Abstract
Conjugated linoleic acid (CLA) is widely used as a “nutraceutical” for weight loss. CLA has anticancer effects in preclinical models, and we demonstrated in vitro that this can be attributed to the suppression of fatty acid (FA) synthesis. We tested the hypothesis that administration of CLA to breast cancer patients would inhibit expression of markers related to FA synthesis in tumor tissue, and that this would suppress tumor proliferation. Women with Stage I–III breast cancer were enrolled into an open label study and treated with CLA (1:1 mix of 9c,11t- and 10t,12c-CLA isomers, 7.5 g/d) for ≥10 days before surgery. Fasting plasma CLA concentrations measured pre- and post-CLA administration, and pre/post CLA tumor samples were examined by immunohistochemistry for Spot 14 (S14), a regulator of FA synthesis, FA synthase (FASN), an enzyme of FA synthesis, and lipoprotein lipase (LPL), the enzyme that allows FA uptake. Tumors were also analyzed for expression of Ki-67 and cleaved caspase 3. 24 women completed study treatment, and 23 tumors were evaluable for the primary endpoint. The median duration of CLA therapy was 12 days, and no significant toxicity was observed. S14 expression scores decreased (p = 0.003) after CLA administration. No significant change in FASN or LPL expression was observed. Ki-67 scores declined (p = 0.029), while cleaved caspase 3 staining was unaffected. Decrements in S14 or Ki-67 did not correlate with fasting plasma CLA concentrations at surgery. Breast tumor tissue expression of S14, but not FASN or LPL, was decreased after a short course of treatment with 7.5 g/day CLA. This was accompanied by reductions in the proliferation index. CLA consumption was well-tolerated and safe at this dose for up to 20 days. Overall, CLA may be a prototype compound to target fatty acid synthesis in breast cancers with a “lipogenic phenotype”.
Keywords: Breast cancer, Fatty acid, Metabolism, Conjugated linoleic acid
Introduction
Approximately 230,000 women are predicted to be diagnosed in the USA with invasive breast cancer in 2012 and 39,500 deaths are expected [1], indicating a need for new therapeutic approaches. We and others have demonstrated that most breast cancer cell lines, as well as breast cancer tumors, have notably high expression of the enzymes of de novo fatty acid (FA) synthesis (reviewed in [2]), and are dependent on FA for cell growth and survival. We recently determined that, in addition to the pathway of FA synthesis, a second pathway for FA acquisition by cellular uptake of exogenous FAs is universally expressed by breast cancers [3]. Inhibition of either FA synthesis or cellular uptake of pre-formed FA can inhibit cancer cell growth and survival, and dual inhibition can yield an enhanced in vitro antiproliferative effect [3, 4]. The dependency of breast cancer cells on FA may therefore provide novel therapeutic targets. Although obesity has been linked to increased breast cancer incidence [5] and adverse prognosis (reviewed in [6]), interventional studies thus far have not revealed a survival advantage of a low fat diet for breast cancer patients [7, 8].
Conjugated linoleic acids (CLA) are a group of 18 carbon, diunsaturated FA found in meat and milk derived from ruminants and are also sold as a nutraceutical for weight loss. CLA was shown to exhibit anticancer effects in pre-clinical models of mammary cancer both in vivo and in vitro [9, 10]. Harvatine and Bauman observed, in bovine mammary gland, that CLA suppressed the expression of key genes involved in FA synthesis, such as S14 (THRSP, Spot 14), a nuclear protein involved in the regulation of genes coding lipogenic enzymes, fatty acid synthase (FASN) a key enzyme for the synthesis of long-chain saturated FA, and lipoprotein lipase (LPL) the secreted enzyme that releases free FA from circulating lipoproteins [11]. We demonstrated that CLA similarly inhibits lipid synthesis in breast cancer cells by suppressing S14 and FASN gene expression, and that this metabolic effect is a major cause of the antiproliferative action of CLA on breast cancer cells [12, 13].
We undertook a proof of principle study that addressed the hypothesis that short-term CLA administration may suppress the expression of S14, FASN, and/or LPL in human breast tumors. The primary objectives of this study (ClinicalTrials.Gov #NCT00908791) were to (a) determine whether ≥ 10 days of CLA administration suppresses expression of S14, FASN, and/or LPL, and (b) define the impact of CLA on biomarkers of tumor cell proliferation (Ki-67) and apoptosis (cleaved caspase 3). Secondary objectives were to (a) confirm the safety of short-term treatment with 7.5 g CLA/d, and; (b) assess the relationship of such effects with circulating concentrations of the free (unesterified) c9, t11- and t10, c12-CLA isomers.
Methods
Patient population
Women with invasive breast cancer were recruited from the Breast Oncology Clinic at the Norris Cotton Cancer Center at The Geisel School of Medicine at Dartmouth from June 2009 to March 2011. The study protocol and informed consent were approved by the Committee for the Protection of Human Subjects of Dartmouth College. All patients gave written informed consent before entry into the study.
