changes in morphine tolerance
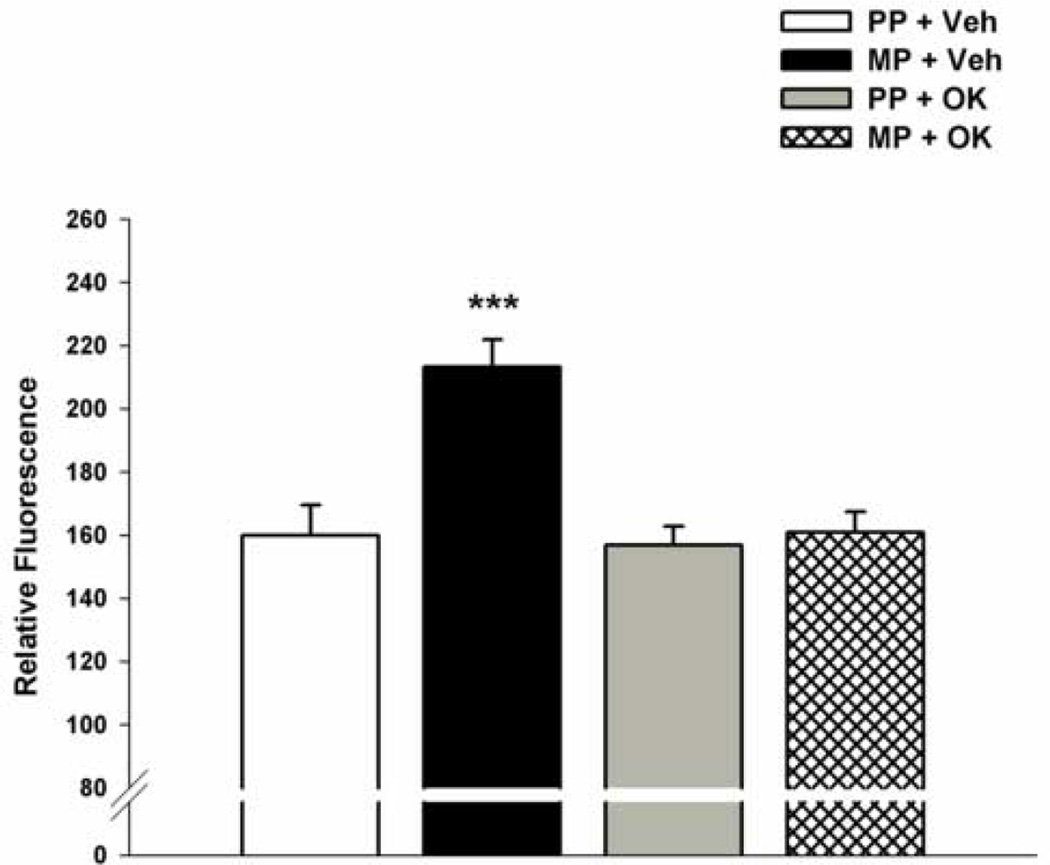
As seen in Fig. 1, the periaqueductal gray (PAG) from morphine-pelleted mice exhibited a significant increase in phosphatase activity compared to the same tissue extracted from placebo-pelleted mice [F(3,20)= 11.9]. The relative fluorescence reflecting the total phosphatase activity in the PAG from morphine-pelleted mice was measured as 213.2 ± 8.7 versus 159.9 ± 9.6 in placebo-pelleted mice, at 72-hr from pellet implantation. Post-hoc analyses indicated that the 33% increase in the total phosphatase activity in the PAG from morphine-pelleted mice versus placebo-pelleted mice was significant (P < 0.001). I.c.v. administration of okadaic acid (3 pmol/mouse) blocked the increase in the phosphatase activity observed in the PAG from morphine-pelleted mice. The relative fluorescence reflecting the total phosphatase activity in the PAG from okadaic acid-treated morphine-pelleted mice was decreased to 160.9 ± 6.5 (Fig. 1). Post-hoc analyses indicated that the total phosphatase activity measured in the PAG from okadaic acid-treated morphine-pelleted was not different from non-treated morphine-pelleted or placebo-pelleted mice (P > 0.05). That treatment with okadaic acid, at a dose of 3 pmol/mouse, had no effect on phosphatase activity in the PAG from placebo-pelleted mice (P > 0.05). It is noteworthy that no significant differences in phosphatase activity were detected in the medulla or spinal cords of placebo- versus morphine pelleted mice.
Figure 1. Total phosphatase activity in periaqueductal gray (PAG) from placebo and morphine-pelleted mice.
Mice (n= 6–10) were surgically implanted with placebo pellets (PP), or 75 mg morphine pellets (MP). Seventy-two hours later, vehicle or okadaic acid (OK; 3 pmol/mouse) was injected i.c.v. The mice were euthanized 30-min later and the PAG was extracted and prepared for total phosphatase assay as shown under Materials and Methods. Data are expressed as mean relative Fluorescence units ± S.E.M. *** significantly different from placebo-pelleted group at P < 0.05
2.2. Effects of phosphatase inhibition on morphine antinociceptive tolerance
Morphine administered s.c. elicited dose-dependent antinociception in the tail immersion test in both placebo and morphine pellet-implanted mice at 72-h following implantation (Table 1). Morphinepelleted mice showed a 5.5-fold tolerance to the antinociceptive effect of acute morphine compared to placebo-pelleted mice in the tail immersion test. Administration of okadaic acid (3 pmol/mouse; i.c.v.) to morphine-pelleted mice resulted in a 14.7-fold tolerance to morphineinduced antinociception compared to the placebo-pelleted mice (Table 1). When administered to placebo-pelleted mice at a dose of 3 pmol/mouse, okadaic acid had no effect on morphineinduced antinociception.
Table 1.
