Abstract
Despite numerous examples of the effects of the human gastrointestinal microbiome on drug efficacy and toxicity, there is often an incomplete understanding of the underlying mechanisms. Here, we dissect the inactivation of the cardiac drug digoxin by the gut Actinobacterium Eggerthella lenta. Transcriptional profiling, comparative genomics, and culture-based assays revealed a cytochrome-encoding operon up-regulated by digoxin, inhibited by arginine, absent in non-metabolizing E. lenta strains, and predictive of digoxin inactivation by the human gut microbiome. Pharmacokinetic studies using gnotobiotic mice revealed that dietary protein reduces the in vivo microbial metabolism of digoxin, with significant changes to drug concentration in the serum and urine. These results emphasize the importance of viewing pharmacology from the perspective of both our human and microbial genomes.
Main Text
Humans are home to large and diverse microbial communities, the most abundant of which resides in the gastrointestinal tract. Recent studies have highlighted the clinical relevance of the biotransformations catalyzed by the human gut microbiome, including alterations to the bioavailability, activity, and toxicity of therapeutic drugs (1, 2). Although >40 drugs are metabolized by the gut microbiome, little is known about the underlying mechanisms. This knowledge is critical to enable the rational design of pharmaceutical or dietary interventions.
The inactivation of the cardiac drug digoxin provides a promising starting point for understanding microbial drug metabolism. Digoxin and other cardiac glycosides have been widely used for hundreds of years to treat heart failure and arrhythmias. Therapeutic effects are accomplished indirectly when inhibition of the Na+/K+ ATPase in cardiac myocytes raises the intracellular Ca2+ concentration (3). Digoxin has a narrow therapeutic range (0.5–2.0 ng/mL) (3), and some patients excrete the inactive digoxin metabolite, dihydrodigoxin, in which the lactone ring is reduced (fig. S1A) (4). This modification disrupts ring planarity, which is thought to shift positioning within the binding pocket of the Na+/K+ ATPase, resulting in decreased target affinity (5). Co-administration of broad spectrum antibiotics increases serum digoxin (4), and Eggerthella lenta reduces digoxin in vitro (6). Prior to this work, the molecular mechanism of digoxin reduction and the factors that alter microbial drug inactivation in vivo were unknown.
We confirmed that E. lenta DSM2243, the type strain, reduces digoxin in vitro (7), and that arginine inhibits this reaction (Fig. 1A). The growth of E. lenta DSM2243 was stimulated by arginine supplementation (Fig. 1A,S2), indicative of using the arginine dihydrolase pathway for ATP (8). Citrulline (an intermediate upstream of ATP production) stimulated growth, whereas ornithine (an end product) did not (figs. S2,S3).
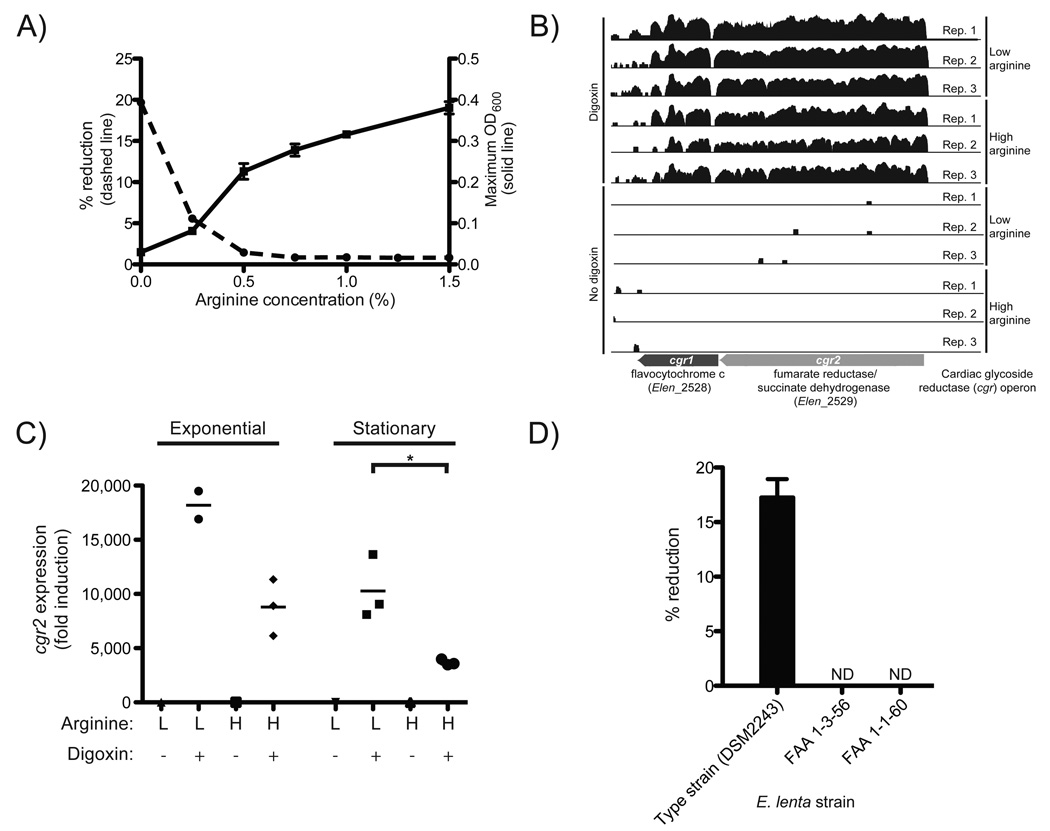
Fig. 1. Discovery of a bacterial operon induced by digoxin.