Study design
This was a single arm, open-label, single-institution study in women with histologically proven invasive non-meta-static breast cancer. Prior to CLA treatment, study participants had 20 mL blood drawn after an eight-hour fast to assess baseline CLA concentrations. Tumor marker analysis before CLA consumption was performed on the core biopsy specimens. Participants were asked to consume 7.5 g of CLA per day, taken in two equal doses between 8 am and noon and between 8 pm and midnight. CLA was taken for a minimum of 10 days before tumor resection. This dose of CLA was based on published work showing that consuming 6.8 g of CLA per day for 3 months was safe [14], and that consuming 12 g of CLA per day for 24 months was equally well-tolerated [15].
Participants were permitted to take CLA for up to 28 days. The last dose of CLA before surgery was taken close to midnight. A second 20 mL fasting blood sample was obtained on the morning of surgery for measurement of post-CLA concentrations. CLA dose modifications were not permitted. Participants who omitted CLA doses for more than one day due to potential drug related adverse events were to be discontinued from the study and considered inevaluable for the primary study endpoint. Participants were interviewed in the clinic 2 weeks post-surgery, and a blood sample was collected to check for potential toxicities. Subjects were contacted by telephone to capture any delayed symptoms of adverse effects of the CLA treatment 4 weeks after surgery.
The primary outcome for the sample size calculation was defined as the change in expression of S14 in tumor following administration of CLA, summarized as the ratio of the number of responders to the total number of evaluable patients. The half width of a 95 % confidence interval for the response rate was computed using exact methods based on the binomial distribution. Based on a sample size of 24, and a projected response rate of 0.4, the 95 % confidence interval had an expected half width of 0.196, which would exclude response rates smaller than 0.2.
Patient eligibility criteria
Eligible study participants met the following criteria (i) women over 18 years of age with histologically confirmed resectable invasive adenocarcinoma of the breast (Stage I, II, or III as defined by the AJCC TNM Staging System), (ii) acceptable hepatic and renal function as evidenced by hepatic transaminases (AST or ALT), total bilirubin and serum creatinine all ≤1.5 times the upper limit of normal were required. Ineligibility criteria included: receiving radiation or chemotherapy; pregnancy or lactation; gastrointestinal abnormalities preventing intake of oral medications; intravenous alimentation, or prior abdominal surgical procedures affecting nutrient absorption; a serious uncontrolled medical disorder, an active infection, or a history of prior CLA supplementation.
Capsule and plasma CLA analyses
Gel capsules (Clarinol) nominally containing 750 mg of a 1:1 mixture of 9c,11t- and 10t,12c-CLA isomers from a single production batch were purchased from Progressive Laboratories (Irving TX). Venous blood samples (20 mL) were collected in heparinized tubes and kept on ice until centrifugation. Plasma was separated and stored at −70 °C. Extraction and analysis of capsule and plasma CLA by Ag+HPLC were as described [13].
Tumor tissue pharmacodynamics
FASN, S14, and LPL expression were assessed by immunohistochemistry (IHC) in diagnostic breast cancer core biopsies and in the resected breast tumor after the participant took CLA for ≥10 days. Tissue samples were formalin-fixed and paraffin-embedded. Immunohistochemical scoring systems are summarized in Table 1. S14 and LPL were detected with mouse monoclonal antibodies that we produced and validated [5, 13], and FASN was detected with an affinity-purified rabbit polyclonal antibody (Novus Biologicals, Littleton, CO), with Citra antigen retrieval as described [3]. Ki-67 was detected with a pre-diluted antibody (Ready-to-Use Primary Antibody (MM1 clone); Vision BioSystems Bond, Norwell MA) using antigen retrieval with ER2 (epitope retrieval solution) for 30 min. Slides immunostained for Ki-67 were digitally scanned at high resolution and montaged together with the Surveyor© Automated Specimen Scanning stage control bundled software (Objective Imaging Ltd., Cambridge UK). Using Media Cybernetics™ Image Pro plus software, the Ki-67-positive tumor cell nuclei were thresholded out and the average % per unit area of Ki-67-positive tumor nuclei in five randomly selected regions was recorded. Cleaved caspase 3 was detected with a polyclonal antibody (1:200 dilution; BioCare Medical, Concord CA) using antigen retrieval for 20 min. The number of viable, intact tumor cells with cytoplasmic immunostaining for cleaved caspase 3 were averaged in five 40 × high power fields per case (both pre-treatment and post-treatment).
Table 1.
Criteria for assignment of immunohistochemical expression scores
| Antibody | Cell compartment | Score 0 | Score 1+ (weak) | Score 2+ (strong) |
|---|---|---|---|---|
| S14 | Nucleus | No immunostaining | Blush, faint or low intensity, diffuse | Intense, usually diffuse; can be focal if intense |
| FASN | Cytoplasm | No immunostaining | Blush, faint or low intensity, diffuse | Intense, usually diffuse; can be focal if intense |
| LPL | Cytoplasm | No immunostaining | Focal or diffuse of low intensity (no perinuclear intensity) | Intense staining which must be perinuclear, even if focal |
CLA safety, toxicity and adverse effects
Adverse events (AEs) were tabulated according to type and grade. In addition, the percent of participants experiencing any adverse event of any grade and the percent with any grade three or worse adverse event were recorded. Laboratory evaluations included hematology and serum chemistry profiles assessed before CLA consumption, immediately pre-operatively, and two weeks post-operatively. The incidence of AEs during treatment was categorized by system organ class, preferred term, severity (based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0), and relationship to the study drug.