Morphine antinociceptive tolerance.
| Treatment | Morphine ED50 value (mg/kg (95% C.L.)) |
Potency Ratio (95% C.L.) |
|
|---|---|---|---|
| PP + Veh | 6.9 (6.3 to 7.6) | ||
| MP + Veh | 38.2 (34.4 to 42.4)* | vs. PP + Veh | 5.5 (4.7 to 5.9)a |
| PP + OK | 7.1 (6.2 to 8.0) | ||
| MP + OK | 104.6 (93.9 to 116.6)‡ | vs. PP + OK | 14.7 (12.4 to 16.9)a |
| PP + Bis | 6.4 (4.8 to 6.9) | ||
| MP + Bis | 6.3 (5.4 to 7.2) | vs. PP + Bis | 1.0 (0.9 to 01.0) |
| MP + OK + Bis | 28.4 (24.2 to 33.4)§ | vs. PP + Bis | 4.4 (3.9 to 5.3)a |
| PP + Go6976 | 6.6 (5.2 to 7.1) | ||
| MP + Go6976 | 6.0 (5.3 to 6.8) | vs. PP + Go6976 | 0.9 (0.8 to 0.9) |
| MP + OK + Go6976 | 42.8 (37.1 to 49.3)# | vs. PP + Go6976 | 6.5 (5.8 to 7.4)a |
| PP + PKI-(14–22)-amide | 6.5 (5.1 to 7.3) | ||
| MP + PKI-(14–22)-amide | 6.0 (5.3 to 6.8) | vs. PP + PKI-(14–22)-amide | 0.9 (0.8 to 0.9) |
| MP + OK + PKI-(14–22)-amide | Incalculableb | ||
Mice were surgically implanted with placebo pellets (PP) or morphine pellets (MP). At 72-h baseline measures were obtained before i.c.v. administration of vehicle, okadaic acid (OK; 3 pmol/mouse), bisindolylmaleimide I (Bis; 44.4 nmol/mouse), Go6976 (4 nmol/mouse) or PKI-(14–22)-amide (2.5 nmol/mouse). Immediately afterwards, the mice were challenged with various doses of morphine s.c. for construction of dose-response curves for calculation of ED50 values and potency ratios.
Significantly different than PP + Veh group based on non-overlapping 95% C.L.s
Significantly different than PP + OK group based on non-overlapping 95% C.L.s
Significantly different than PP + Bis group based on non-overlapping 95% C.L.s
Significantly different than PP + Go6976 group based on non-overlapping 95% C.L.s
Significantly different potency ratio since lower 95% C.L. is greater than 1
The ED50 value for the MP + OK + PKI-(14–22)-amide group was incalculable since the highest %MPE obtained within the range of morphine doses testwas less than 50%.
2.3. Effects of phosphatase inhibition on the ability of PKC inhibitors to reverse morphine antinociceptive tolerance
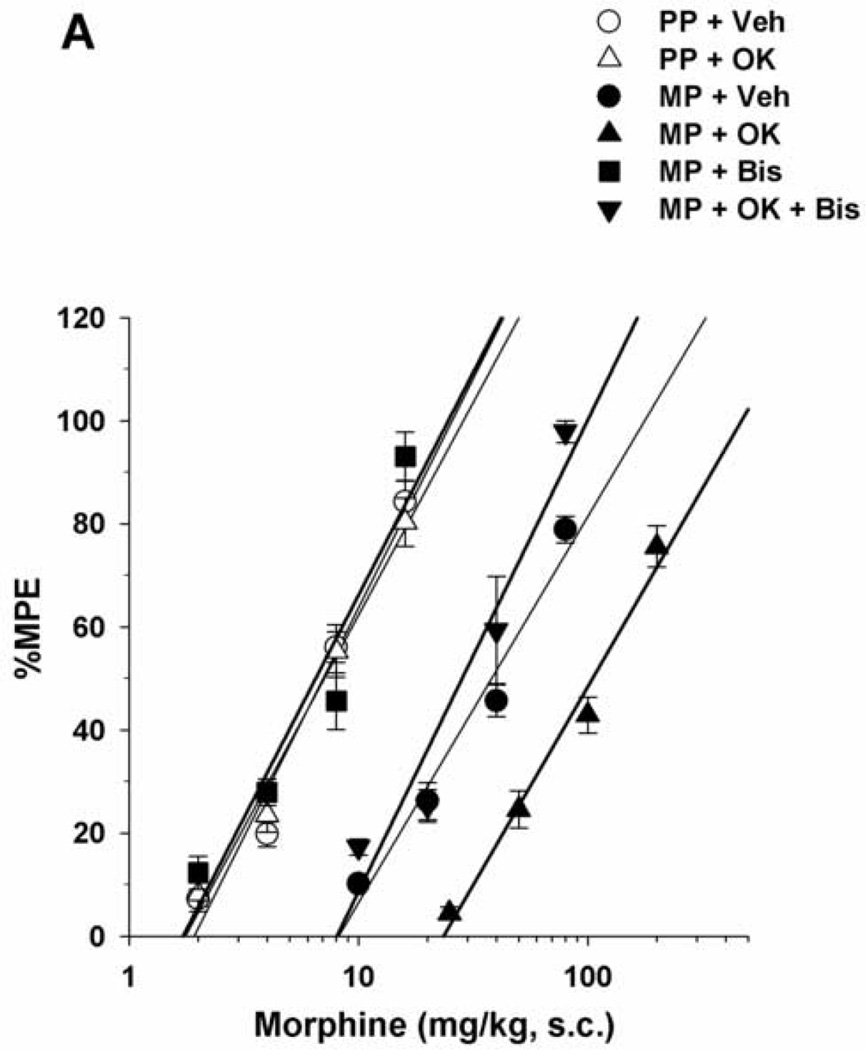
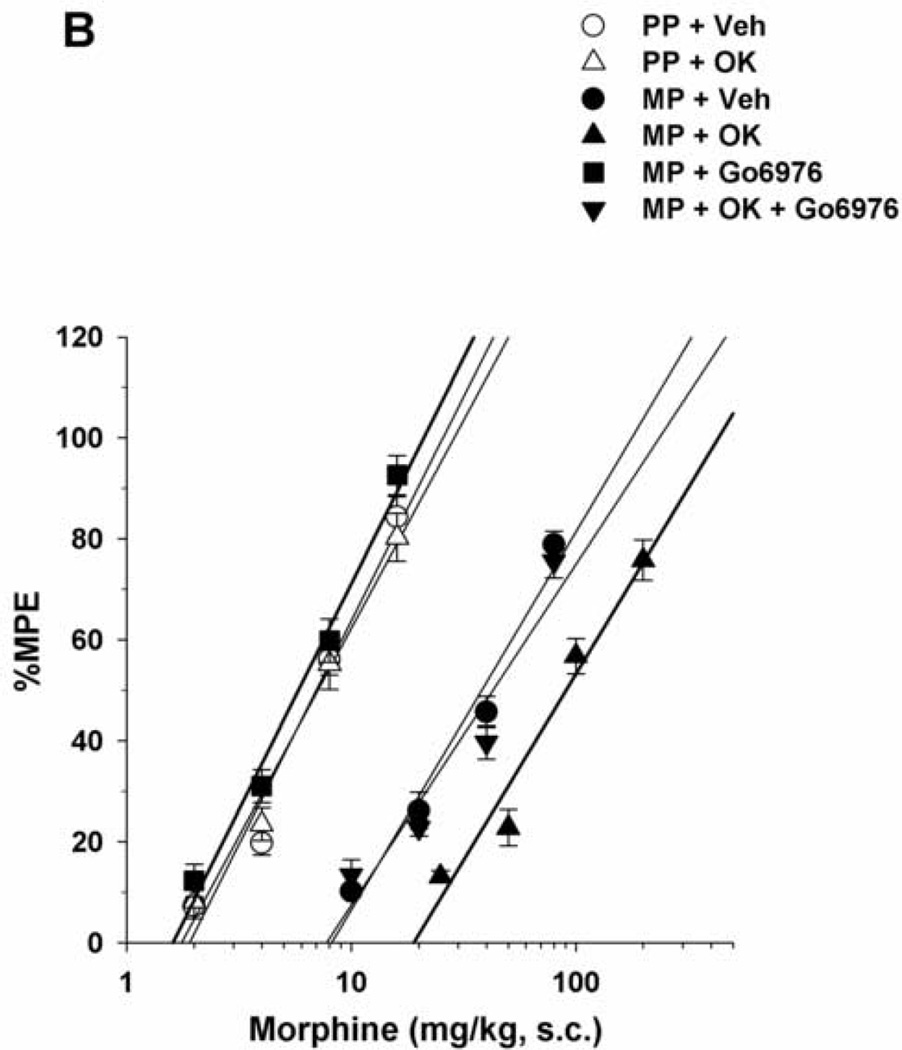