(A) Arginine stimulates the growth of E. lenta DSM2243 in vitro while blocking the reduction of digoxin. Maximum OD600 (solid line; values are the mean±sem; n=3) and digoxin % reduction efficiency (dashed line; values are the mean; n=2) after 48 hours of growth. (B) RNA-Seq profiles of the cardiac glycoside reductase (cgr) operon are shown with/without digoxin during exponential growth in medium containing low/high arginine. The height is proportional to the natural log of the number of unambiguous sequencing reads mapped to each base. (C) cgr2 transcription as determined by qRT-PCR. Asterisks indicate statistical significance by Student’s t test (P<0.05). Horizontal lines are the mean; n=2–3. (D) Identification of 2 strains of E. lenta incapable of reducing digoxin. Values are the mean±sem; n=3. ND=no reduction detected.
E. lenta cultures were grown anaerobically in rich medium supplemented with low- and high-levels of arginine (0.25% and 1.25%, respectively) in the presence or absence of digoxin (10 µg/mL) and we performed RNA-Seq on the resultant cellular biomass (figs. S4–6, table S1). A two-gene operon was highly up-regulated after exposure to digoxin during exponential growth (>100-fold; Fig. 1B, tables S2,S3). These two genes, referred to here as the cardiac glycoside reductase (cgr) operon (gene labels: cgr1 and cgr2), encode proteins that are homologous to bacterial cytochromes and are therefore potentially capable of using digoxin as an alternative electron acceptor. Incubation of E. lenta with multiple cardiac glycosides and their reduced forms revealed that the cgr operon is broadly responsive to compounds with an α,β-unsaturated butyrolactone ring (figs. S7–9, table S5).
Digoxin induction was increased in low arginine conditions during both exponential and stationary phase, relative to cultures exposed to high levels of arginine (fig. S10A,B). cgr induction by digoxin, and the growth phase-dependent effects exerted by arginine were confirmed on independent samples using qRT-PCR (Figs. 1C,S7C, table S4). Unlike arginine, ornithine did not repress cgr2 expression (fig. S11). These results are consistent with the hypothesis that arginine represses cgr operon expression, thereby inhibiting digoxin reduction.
Next, we tested three strains of E. lenta (DSM2243, FAA 1-3-56, and FAA 1-1-60) (9, 10), for digoxin reduction; the type strain was the sole strain capable of digoxin reduction in vitro (Fig. 1D). Comparative genomics revealed that the type strain was nearly indistinguishable from the other two strains using common marker genes (fig. S12). Reciprocal BLASTP comparisons of all protein-coding sequences of the three fully sequenced E. lenta strains revealed that the type strain shared 79.4% and 90.5% of its proteome with strains FAA 1-3-56 and FAA 1-1-60, respectively (fig. S12). The cgr operon was unique to the type strain (table S6); furthermore, the two non-reducing E. lenta strains were missing three genomic loci which were also up-regulated by digoxin, and are predicted to encode membrane transporters for the uptake of small molecules and glycosides (fig. S13). Arginine did not significantly decrease the expression level of these transporters (fig. S14).