Statistics
Two pathologists independently scored the staining in randomized sample sequences, and were blinded to the timing of the samples (pre- or post-CLA administration). The scores were 100 % concordant between the two readers. The proportions in each category for S14, FASN, and LPL were compared before and after CLA treatment using McNemar or Bowker’s test for paired binary or ordinal categorical data. The change from pre-treatment to post-treatment samples was then characterized as a decrease (−1), stable (0) or increase (1). The effect of the CLA on the ordinal change variables was assessed using a cumulative proportional odds mixed model for ordinal data with random intercepts for the individual and a logit link (SAS 9.2 Institute Inc. Cary N.C. SAS/STAT). Confidence intervals and p-values for the odds of changes in staining per one standard deviation increment of CLA were calculated for each outcome.
Results
Patients
Between June 10, 2009 and March 30, 2011, 39 potential participants were identified. There were 8 screening failures: 4 were excluded due to poorly controlled hypertension, 2 were excluded because of abnormal liver function tests, 1 had an abnormal electrocardiogram, and 1 had concerns about privacy. Seven had breast cancer surgery before taking CLA for the required minimum 10 day period and were inevaluable for the primary study endpoint. The median age of evaluable study participants was 55 years (range 34–80). The median duration of CLA treatment was 12 days (range 9.5–20.5). Patient characteristics are shown in Table 2.
Table 2.
Characteristics of patients evaluable for the primary study endpoint
| Age (n = 23) | |
| Median | 55 |
| Range | 34–80 |
| Less than 50 (%) | 25 |
| 50–69 (%) | 67 |
| 70 or older (%) | 8 |
| Tumor Size (cm)a | |
| Median | 1.6 |
| Range | 0.55–8 |
| Histology of core biopsy (%) | |
| Invasive lobular carcinoma (ILCA) | 4 |
| ILCA with ductal carcinoma in situ features (ILCA with DCIS) | 4 |
| Invasive ductal carcinoma (IDCA) | 52 |
| IDCA with DCIS | 13 |
| IDCA with lobular features | 13 |
| DCIS | 4 |
| Lobular carcinoma in situ (LCIS) | 4 |
| Mucinous/Colloid | 4 |
| Histology of surgical specimen (%) | |
| Invasive lobular carcinoma (ILCA) | 4 |
| Invasive ductal carcinoma (IDCA) | 74 |
| IDCA with lobular features | 4 |
| IDCA with medullary features | 9 |
| Invasive pleomorphic carcinoma | 4 |
| Mucinous/colloid | 4 |
| Grade (ductal cancers only, %)b | |
| I | 9 |
| II | 35 |
| III | 43 |
| Not evaluable | 13 |
| Number of positive lymph nodes (%) | |
| None | 70 |
| 1–3 | 26 |
| 4–10 | 4 |
| 10 or more | 0 |
| Median | 0 |
| Estrogen receptor status (%)c | |
| Positive (+) | 73 |
| Negative (−) | 23 |
| Equivocal | 1 |
| Progesterone receptor status (%)c | |
| Positive (+) | 55 |
| Negative (−) | 36 |
| Equivocal | 9 |
| Her-2 score by FISH (%)d | |
| Negative | 79 |
| Positive | 8 |
| Unknown | 12 |
| Tumor side (%) | |
| Left | 48 |
| Right | 52 |
| Presence of necrosis (%) | |
| Yes | 29 |
| No | 42 |
| Not evaluable | 29 |
| Presence of calcifications (%) | |
| Yes | 29 |
| No | 42 |
| Not evaluable | 29 |
| Presence of vascular invasion (%) | |
| Yes | 23 |
| No | 77 |
Two patients are not included: one had 1.5 cm tumors right and left, one had three tumors on the right (2, 1, 0.8 cm)
Graded by the Nottingham system
One patient had two tumors and is not included: one ER/PR + and the other ER/PR−
Her-2 data were not available for 4 of the 23 patients
CLA capsule isomeric purity and content
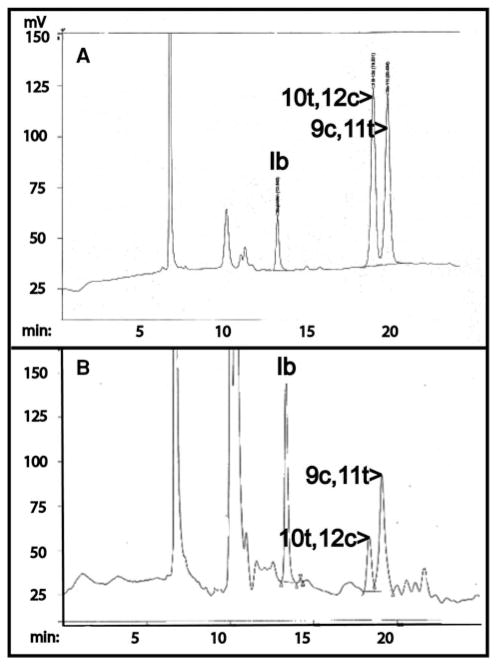
A representative chromatogram of the CLA batch sample analyzed by Ag+HPLC is shown in Fig. 1a. The capsules contained two major CLA peaks representing the 10t,12c and the 9c,11t isomers, as determined by co-elution of the peaks with pure isomer preparations (not shown). The relative representation was 47:53 for the 9c,11t- and 10t,12c-CLA isomers, very close to the 50:50 ratio described by the manufacturer. The average amount of CLA per capsule (n = 6) was 797.4 mg. All capsules used in the clinical trial were from the production batch analyzed in the chromatogram.