Morphine-pelleted mice, injected with vehicle i.c.v., showed a 5.5-fold tolerance to antinociception compared to placebo-pelleted mice as shown in the tail immersion test (Table 1). I.c.v. administration of the PKC inhibitors bisindolylmaleimide I (44.4 nmol/mouse) or Go6976 (4 nmol/mouse) completely reversed morphine antinociceptive tolerance. Acutely administered morphine s.c. was equally potent in both placebo- and morphine-pelleted mice injected with bisindolylmaleimide I (Table 1, Fig. 2A) or Go6976 (Table 1, Fig. 2B). On the other hand, administration of bisindolylmaleimide I or Go6976 to morphine-pelleted mice previously treated with okaidaic acid and exhibiting a higher level of antinociceptive tolerance (14.7-fold decrease in morphine’s potency; Table 1), only decreased the tolerance level to the level observed in non-okadaic acid-treated tolerant mice (5.5-fold decrease in morphine’s potency; Table 1, Fig. 2A and 2B). It is noteworthy that the administration of either of the PKC inhibitors could not bring morphine’s potency in okadaic-acid-treated mice to the same level observed in placebo-pelleted mice. Neither inhibitor was active in placebo-pelleted mice treated with okadiac acid. The selected doses of the inhibitors were shown to produce maximum reversal of morphine tolerance in our previous studies (Smith et al., 2003; 2006; Javed et al., 2004; Dalton et al., 2005).
Figure 2.
(A) Reversal of morphine tolerance with bisindolylmaleimide I in okadaic acid-treated mice. Mice were surgically implanted with placebo pellets (PP), or 75 mg morphine pellets (MP). Seventy-two hours later, vehicle (Veh), bisindolylmaleimide I (Bis; 44.4 nmol/mouse) or okadaic acid (OK; 3 pmol/mouse) was injected i.c.v. immediately followed by s.c. morphine. Tail immersion latencies were obtained 30 min later. (B) Reversal of morphine tolerance with Go6976 in okadaic acid-treated mice. Mice were surgically implanted with PP, or 75 mg MP. Seventy-two hours later, Veh, Go6976 (4 nmol/mouse) or OK (3 pmol/mouse) was injected i.c.v. immediately followed by s.c. morphine. Data are expressed as mean % MPE ± S.E.M. Each data point represents 6–10 mice.