Strain-level variation provides an explanation for the difficulties in predicting dihydrodigoxin levels in cardiac patients by the presence or absence of E. lenta (6, 11). We used qPCR to measure the relative abundance of the cgr operon to the E. lenta 16S rRNA gene (the “cgr ratio”) in microbial community DNA from 20 unrelated healthy people, along with ex vivo digoxin reduction assays. The results stratified our cohort into low reducers (12.82±10.68% reduction; n=6) and high reducers (96.25±7.69% reduction; n=14) (Fig. 2A). The cgr ratio was significantly increased for the high reducers (1.058±0.562) when compared to low reducers (0.425±0.582; P<0.05, Student’s t test) (Fig. 2B,S15). Linear regression of reduction efficiency with the cgr ratio revealed a significant correlation (R2=0.22, P<0.05), whereas the abundance of E. lenta failed to predict the extent of reduction (R2=0.06, P=0.30). The optimal cgr ratio cutoff (0.6) predicted digoxin reduction efficiency with a sensitivity of 86%, specificity of 83%, and precision of 92%.
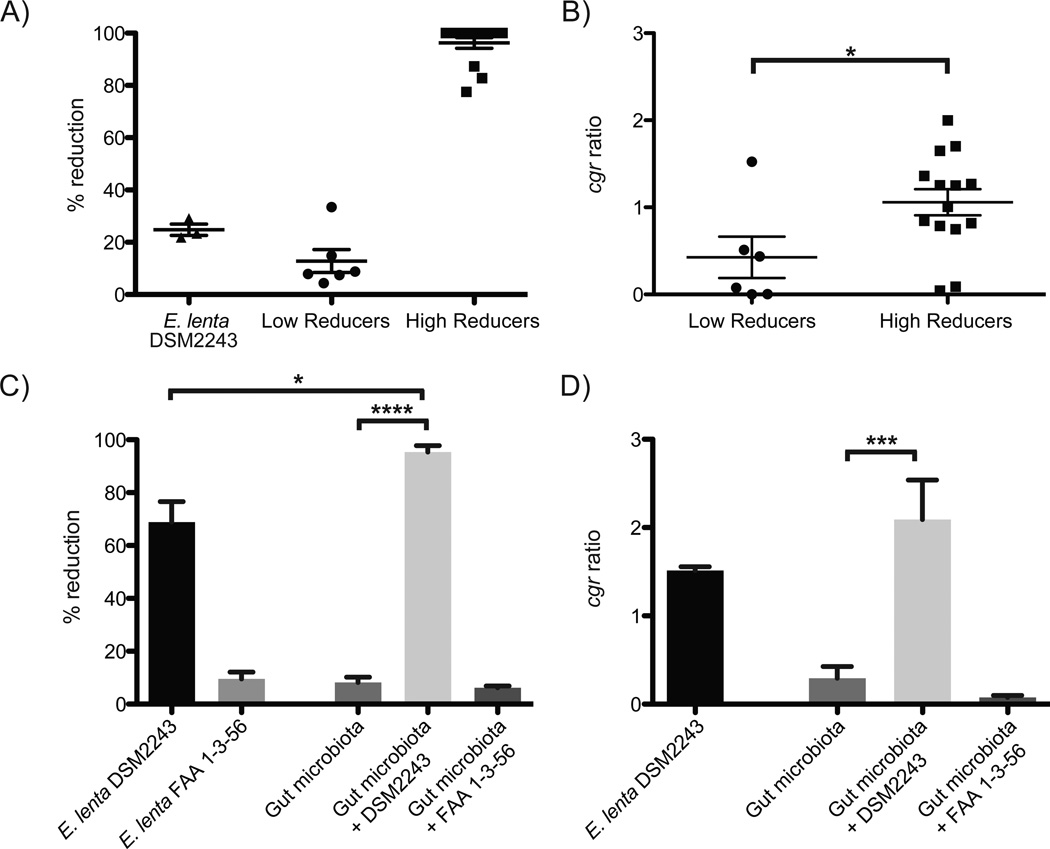
Fig. 2. A microbial biomarker predicts the inactivation of digoxin.