Fig. 1.
Ag+HPLC analysis of CLA capsules: The two isomer and internal standard peak identities were determined by assessment of pure standards. Ib indicates the ibuprofen internal standard. a Chromatography of a CLA gelcap extract. The CLA isomer peaks shown represent 300 nanograms loaded onto the column. b Chromatogram of a fasting plasma extract obtained on the morning of breast surgery. The two peaks representing the CLA isomers are readily detectable
Free CLA concentrations in plasma
We obtained fasting venous blood samples before initiation of CLA administration and on the morning of surgery for determination of plasma free CLA isomer concentrations. Mean concentrations of 10t, 12c-CLA were very low before CLA supplementation (0.11 ± 0.02 mg/L (±SEM)), with undetectable concentrations in 7 of 23 patients. In contrast, concentrations (mean ± SEM) of the 9c,11t-isomer were higher in baseline samples (0.58 ± 0.07 mg/L, p < 0.0001 compared to the baseline 10t,12c-CLA level). After CLA administration, fasting free CLA concentrations rose to 2.17 ± 0.08 and 1.10 ± 0.16 mg/L for 9c,11t- and 10t,12c-CLA, respectively (mean ± SEM; p < 0.0001 compared to baseline in each case). Increments varied considerably among individuals, with minimum and maximum values of 0.06/5.63 and 0.06/3.21 mg/L for the 9c,11t- and 10t,12c-isomers, respectively. A representative plasma chromatogram is shown in Fig. 1b, and the complete plasma CLA data set may be found in Supplementary data Table S1.
Expression of proteins related to lipid metabolism in tumor tissue
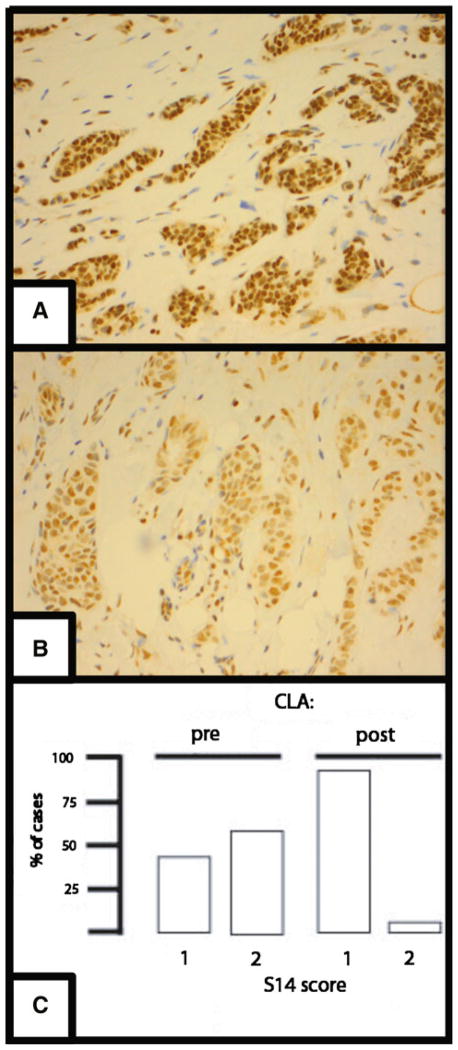
Immunohistochemical (IHC) staining for S14, FASN, and LPL was graded 0, 1, or 2. The numbers (%) of tumor samples receiving an IHC score for S14 pre-CLA treatment of 0, 1, or 2 were 0 (0),11 (46), and 13 (54), whereas the distribution after treatment was 0 (0), 22 (92), and 2 (8). An example of S14 IHC and a graphical presentation of the data are presented in Fig. 2. Notably, of the 10 tumors with an initial score of 1, none declined post-treatment, while of the 13 with a high baseline score of 2, 12 declined after CLA ingestion. We applied a logistic regression analysis to test the hypothesis that ER status was related to S14 reduction or Ki-67 reduction. This showed that odds for being ER− (95 % CI) increased by 1.61 (−0.55, 14.05) (p = 0.28) with one unit increase of S14.
Fig. 2.