2.4. Effects of phosphatase inhibition on the ability of PKA inhibitors to reverse morphine antinociceptive tolerance
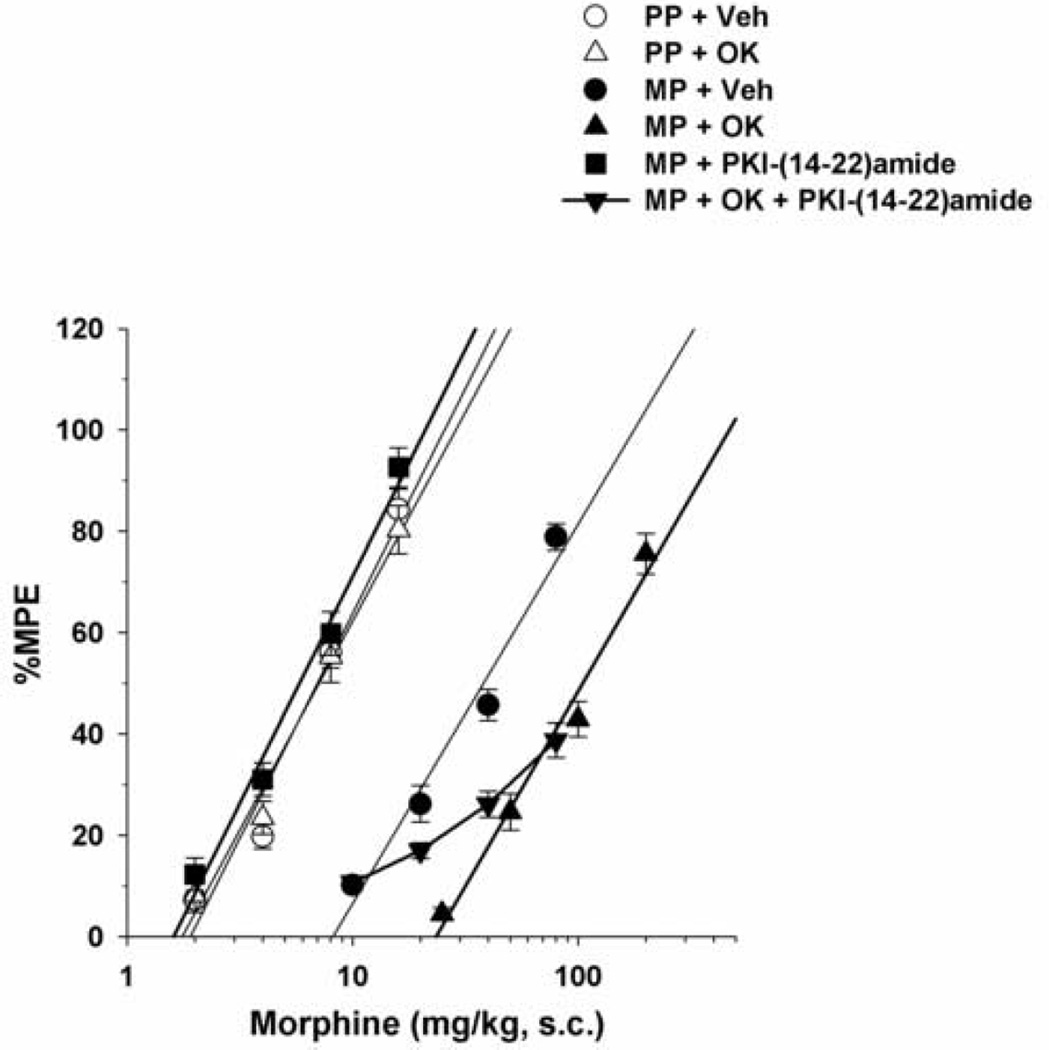
I.c.v. administration of the PKA inhibitor PKI-(14–22)-amide (2.5 nmol/mouse) completely reversed morphine antinociceptive tolerance. Acutely administered morphine s.c. was equally potent in both placebo- and morphine-pelleted mice injected with PKI-(14–22)-amide (Table 1, Fig. 3). Conversely, PKI-(14–22)-amide appeared to reverse the effect of okadaic acid to enhance morphine tolerance only when mice were challenged with 10 and 20 mg/kg morphine s.c. Yet at higher morphine doses of 40 and 80 mg/kg, okadaic acid enhanced tolerance was not reversed at all. The curve differed significantly from parallel from the other curves, thus not allowing statistical comparison between the ED50 and potency-ratio values. These data indicate that that PKA contributed partially to this effect (Fig. 3). It is worth mentioning that PKI-(14–22)-amide had no effect when tested in placebo-pelleted mice treated with okadiac acid.
Figure 3. Effects of PKI-(14–22)-amide on morphine tolerance in okadaic acid-treated mice.
Mice were surgically implanted with placebo pellets (PP), or 75 mg morphine pellets (MP). Seventy-two hours later, vehicle (Veh), PKI-(14–22)-amide (2.5 nmol/mouse) or OK (3 pmol/mouse) was injected i.c.v. immediately followed by s.c. morphine. Data are expressed as mean % MPE ± S.E.M. Each data point represents 6–10 mice.