(A) Liquid chromatography/mass spectrometry (LC/MS) was used to quantify digoxin reduction in the fecal microbiomes of 20 unrelated individuals. (B) The cgr ratio was significantly different between low and high reducers. Data represent qPCR using the cgr2 gene, and E. lenta specific 16S rDNA primers (table S4). (C) Five low reducing fecal microbial communities were incubated for five days in the presence or absence of E. lenta DSM2243 or FAA 1-3-56. LC/MS was used to quantify the completion of digoxin reduction. Supplementation with the non-reducing strain of E. lenta did not significantly affect digoxin reduction efficiency. (D) The cgr ratio was obtained for each of the low reducing microbial communities post incubation. Outliers were identified using Grubbs’ test (P<0.01) and removed. Values are the mean±sem. Points in A,B represent biological replicates. Asterisks indicate statistical significance by Student’s t test (*=P<0.05; ***=P<0.001; ****=P<0.0001).
Co-culture of E. lenta with the fecal microbiome enhanced the efficiency of digoxin reduction. Each low-reducing fecal samples was incubated with the type (reducing) and FAA 1-3-56 (non-reducing) strains of E. lenta. The communities incubated with the type strain reduced more digoxin (95.39±2.41%) than the type strain alone (68.91±7.70%; P<0.05, Mann-Whitney test) (Fig. 2C). The cgr ratio was significantly elevated after co-culture (Fig. 2D), and was tightly linked to reduction efficiency (R2=0.74, P<0.0001). An explanation for the observed microbial synergy is that the fastidious growth of E. lenta is promoted by growth factors supplied by the gut microbiota, a phenomena that is known to impact the metabolism of environmental pollutants by soil microbial communities (12), along with competition for arginine that boosts digoxin reduction by E. lenta. Consistent with these hypotheses, the abundance of the E. lenta type strain was significantly increased in the presence of a complex microbial community (1.6e6±4.8e5 vs. 1.8e5±8.4e3 in isolation; P<0.05, Mann-Whitney test), and arginine supplementation suppressed the reduction of digoxin during co-culture (S16).
Diet could also explain inter-individual variations in digoxin reduction. In vitro growth of E. lenta showed that while arginine stimulated cell growth, it decreased cgr operon expression, and prevented the conversion of digoxin to dihydrodigoxin (Figs. 1A,C,S10). These observations led us to hypothesize that increased consumption of dietary protein, and the corresponding increase in arginine, would inhibit the in vivo reduction of digoxin by E. lenta. Germ-free adult male Swiss-Webster mice were colonized with the type strain prior to being fed diets differing only in the amount of total protein (n=5 mice/group; tables S7,S8; fig. S17A). E. lenta colonized mice on both diets (fig. S18A), and exhibited high levels of expression of the cgr operon (fig. S18B). Quantification of serum and urine digoxin (7) revealed significant increases on the high protein diet, indicative of suppressed digoxin reduction by E. lenta (Fig. 3A,B). These trends were also consistent with fecal analysis of samples from each group of mice 4–16 hours following digoxin administration (Fig. 3). We also confirmed that the high protein diet significantly elevated the level of amino acids in the distal small intestine (7), resulting in a fold increase of 1.71±0.06 (p<0.001, Wilcoxon test; tables S9,10).