S14 Immunohistochemistry. a Example of a breast tumor biopsy sample obtained before CLA administration and immunostained for S14. Brown pigment is immunostain, blue is hematoxylin counterstain. The staining is primarily nuclear and was given a score of 2. b A sample of the same tumor shown in panel A obtained at surgery, after CLA administration, and stained for S14, yielding a score of 1. c Distribution of breast tumor S14 immunohistochemical scores pre- and post-CLA administration. The percentage of cases with scores of 1 or 2 is presented: no case was scored at 0. Median scores declined significantly (p = 0.003 by exact McNemar test for paired binary data). Of the 10 tumors with an initial score of 1, none declined post-treatment, while of the 14 with a baseline score of 2, 13 declined after CLA ingestion
In contrast to the decline of S14, immunohistochemical scores for FASN and LPL did not statistically significantly change after CLA ingestion. The numbers (%) of tumor samples receiving an immunostaining score for FASN pre-CLA treatment of 0, 1, or 2 were 0 (0 %), 12 (50), 12 (50), whereas the distribution after treatment was 0 (0),14 (58), 10 (42) (McNemar’s p = 0.41). The numbers (%) of tumor samples receiving an immunostaining score for LPL pre-treatment of 0, 1, or 2 were 2 (8), 16 (67), and 6 (25), whereas the distribution after treatment was 0 (0), 19 (79), and 5 (21) (Bowker’s p = 0.48).
Markers of tumor cell proliferation and apoptosis
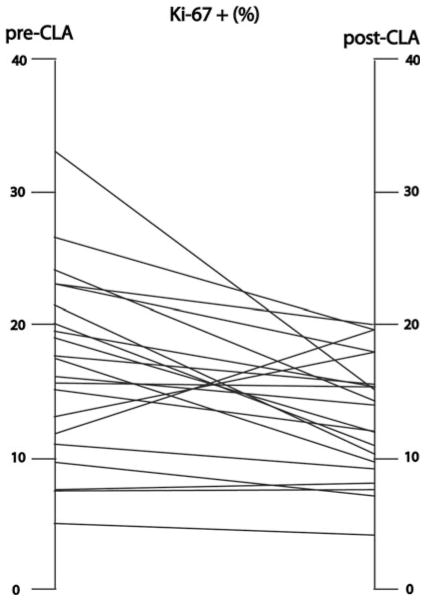
We estimated the status of tumor proliferation by calculating the percentage of cells expressing Ki-67. Baseline proliferation indices varied among cases, from a minimum of 2.6 % to a maximum of 33.0 %. Post-treatment indices varied between 3.7 % and 23.5 %, however, 16 cases showed a decline in Ki-67 expression as compared to the baseline value, while 5 showed an increase (Fig. 3). The signed rank test for paired data revealed this difference to be statistically significant (p = 0.029). Among cases showing a decline, the mean decrement was 6.4 %. Among the cases that showed an increase in Ki-67 after treatment, the S14 score did not change in 3 and fell in 2. Three were ER+, and 2 were ER−, and none showed Her2 amplification by FISH. We applied a logistic regression analysis to test the hypothesis that ER status was related to Ki-67 reduction. This showed that odds for being ER− (95 % CI) increased by 1.61 (−0.55, 14.05) (p = 0.28) with one unit increase of S14. The odds ratio (95 % CI) was 0.18 (0.009, 3.61) (p = 0.26) for log(Ki-67).
Fig. 3.
Ki-67 immunostaining of breast tumor tissue. Five separate microscopical fields from each sample were scanned digitally and the percentage of stained cells was computed. Each line connects the mean score for each tumor before, and at the conclusion of, CLA administration. Of the 21 pairs of tumor tissue that were evaluable for Ki-67, 16 showed a decline, while 5 demonstrated an increase, in the Ki-67 score (p = 0.029 by signed rank test). There was insufficient paired tumor tissue for analysis in 4 cases, while one patient had bilateral evaluable tumors
We assessed tumor cell apoptosis by immunostaining for cleaved caspase 3. The mean number of cleaved caspase 3 staining cells per microscope field did not significantly differ pre-/post CLA (1.16 ± 0.51 vs 1.37 ± 0.38; p = 0.61).
We applied ANOVA to test the association between the post-CLA log(Ki-67) score and that of S14, yielding a non-statistically significant p value (0.14). The same model was used to analyze the change of S14 and that of log(Ki-67) before and after CLA administration for those cases showing a decrease in the S14 score (p = 0.15).
We assessed for correlations between post-treatment concentrations of each CLA isomer, or the increment in the concentrations over the baseline concentration, with reductions in S14 or Ki-67 scores and found no statistically significant relationships between these variables. After categorizing change of S14 into three groups: −1 for decrease, 0 for the same, and 1 for increase, we used ANOVA to compare outcomes among the three groups. Pair-wise p-values were adjusted by the Tukey method. This yielded non-statistically significant adjusted p values for S14 and the final 9c,10t-CLA concentration (p = 0.44), S14 and the final 10t,12c-CLA concentration (p = 0.20), S14 and the increment in 9c,10t-CLA concentration (p = 0.47), and S14 and the increment in the 10t,12c-CLA concentration (p = 0.21). The analysis yielded non-significant Pearson’s correlation coefficients (r) values for Ki-67 scores and the final 9c,10t-CLA concentration (r = −0.28, p = 0.22), Ki-67 and the final 10t,12c-CLA concentration (r = −0.31, p = 0.17), Ki-67 and the increment in 9c,10t-CLA concentration (r = −0.27, p = 0.23), and Ki-67 and the increment in the 10t,12c-CLA concentration (r = −0.32, p = 0.16).