3. Discussion
3.1. Phosphatase inhibition increases the level of morphine antinociceptive tolerance
The phosphorylation state of a protein is the product of both the rate of phosphorylation by kinases, and the rate of dephosphorylation by phosphatases. While relatively less is known about phosphatases than kinases, it is evident that intracellular regulation of phosphatase activity is extremely complex, involving a combination of their targeting and regulatory subunits as well as endogenous inhibitors (Cohen and Cohen, 1989). Because the intrinsic catalytic activity and intracellular concentrations of most kinases and phosphatases are approximately the same (Cohen, 1992), inhibition of phosphatase activity will tend to promote the phosphorylated state of substrate proteins. The hypothesis was tested that if enhanced kinase activity contributes to the expression of opioid tolerance, then inhibited phosphatase activity might further enhance the degree of tolerance, since it has been shown in our lab and others that inhibition of protein kinases reverses the expression of tolerance to morphine antinociception (Narita et al., 1994; Bernstein and Welch 1997; Javed et al., 2004; Smith et al., 2007). PP1 and PP2A are the two most commonly expressed protein phosphatases in the central nervous system. Acute morphine antinociception was blocked by very low picogram doses of okadaic acid (PP2A:PP1 inhibitory ratio 82.4:1; Haystead et al., 1989) or cantharidin (PP2A:PP1 inhibitory ratio 11.8:1; Honkanen, 1993), indicating that inhibition of PP2A allowed for increases in phosphorylation to inhibit antinociception (Moncada et al., 2003). However, high nanogram doses of okadaic acid or calyculin-A that inhibit both PP1 and PP2A did not alter morphine-induced antinociception, indicating that PP1 may counterbalance the effects of PP2A inhibition. Our hypothesis for these studies was that okadaic acid, which is a shellfish toxin that potently inhibits serine/threonine-specific protein phosphatases and can penetrate cells easily, when used at lower picogram doses will enhance the level of morphine tolerance due to selective inhibition of PP2A. Our data demonstrate that i.c.v. administration of okadaic acid at a dose of 3 pmol/mouse (a dose that had no effects on morphine antinocicpetion in placebo-pelleted mice) significantly enhanced the level of morphine antinociceptive tolerance in morphine-pelleted mice. Our results also show a significant increase in the level of phosphatase activity in the PAG from morphine tolerant mice versus placebo-pelleted mice. This increase was blocked in the okadaic acid-treated morphine-pelleted mice. It could thus be suggested that, under the tolerant state, an intracellular homeostatic regulation occurs to maintain a balance between the phosphorylation (kinases) and de-phosphorylation (phosphatases) states. This involves an increase in the phosphatase activity to counter-balance the enhanced kinase activity that contributes to the expression of opioid tolerance. Conversely, we did not observe any significant changes in the phosphatase activity in the medulla or spinal cord from morphine-pelleted compared to those from placebo-pelleted mice. Although opioids act at sites throughout the nervous system, the PAG appears to be a key structure for the development of tolerance to morphine antinociception. Tolerance to the antinociceptive effect of systemically administered morphine is prevented by selectively blocking opioid receptors in the PAG (Lane et al., 2005), and the antinociception produced by microinjection of morphine into the PAG is reduced with repeated administration (Jacquet and Lajtha, 1976, Siuciak and Advokat, 1987 and Tortorici et al., 1999; Morgan et al., 2005). Okadaic acid injected i.c.v. appeared to inhibit a specific phosphatase isoform in the PAG of morphine tolerant mice to decrease elevated phosphatase activity to basal activity similar to placebo-pelleted mice. In corresponding fashion, this same dose of okadaic acid enhanced the level of morphine tolerance presumably by allowing kinases to continue to increase the phosphorylation state. Alternatively, okadaic acid administered i.c.v. had no effect on basal phosphatase activity in the PAG of placebo-pelleted mice since this specific phosphatase isoform appears to be quiescent in opioid-naïve mice. The assay did demonstrate that the tissue samples from each region contained measurable phosphatase activity. However, concentrations of okadaic acid used in these studies were not high enough to inhibit the activity of these other phosphatase isoforms. Consistent with these data were the findings that okadaic acid i.c.v. had no effect on the morphine’s potency in placebo-pelleted mice.
3.2. Phosphatase inhibition reduces the ability of PKC inhibitors to reverse morphine antinociceptive tolerance
We and others have previously reported on the implication of PKC in mediating morphine tolerance and the ability of PKC inhibitors to reverse morphine tolerance (Bailey et al., 2006). Granados-Soto et al. (2000) demonstrated that rats infused with morphine for 5-days had significantly higher levels of PKCα and PKCγ in the dorsal spinal horn. The higher levels of PKCα and PKCγ as well as morphine antinociceptive tolerance were prevented when the PKC inhibitor chelerythrine was co-infused with morphine during the 5-day treatment. In addition, it was reported that PKCγ null mutant mice exhibit significantly less tolerance than wild-type controls (Zeitz et al., 2001). Moreover, intrathecal co-infusion of antisense oligonucleotide to PKCα during the 5-day intrathecal morphine infusion was shown to prevent the development of morphine tolerance (Hua et al., 2002). We previously reported that chemical inhibitors of PKC, bisinolylmaleimide I and Go7874, administered i.c.v., completely reversed morphine antinociceptive tolerance in 72-h morphine-peletted mice challenged with s.c. morphine (Javed et al., 2004). We further found that the injection of theses PKC inhibitors reinstated antinociception, hypothermia and Straub tail in mice implanted with morphine pellets 72-h earlier (Smith et al., 2006). In the present study, another hypothesis was tested that phosphatase inhibition will alter the ability of PKC inhibitors to reverse morphine antinociceptive tolerance. Our data showed that the PKC inhibitors, bisinolylmaleimide I and Go6976 were able to only decrease the level of tolerance in okadaic acid-treated mice (14.7-fold) to the level observed in non-okadaic acid-treated tolerant mice (5.5-fold). Both inhibitors could not bring morphine’s potency in okadaic-acid-treated mice to the same level observed in placebo-pelleted mice. It could have been thought that if tolerance is due to PKC phosporylation of morphine receptors, then when all phosphatases are inhibited the receptor should stay phosphorylated and tolerance should not be reversed at all. However, since okadaic acid at the tested dose (3 pmol/mouse) is believed to inhibit only PP2A, only partial reversal of tolerance was observed with bisinolylmaleimide I. The PKC will be then phosphorylating the sites that were de-phosphorylated by the non-inhibited phosphatases. We further suggest that the increase in phosphatase activity in the tolerance state (in response to enhanced kinase activity) played an important synergistic role with PKC inhibitors in our previous studies which showed complete reversal of morphine tolerance. It is also possible that phosphatase inhibition produces a high state of tolerance which is not reversed by PKC inhibition alone. We have previously shown that PKC inhibitors, which completely reversed 5- to 8-fold morphine tolerance, can only partially reverse a 45-fold tolerance level achieved by a 75-mg morphine pellet and twice daily morphine injections over 3 days (Smith et al., 2003).