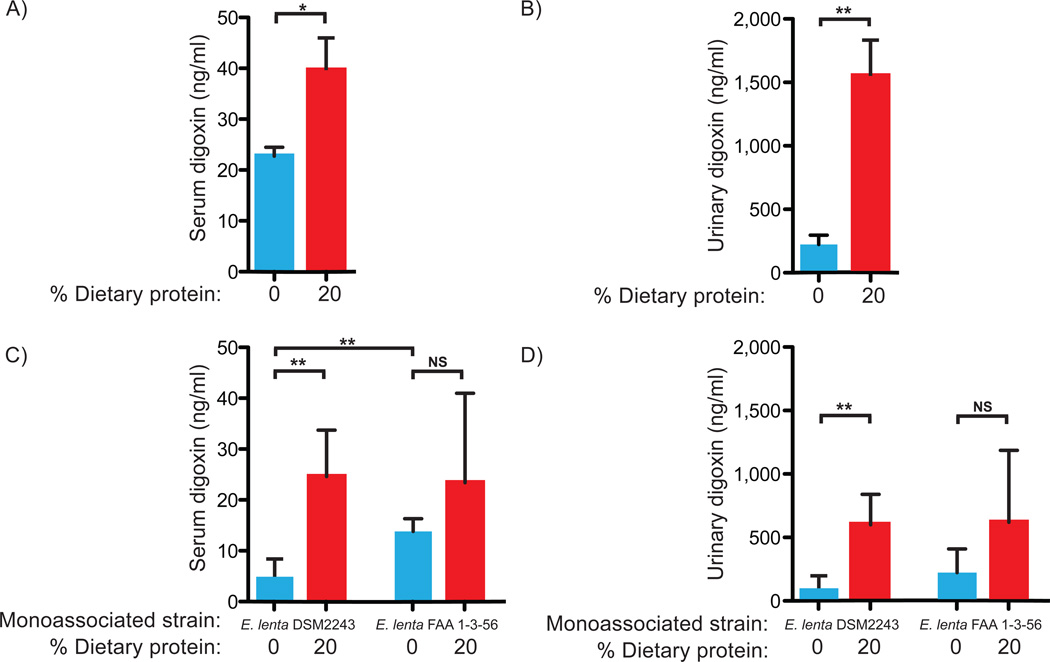
Fig. 3. Dietary protein blocks the inactivation of digoxin.
Serum (A) and urinary (B) digoxin levels from the type strain experiment. Fecal digoxin levels showed a consistent trend: the mean area under the curve was 6.226 ng digoxin*h/mL in germ-free mice, 3.576 for mice on the 0% protein diet, and 6.364 for mice on the 20% protein diet. Serum (C) and urinary (D) digoxin levels from each group. Digoxin levels were quantified by ELISA (7). Values are the mean±sem. Asterisks indicate statistical significance by Student’s t test (*=P<0.05; **=P<0.01). n=4–5 mice/group. NS=not significant.
We controlled for the indirect effects of host diet and colonization that might alter digoxin pharmacokinetics irrespective of reduction by E. lenta. Germ-free mice were colonized with either the digoxin-reducing type strain or the non-reducing FAA 1-3-56 strain, and subsequently fed the same two diets (fig. S17B). As seen before, we detected colonization with both strains, high cgr operon expression, and elevated serum and urine digoxin on the high protein diet for mice colonized with the type strain (Figs. 3C,D,S18C,D). Diet did not significantly impact the serum or urine digoxin levels of mice colonized with the non-reducing strain (Fig. 3C,D). Serum digoxin was significantly lower in mice colonized with the type strain on the 0% protein diet, relative to those colonized with the non-reducing strain (4.91±1.56 ng/mL vs. 13.8±1.25; P<0.01, Student’s t test, Fig. 3C). Together, these results suggest that the increased level of free amino acids available to E. lenta inhibited the activity of the cgr operon, increasing the bioavailability of digoxin.
An expanded model of digoxin pharmacokinetics is now emerging: colonization by distinct strains of E. lenta, microbial interactions, and host diet act together to influence drug levels (fig. S19). Follow-up studies in cardiac patients are necessary to determine if rapid qPCR-based biomarker assessments of the gut microbiome can guide dosage regimes. It may also be possible to provide dietary guidelines or supplements that prevent microbial drug metabolism. More broadly, our results emphasize that a comprehensive view of pharmacology includes the structure and activity of our resident microbial communities, and a deeper understanding of their interactions with each other, with their host habitat, and with the nutritional milieu of the gastrointestinal tract.
Supplementary Material
Acknowledgements
Bogdan Budnik and Sunia Trauger for LC/MS analyses; Vladimir Yeliseyev, Alice Liou, and Rachel Carmody for mouse studies; Claire Reardon and Christian Daly for sequencing support; Corinne Maurice, Lawrence David, Rachel Dutton, Ben Wolfe, Julie Button, Marie Elliot, Yves Falanga, Richard Losick, Andrew Murray, and Bodo Stern for helpful discussions. Mouse experiments were done with the generous support of the Harvard Digestive Diseases Center and the University of North Carolina gnotobiotic cores. This work was supported by grants from the National Institutes of Health (P50 GM068763) and the Harvard Digestive Diseases Center (2P30DK034854-26). HJH is supported by the Canadian Institutes of Health Research (MFE-112991). RNA-Seq data is deposited in the Gene Expression Omnibus (GEO) database (accession GSE43919).