We also performed ANCOVA to examine the relationship between the final Serum CLA concentrations or the change in CLA concentrations pre- and post-treatment with responses of the categorical biomarker outcomes (S14, FASN, LPL). A similar analysis using linear regression models was performed for the continuous biomarker outcomes (caspase-3, Ki-67). We included BMI, tumor size, tumor grade, and number of positive lymph nodes as controlling variables. The results did not reveal any statistically significant relationships.
CLA toxicity and adverse effects
Most reported AE’s were Grade I in severity with nausea (15 %) and fatigue (11 %) being most common. The majority of Grade II/III AEs were determined to be unrelated to CLA administration. No Grade IV toxicities were observed. A tabulation of all AEs is found in supplemental Table S2.
Discussion
We conducted a proof of principle study with the primary objective of evaluating the effect of this CLA dose on selected biomarkers of FA metabolism (S14, FASN, LPL). The major findings in this clinical trial were that preoperative administration of at least a 10 day course of treatment with of 7.5 g/d CLA significantly reduced expression of S14 and reduced the proliferation marker Ki-67 in primary invasive breast cancer tissue. However, we noted that only those cases with the highest S14 IHC score (=2) in the pretreatment tumor tissue sample showed suppression. This suggests that the initial metabolic status of breast cancer cells may govern their response to short-term CLA at this dose.
Compared to normal mammary epithelium, the majority of breast cancers exhibit high levels of FA-synthesizing enzymes and demonstrate dependence on FA for growth and survival [2]. The product of the S14 gene is a nuclear protein that has been shown to promote expression of genes coding FA-synthesizing enzymes, including FASN, in cultured human breast cancer and hepatoma cells, as well as in primary cultures of rat hepatocytes [16–18]. We have previously demonstrated that S14 knockdown has anti-proliferative and pro-apoptotic effects in breast cancer cell lines [4], and that high S14 expression in primary human breast tumors strongly predicts the likelihood of recurrence and reduced survival. Indeed, no tumor with low S14 expression recurred with prolonged follow-up [19].
In the current study, CLA suppressed expression of S14, but not of FASN or LPL. We acknowledge the relatively small size and the relatively short duration of treatment as limitations to our study. In bovine mammary and mouse adipose tissue, CLA had been shown to inhibit expression of both the S14 and FASN genes [11, 20]. In several other models, including primary rat hepatocytes, intact rodent liver, and breast cancer cells, conditions that reduce S14 expression are also associated with reduced expression of the lipogenic enzymes, including FASN [4, 16, 17, 21]. In these systems, S14 gene expression was more profoundly inhibited than was observed in our clinical trial, perhaps secondary to a dose/concentration effect. Further exploitation of this suppression in vivo may require an escalating dosing study design.
In contrast to the data from cultured cells, levels of FASN mRNA and enzyme activity in a variety of tissues, including mammary gland, from an S14 knock-out mouse were normal, while mammary FA synthesis was abrogated [22]. It is therefore possible that FA synthesis was reduced in the breast cancers of our patients with reduced post-treatment S14 expression despite the preserved levels of FASN protein as evaluated by IHC. This is consistent with a recent report that S14 may directly modulate the activity of cytosolic acetyl CoA-carboxylase, the rate-limiting enzyme of FA synthesis, and thus could down-regulate FA synthesis independently of lipogenic enzyme content by this mechanism [23]. Analysis of an animal model of breast cancer will thus be required to define the relationships between S14, FASN, and lipogenesis in breast cancer tissue exposed to CLA.
In addition to de novo FA biosynthesis, breast cancers may utilize diet-derived fat from the bloodstream, and we have demonstrated that LPL is universally expressed in breast tumors [3]. In addition to the suppressing S14 and FASN mRNA levels, CLA has been shown to suppress LPL mRNA content in bovine mammary and mouse adipose tissues [11, 20]. LPL expression was not suppressed, however, by CLA treatment in this clinical study. S14 and LPL may be independently regulated and/or may exhibit different dose–response relationships to CLA.
A 1:1 mix of c9, t11 and t10, c12 CLA isomers is marketed as a dietary supplement that is widely used to promote weight loss in humans. A 2 year clinical study in healthy volunteers showed reduced adiposity with no toxicity with CLA doses up to 7.5 g/day [15], and the current short-term study was consistent with those previously published data.
It is likely that the orally-ingested CLA is handled as other FAs, by esterification in the gut, export in lipoprotein particles, delivery to adipose and other tissues, and subsequent export from adipose stores during fasting. Given this complex pharmacology, it not surprising that circulating fasting CLA levels did not correlate with the pharmacodynamic effects.