3.3. Phosphatase inhibition blocks the ability of PKA inhibitors to reverse morphine antinociceptive tolerance
Accumulating evidence suggests an important role for increased PKA activity in the development of opioid tolerance. Shen et al. (2000) demonstrated that i.c.v. administration of the anti-sense oligodeoxynucleuotide to PKA mRNA blocked the antinociceptive tolerance to morphine in mice (Shen et al., 2000). We previously reported that i.c.v. administration of the inhibitors of PKA, PKI-(14–22)-amide and 4-cyano-3-methylisoquinoline and KT-5720 completely reversed 5- to 8-fold and only partly 45-fold morphine antinociceptive tolerance (Smith et al., 2003; Javed et al., 2004; Dalton et al., 2005). We also demonstrated that PKA inhibition reinstated antinociception, hypothermia and Straub tail in mice implanted with morphine pellets 72-h earlier (Smith et al., 2006). However, in the present study, the specific and cell permeable myristoylated inhibitor of PKA, PKI-(14–22)-amide (selective peptide fragment of the autoregulatory domain of PKA; 2.5 nmol/mouse) was inactive at reversing the high level of antinociceptive tolerance observed in okadaic acid-treated morphine tolerant mice. PKI-(14–22)-amide only resulted in a partial reversal of the effect of okadaic acid to enhance morphine tolerance at the lower 10 and 20 mg/kg morphine s.c. challenge doses. However, okadaic acid-enhanced tolerance was not reversed at all at 40 and 80 mg/kg or higher morphine doses, indicating that PKA only partially contributed to this effect. Unfortunately, the slope of the morphine dose-response curve made it impossible to compare ED50 values or potency-ratio values between other treatment groups. Yet the data indicates that PKA may contribute a small portion the enhanced tolerance observed after phosphatase inhibition by okadaic acid. It is worth mentioning that PKI-(14–22)-amide was tested at up to a 5-fold higher dose than the dose that reversed moderate levels of morphine tolerance (5- to 8-fold decrease in morphine’s potency) in our previous studies (Dalton et al., 2005). These results suggest that enhanced morphine tolerance due to phosphatase inhibition involves only a partial activation of the adenylyl cyclase pathways. This provides evidence that multiple signaling pathways are involved in different levels of opioid tolerance.
3.4. Perspectives
Previous reports show that moderate levels of morphine tolerance can be reversed by either PKC or PKA inhibition. We now show that tolerance to morphine is increased by phosphatase inhibition. Taken together, these findings show the importance of the balance between theses enzymes in expressing opioid tolerance. This balance between kinases and phosphatases can be disrupted by blocking one or the other, showing that either may be altered with tolerance development. While changes have been reported to occur in kinase activity in the brains of morphine-tolerant animals, we now demonstrate that changes also occur in phosphatase activity with tolerance development. Further studies are needed to better understand the role played by different protein phosphatase isomers in opioid tolerance and to clarify the relationship, if there is any, between the increase in kinase and in phosphatase activity. We propose that increase in phosphatase activity may be compensatory to the increase in kinase activity.
4. Experimental Procedure
4.1. Animals
Male Swiss Webster mice (Harlan Laboratories, Indianapolis, IN) weighing 25–30 g were housed 6 to a cage in animal care quarters and maintained at 22 ± 2 °C on a 12-hr light-dark cycle. Food and water were available ad libitum. The mice were brought to a test room (22 ± 2 °C, 12-hr light-dark cycle), marked for identification and allowed 18-hr to recover from transport and handling. Protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University Medical Center and comply with the recommendations of the IASP (International Association for the Study of Pain).
4.2. Surgical implantation of pellets
Mice were anesthetized with 2.5% isoflurane before shaving the hair around the base of the neck. Adequate anesthesia was noted by the absence of the righting-reflex and lack of response to toe-pinch, according to IACUC guidelines. The skin was cleansed with 10% povidone iodine (General Medical Corp., Prichard, WV) and rinsed with alcohol before making a 1 cm horizontal incision at the base of the neck. The underlying subcutaneous space toward the dorsal flanks was opened using a sterile glass rod. Maintenance of a stringent aseptic surgical field minimized any potential contamination of the pellet, incision and subcutaneous space. A placebo pellet or 75 mg morphine pellet was inserted in the space before closing the site with Clay Adams Brand, MikRon® AutoClip® 9mm Wound Clips (Becton Dickinson and Co., Sparks, MD) and again applying iodine to the surface. The animals were allowed to recover in their home cages where they remained throughout the experiment.
4.3. Intracerebroventricular injections
Intracerebroventricular (i.c.v.) injections were performed as described by Pedigo et al. (1975). Mice were anesthetized with isoflurane and a horizontal incision was made in the scalp. A free-hand 5 µl injection of drug or vehicle was made in the lateral ventrical (2 mm rostral and 2 mm lateral at a 45° angle from the bregma). The extensive experience of this laboratory has made it possible to inject drugs with greater than 95% accuracy. Immediately after testing, the animals were euthanized to minimize any type of distress, according to IACUC guidelines.
4.4. Tail immersion test
The warm-water tail immersion test was performed according to Coderre and Rollman (1983) using a water bath with the temperature maintained at 56 ± 0.1 °C. Before injecting the mice, a base-line (control) latency was determined. Only mice with a control reaction time from 2- to 4-sec were used. The average baseline latency for these experiments was 3.0 ± 0.1 sec. The test latency after drug treatment was assessed at the appropriate time, and a 10-sec maximum cut-off time was imposed to prevent tissue damage. Antinociception was quantified according to the method of Harris and Pierson (1964) as the percentage of maximum possible effect (% MPE) which was calculated as: %MPE = [(test latency – control latency) / (10– control latency)] × 100. Percent MPE was calculated for each mouse using at least 6 mice per drug.