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Haiser HJ, Turnbaugh PJ. Is it time for a metagenomic basis of therapeutics? Science. 2012;336:1253–1255. doi: 10.1126/science.1224396. [DOI] [PubMed] [Google Scholar]
- 2.Wallace BD, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman LS, et al. Goodman & Gilman's the pharmacological basis of therapeutics. ed. 12th. New York: McGraw-Hill; 2011. [Google Scholar]
- 4.Lindenbaum J, et al. Inactivation of digoxin by the gut flora: reversal by antibiotic therapy. N. Engl. J. Med. 1981;305:789–794. doi: 10.1056/NEJM198110013051403. [DOI] [PubMed] [Google Scholar]
- 5.Farr CD, et al. Three-dimensional quantitative structure-activity relationship study of the inhibition of Na(+),K(+)-ATPase by cardiotonic steroids using comparative molecular field analysis. Biochemistry. 2002;41:1137–1148. doi: 10.1021/bi011511g. [DOI] [PubMed] [Google Scholar]
- 6.Dobkin JF, et al. Digoxin-inactivating bacteria: identification in human gut flora. Science. 1983;220:325–327. doi: 10.1126/science.6836275. [DOI] [PubMed] [Google Scholar]
- 7.Materials and methods are available as supplementary materials on Science Online.
- 8.Sperry JF, Wilkins TD. Arginine, a growth-limiting factor for Eubacterium lentum. J. Bacteriol. 1976;127:780–784. doi: 10.1128/jb.127.2.780-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders E, et al. Complete genome sequence of Eggerthella lenta type strain (IPP VPI 0255) Stand. Genomic Sci. 2009;1:174–182. doi: 10.4056/sigs.33592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson KE, et al. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathan VI, Wiederman J, Dobkin JF, Lindenbaum J. Geographic differences in digoxin inactivation, a metabolic activity of the human anaerobic gut flora. Gut. 1989;30:971–977. doi: 10.1136/gut.30.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maymo-Gatell X, Chien Y, Gossett JM, Zinder SH. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- 13.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ning Z, Cox AJ, Mullikin JC. SSAHA: a fast search method for large DNA databases. Genome Res. 2001;11:1725–1729. doi: 10.1101/gr.194201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Let.t. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 16.Untergasser A, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiele B, et al. Analysis of amino acids without derivatization in barley extracts by LC-MS-MS. Anal. Bioanal. Chem. 2008;391:2663–2672. doi: 10.1007/s00216-008-2167-9. [DOI] [PubMed] [Google Scholar]
- 19.Okarma TB, Tramell P, Kalman SM. The surface interaction between digoxin and cultured heart cells. J. Pharmacol. Exp. Ther. 1972;183:559–576. [PubMed] [Google Scholar]
- 20.Gabel LP, Bihler I, Dresel PE. Induction of failure in gas-perfused hearts by intermittent administration of Krebs solution. The effect of digitalis glycosides. Circ. Res. 1967;21:263–269. doi: 10.1161/01.res.21.3.263. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs WA, Hoffmann A. The relationship between the structure and the biological action of the cardiac glucosides. J. Biol. Chem. 1927;74:482–486. [Google Scholar]
- 22.Brown BT, Wright SE. Hydrogenation of Digitalis Genins and Anhydrogenins. J. Pharm. Pharmacol. 1961;13:262–267. [Google Scholar]
- 23.Qazzaz HMAM, El-Masri MA, Valdes R. Secretion of a lactone-hydrogenated ouabain-like effector of sodium, potassium-adenosine triphosphatase activity by adrenal cells. Endocrinology. 2000;141:3200–3209. doi: 10.1210/endo.141.9.7664. [DOI] [PubMed] [Google Scholar]
- 24.Belz GG, Breithaupt-Grogler K, Osowski U. Treatment of congestive heart failure--current status of use of digitoxin. Eur. J. Clin. Invest. 2001;31(Suppl 2):10–17. doi: 10.1046/j.1365-2362.2001.0310s2010.x. [DOI] [PubMed] [Google Scholar]
- 25.Furstenwerth H. Ouabain - the insulin of the heart. Int. J. Clin. Pract. 2010;64:1591–1594. doi: 10.1111/j.1742-1241.2010.02395.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.