Paradoxically, concerns about enhanced mammary epithelial proliferation and accelerated tumorigenesis in mouse breast cancer models has been suggested by studies feeding 0.5–1.0 % 10t,12c-CLA to MMTV-Her2 [24] or MMTV-PyMT [25] mice. In these studies, CLA was administered as a potential chemopreventive agent, rather than as a potential therapy for established tumors. Further recent work using mice, however, indicated that mammary epithelial proliferation does not occur with diets lower in CLA (~0.2 %), which nonetheless are sufficient to suppress lipid metabolism [26]. Species- and dose-related differences in the impact of CLA on mammary epithelial cells and tumorigenesis therefore remain to be resolved.
Our overall findings indicate that a short course of CLA did reduce expression of S14 and Ki-67 without significant toxicity. Based on these results, CLA may represent a prototype drug for targeting the dependence of breast tumors on FA. This metabolic dependency should be explored as it opens up the possibility of a future line of novel investigative drugs for breast cancer management.
Supplementary Material
Acknowledgments
Support was provided by a Norris Cotton Cancer Center Prouty Grant (WK, BE), a grant from the National Cancer Institute P30CA023108 (LDL), and NIH grant RO1CA126618 (WK). Immunohistochemistry was performed by Rebecca O’Meara HT (ASCP) in the Department of Pathology Translational Research Shared Resource Laboratory of the Geisel School of Medicine at Dartmouth, the Dartmouth Hitchcock Medical Center and the Norris Cotton Cancer Center.
Abbreviations
- Ag+HPLC
Silver ion high pressure liquid chromatography
- CLA
Conjugated linoleic acid
- FA
Fatty acid
- FASN
Fatty acid synthase
- IHC
Immunohistochemistry
- LPL
Lipoprotein lipase
- S14
Spot 14, aka THRSP
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-013-2446-9) contains supplementary material, which is available to authorized users.
Conflict of interest None of the authors discloses any conflict of interest.
Contributor Information
Margit M. McGowan, Section of Hematology/Oncology, Department of Medicine, Dartmouth-Hitchcock Medical Center (MM & GS) and White River Junction VA Hospital (NK), and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Burton L. Eisenberg, Section of Surgical Oncology, Department of Surgery, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Lionel D. Lewis, Section of Clinical Pharmacology, Department of Medicine, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Heather M. Froehlich, Department of Pathology, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Wendy A. Wells, Department of Pathology, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Alan Eastman, Dept of Pharmacology, The Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA.
Nancy B. Kuemmerle, Section of Hematology/Oncology, Department of Medicine, Dartmouth-Hitchcock Medical Center (MM & GS) and White River Junction VA Hospital (NK), and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Kari M. Rosenkrantz, Section of Surgical Oncology, Department of Surgery, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Richard J. Barth, Jr., Section of Surgical Oncology, Department of Surgery, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Gary N. Schwartz, Section of Hematology/Oncology, Department of Medicine, Dartmouth-Hitchcock Medical Center (MM & GS) and White River Junction VA Hospital (NK), and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Zhongze Li, The Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA.
Tor D. Tosteson, Dept of Family and Community Medicine, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
Bernard B. Beaulieu, Jr., Section of Clinical Pharmacology, Department of Medicine, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756, USA
William B. Kinlaw, Email: William.B.Kinlaw.III@Dartmouth.EDU, Section of Endocrinology, Department of Medicine, Dartmouth-Hitchcock Medical Center and the Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, 606 Rubin Building, Lebanon, NH 03756, USA
References
- 1.American Cancer Society, I. Breast Cancer Facts & Figures 2011–2012. American Cancer Society; 2012. [Google Scholar]
- 2.Menendez J, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 3.Kuemmerle N, Rysman E, Lombardo P, Flanagan A, Lipe B, Wells W, Pettus J, Memoli V, Morganelli P, Swinnen J, Timmerman L, Chaychi L, Eisenberg B, Coleman W, Kinlaw W. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel P, Bingham C, McGraw C, Baker C, Morganelli P, Meng M, Jemal A, Moncur J, Kinlaw WB. S14 protein in breast cancer cells: direct evidence for regulation by SREBP-1c, superinduction with progestin, and implication in cell growth. Exp Cell Res. 2005;312:278–288. doi: 10.1016/j.yexcr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Eheman C, Henley J, Ballard-Barbash R, Jacobs E, Schymura M, Noone A, Pan L, Anderson R, Fulton J, Kohler B, Jemal A, Ward E, Plescia M, Ries L, Edwards B. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient activity. Cancer. 2012;118:2238–2266. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chlebowski R. Obesity and breast cancer: adding to the evidence. J Clin Oncol. 2012;30:126–128. doi: 10.1200/JCO.2011.39.7877. [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski R, Blackburn G, Hoy M, Thomson C, Giuliano A, McAndrew P, Hudis C, Butler J, Shapiro A, Elashoff R. Survival analyses from the women’s intervention nutrition study (WINS) evaluating dietary fat reduction and breast cancer outcome. J Clin Oncol. 2008 May 20;26(suppl):abstr 522. [Google Scholar]