4.5. Measurement of phosphatase activity
Animals were euthanized by decapitation at the base of the skull. Brains were rapidly removed and placed on moistened filter paper on wet ice for dissection of the periaqueductal gray (PAG) and medulla. The spinal cords were extracted by injecting normal saline under pressure through a 16-gauge needle into the upper sacral spine, thus forcing the spinal cord out rostrally. Brain tissues and spinal cord segments were flash frozen in liquid nitrogen and stored at −80 °C. For phosphatase activity measurement, tissues were homogenized in ice-cold buffer: 50 mM Tris (pH 7.0), 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.1% Triton × −100, and Complete Protease Inhibitor Cocktail from Roche (Indianapolis, IN, USA; 1 tablet/50 ml of lysis buffer). The homogenate was centrifuged at 15,000 × g at 4 °C for 30 min. Phosphatase activity was measured using a fluorescence intensity quenching assay (IQ® Serine/Threonine Phosphatase Assay; Pierce, Rockford, IL, USA). Fluorophore-labeled peptides were used as enzyme substrates, and phosphatase activity was quantified by direct measurement of the phosphorylation state of the substrate. The fluorescence intensity increased in proportion to the percent de-phosphorylated product. In brief, the tissue sample was incubated with 5 μl of the fluorescent dye-labeled phosphopeptide enzyme substrate at room temperature for 60 min. Reactions were terminated by adding 120 ul IQ reagent mixture. Fluorescence intensity was determined at 560/590 excitation/emission using a Multi-Detection Microplate Reader FLx800™ (Biotek Instruments In., Winooski, VT, USA).
4.6. Drugs and chemicals
The placebo and 75mg morphine pellets were obtained from the National Institute on Drug Abuse (NIDA), Bethesda, MD. Morphine sulfate (Mallinckrodt, St. Louis, MO) was dissolved in pyrogen-free isotonic saline (Baxter Healthcare, Deerfield, IL). Okadaic acid ammonium salt was purchased from Sigma-Aldrich (St. Louis, MO, USA). Bisindolylmaleimide I HCl ((2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide hydrochloride), Go6976 (12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo- 5H-indolo(2,3-a)pyrrolo(3,4-c) carbazole), and myristolated PKI (14–22) amide [PKI-(14–22)-amide; Myr-N-Gly-Arg-Thr-Gly-Arg-Arg-Asn-Ala-Ile-NH2] were purchased from Calbiochem (San Diego, CA, USA). Bisindolylmaleimide I HCl and PKI-(14–22)-amide were dissolved in distilled water, whereas Go6976 was dissolved in 10% dimethyl sulfoxide, 20% emulphor, 70% distilled water. We have previously published on the use of this vehicle for i.c.v. injections (Smith et al., 1999; 2002; 2003; 2006). The control vehicle-injected mice were injected with 10% DMSO, 20% emulphor, 70% distilled water.
4.7. Experimental design and statistical analysis
In the first series of experiments, morphine dose-response curves were generated in placebo- and morphine-pelleted mice 72-h following pellets implantation, to test for the development of tolerance. Baseline measures of tail immersion were obtained prior to subcutaneous (s.c.) morphine administration. Effective dose-50 (ED50) values were calculated using least-squares linear regression analysis followed by calculation of 95% confidence limits (95% C.L.) by the method of Bliss (1967). Tests for parallelism were conducted before calculating the potency-ratio values with 95% C.L. by the method of Colquhoun (1971). Colquhoun notes that a potency-ratio value of greater than one, with the lower 95% C.L. greater than one, is considered a significant difference in potency between groups.
The following experiments then examined the effects of i.c.v. administration of okadaic acid (3 pmol/mouse) on the expression of antinociceptive tolerance to morphine as well as the effects of okadaic acid on the ability of PKC inhibitors (bisindolylmaleimide I; 44.4 nmol/mouse, and Go6976; 4 nmol/mouse) or the PKA inhibitor, PKI-(14–22)-amide (2.5 nmol/mouse) to reverse morphine tolerance. In another series of experiments, placebo- and morphine-pelleted mice treated and untreated with okadaic acid, were assessed for their phosphatase activity in different tissues, including the PAG, medulla and spinal cord.
Data are expressed as mean values ± S.E.M. Analysis of variance (ANOVA) followed by the post hoc “Student-Newman-Keuls” test were performed to assess significance using the Instat 3.0 software (GraphPad Software, San Diego, CA, U.S.A.). P < 0.05 was considered significant.
Acknowledgements
We thank Joshua A. Seager and David L. Stevens for valuable technical assistance during these studies. This work was funded by the National Institute on Drug Abuse grants: DA-01647, K05-DA00480 and DA-020836
Abbreviations
- PKC
protein kinase C
- PKA
protein kinase A
- ED50
50% effective dose
- %MPE
percent maximum possible effect
- i.c.v.
intracerebroventricular
- s.c.