- 8.Pierce J, Natarajan L, Caan B, Parker B, Greenberg E, Flatt S, Rock C, Kealy S, Al-Delaimy W, Bardwell W, Carlson R, Emond J, Faerber S, Gold E, Hajek R, Hollenbach K, Jones L, Karanja N, Madlensky L, Marshall J, Newman V, Ritenbaugh C, Thomson C, Wasserman L, Stefanick M. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the women’s healthy eating and living (WHEL) randomized trial. JAMA. 2007;298:289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ip C, Singh M, Thompson H, Scimeca J. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–1215. [PubMed] [Google Scholar]
- 10.Ip C, Dong Y, Ip M, Banni S, Carta G, Angioni E, Murru E, Spada S, Melis M, Saebo A. Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer. 2002;43:52–58. doi: 10.1207/S15327914NC431_6. [DOI] [PubMed] [Google Scholar]
- 11.Harvatine K, Bauman D. SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J Nutr. 2006;136:2468–2474. doi: 10.1093/jn/136.10.2468. [DOI] [PubMed] [Google Scholar]
- 12.Hughes D, Martel P, Kinlaw W, Eisenberg B. The synthetic triterpenoid CDDO-Im inhibits fatty acid synthase expression and has antiproliferative and proapoptotic effects in human liposarcoma cells. Cancer Invest. 2007;26:118–127. doi: 10.1080/07357900701522612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly C, Olsen A, Lewis L, Eisenberg B, Eastman A, Kinlaw W. Conjugated linoleic acid (CLA) inhibits expression of the Spot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells. Nutr Cancer. 2009;61:114–122. doi: 10.1080/01635580802348666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blankson H, Stakkestad J, Fagertun H, Thom E, Wadstein J, Gudmunsin O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000;130:2943–2948. doi: 10.1093/jn/130.12.2943. [DOI] [PubMed] [Google Scholar]
- 15.Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135:778–784. doi: 10.1093/jn/135.4.778. [DOI] [PubMed] [Google Scholar]
- 16.Brown SB, Maloney M, Kinlaw WB. “Spot 14” protein functions at the pretranslational level in the regulation of hepatic metabolism by thyroid hormone and glucose. J Biol Chem. 1997;272:2163–2166. [PubMed] [Google Scholar]
- 17.Moreau A, Teruel C, Beylot M, Albalea V, Tomasi V, Umbdenstock T, Parmentier Y, Sa-Cahuna A, Suc B, Fabre J, Navarro F, Ramos J, Meyer U, Maurel P, Vilarem M, Pascussi J. A novel pregnane X receptor and S14-mediated lipogenic pathway in human hepatocyte. Hepatology. 2009;49:2068–2079. doi: 10.1002/hep.22907. [DOI] [PubMed] [Google Scholar]
- 18.Kinlaw W, Quinn J, Wells W, Roser-Jones C, Moncur J. S14 in breast cancer: a marker of aggressive disease and a potential therapeutic target. Endocrinology. 2006;147:4048–4055. doi: 10.1210/en.2006-0463. [DOI] [PubMed] [Google Scholar]
- 19.Wells W, Schwartz G, Morganelli P, Cole B, Chambers J, Kinlaw WB. Expression of “Spot 14” (THRSP) predicts disease free survival in invasive breast cancer: immunohistochemical analysis of a new molecular marker. Breast Cancer Res Treat. 2006;98:231–240. doi: 10.1007/s10549-005-9154-z. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Storkson J, Kim S, Sugimoto K, Park Y, Pariza M. Short-term intake of conjugated linoleic acid inhibits lipoprotein lipase and glucose metabolism but does not enhance lipolysis in mouse adipose tissue. J Nutr. 2003;133:663–667. doi: 10.1093/jn/133.3.663. [DOI] [PubMed] [Google Scholar]
- 21.Kinlaw W, Church J, Harmon J, Mariash C. Direct evidence for a role of the “spot 14” protein in the regulation of lipid synthesis. J Biol Chem. 1995;270:16615–16618. doi: 10.1074/jbc.270.28.16615. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Q, Anderson G, Mucha G, Parks E, Metkowski J, Mariash C. The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology. 2005;146:3343–3350. doi: 10.1210/en.2005-0204. [DOI] [PubMed] [Google Scholar]
- 23.Colbert C, Kim C, Moon Y, Henry L, Palnitkar M, McKean W, Fitzgerald K, Deisenhofer J, Horton J, Kwon H. Crystal structure of Spot 14, a modulator of fatty acid synthesis. PNAS. 2010;107:18820–18825. doi: 10.1073/pnas.1012736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ip M, Sibel O, Masso-Welch A, Ip C, Meng X, Ou L, Shoemaker S. The t10, c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis. 2007;28:1269–1276. doi: 10.1093/carcin/bgm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flowers M, Schroeder J, Borowsky A, Besselsen D, Thomson C, Pandey R, Thompson P. Pilot study on the effects of dietary conjugated linoleic acid on tumorigenesis and gene exprtession in PyMT transgenic mice. Carcinogenesis. 2010;31:1642–1649. doi: 10.1093/carcin/bgq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foote M, Giesy S, Bernal-Santos G, Bauman D, Boisclair Y. t10, c12-CLA decreases adiposity in peripubertal mice without dose-related detrimental effects on mammary development, inflammation 3 status and metabolism. Am J Physiol-Regulatory Intergrative & Comparative Physiol. 2010;299:R1521–R1528. doi: 10.1152/ajpregu.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.