subcutaneous
- PAG
periaqueductal gray matter
- Go6976
(12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5- oxo-5H-indolo(2,3-a)pyrrolo(3,4-c) carbazole
- PKI-(14–22)-amide
Myr-N-Gly-Arg-Thr-Gly- Arg-Arg-Asn-Ala-Ile-NH2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol. Sci. 2006;27:558–565. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Barton C, Basbaum AI, Fields HL. Dissociation of supraspinal and spinal actions of morphine: a quantitative evaluation. Brain Res. 1980;188:487–498. doi: 10.1016/0006-8993(80)90047-5. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bernstein MA, Welch SP. Effects of spinal versus supraspinal administration of cyclic nucleotide-dependent protein kinase inhibitors on morphine tolerance in mice Drug and Alcohol Depend. 1997;44:41–46. doi: 10.1016/s0376-8716(96)01320-8. [DOI] [PubMed] [Google Scholar]
- Bliss CI. Statistics in Biology. New York, NY: McGraw-Hill; 1967. p. 439. [Google Scholar]
- Coderre TJ, Rollman GB. Naloxone hyperalgesia and stress-induced analgesia in rats. Life Sci. 1983;32:2139–2146. doi: 10.1016/0024-3205(83)90103-0. [DOI] [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem. Sci. 1992;17:408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen PTW. Protein phosphatases come of age. J. Biol. Chem. 1989;264:21435–21438. [PubMed] [Google Scholar]
- Colquhoun D. Lectures on biostatistics: An introduction to statistics with applications in biology and medicine. Oxford: Clarendon Press; 1971. pp. 327–333. [Google Scholar]
- Dalton GD, Smith FL, Smith PA, Dewey WL. Alterations in brain Protein Kinase A activity and reversal of morphine tolerance by two fragments of native Protein Kinase A inhibitor peptide (PKI) Neuropharmacology. 2005;48:648–657. doi: 10.1016/j.neuropharm.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Freye E, Latasch L. Development of opioid tolerance - molecular mechanisms and clinical consequences. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2003;38:14–26. doi: 10.1055/s-2003-36558. [DOI] [PubMed] [Google Scholar]
- Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL. Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain. 2000;85:395–404. doi: 10.1016/S0304-3959(99)00281-X. [DOI] [PubMed] [Google Scholar]
- Harris LS, Pierson AK. Some narcotic antagonists in the benzomorphan series. J. Pharmacol Exp. Ther. 1964;143:141–148. [PubMed] [Google Scholar]
- Haystead TAJ, Sim ATR, Carling D, Honnor RC, Tsukitani Y, Cohen P, Hardie DG. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Hsu MM, Wong CS. The roles of pain facilitatory systems in opioid tolerance. Acta Anaesthesiol. Sin. 2000;38:155–166. [PubMed] [Google Scholar]
- Hua XY, Moore A, Malkmus S, Murray SF, Dean N, Yaksh TL, Butler M. Inhibition of spinal protein kinase Calpha expression by an antisense oligonucleotide attenuates morphine infusion-induced tolerance. Neuroscience. 2002;113:99–107. doi: 10.1016/s0306-4522(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Javed RR, Dewey WL, Smith PA, Smith FL. PKC and PKA inhibitors reverse tolerance to morphine-induced hypothermia and supraspinal analgesia in mice. Eur. J. Pharmacol. 2004;492:149–157. doi: 10.1016/j.ejphar.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Moncada A, Cendan CM, Baeyens JM, Del Pozo E. Effects of serine/threonine protein phosphatase inhibitors on morphine-induced antinociception in the tail flick test in mice. Eur. J. Pharmacol. 2003;465:53–60. doi: 10.1016/s0014-2999(03)01461-4. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Tierney BW, Ingram SL. Intermittent dosing prolongs tolerance to the antinociceptive effect of morphine microinjection into the periaqueductal gray. Brain Res. 2005;1059:173–178. doi: 10.1016/j.brainres.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Narita M, Feng Y-Z, Makimura M, Hoskins B, Ho IK. A protein kinase inhibitor, H-7, inhibits the development of tolerance to opioid antinociception. Eur. J. Pharmacol. 1994;271:543–545. doi: 10.1016/0014-2999(94)90817-6. [DOI] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Kampine JP, Tseng LF. Role of protein kinase C in desensitization of spinal delta-opioid-mediated antinociception in the mouse. Br. J. Pharmacol. 1996;118:1829–1835. doi: 10.1111/j.1476-5381.1996.tb15610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo NW, Dewey WL, Harris LS. Determination and characterization of the antinociceptive activity of intraventricularly administered acetylcholine in mice. J. Pharmacol. Exp. Ther. 1975;193:845–852. [PubMed] [Google Scholar]
- Shen J, Gomes AB, Gallagher A, Stafford K, Yoburn BC. Role of cAMP-dependent protein kinase (PKA) in opioid agonist-induced mu-opioid receptor downregulation and tolerance in mice. Synapse. 2000;38:322–327. doi: 10.1002/1098-2396(20001201)38:3<322::AID-SYN11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res. 1987;424:311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- Smith FL, Dombrowski DS, Dewey WL. Involvement of intracellular calcium in morphine tolerance in mice. Pharmacol. Biochem. Behav. 1999;62:381–388. doi: 10.1016/s0091-3057(98)00168-3. [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain. 2007;127:129–139. doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Dewey WL. The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res. 2003;985:78–88. doi: 10.1016/s0006-8993(03)03170-6. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Welch SP, Selley D, Sim-Selley L, Dewey WL. Prolonged reversal of morphine tolerance with no reversal of dependence by protein kinase C inhibitors. Brain Res. 2002;958:28–35. doi: 10.1016/s0006-8993(02)03394-2. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Smith PA, Dewey WL, Gabra BH. PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol. Res. 2006;54:474–480. doi: 10.1016/j.phrs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Smith FL, Lohman AB, Dewey WL. Influence of phospholipid signal transduction pathways in morphine tolerance in mice. Br. J. Pharmacol. 1999;128:220–226. doi: 10.1038/sj.bjp.0702771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav. Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Noueihed R. The physiology and pharmacology of spinal opiates. Annu. Rev. Pharmacol. Toxicol. 1985;25:433–462. doi: 10.1146/annurev.pa.25.040185.002245. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain. 2001;94:245–253. doi: 10.1016/S0304-3959(01)00353-0. [DOI] [PubMed] [Google Scholar]