Abstract
Superficial siderosis is caused by recurrent haemorrhage in the subarachnoid space leading to haemosiderin deposition. It typically causes the triad of ataxia, deafness and myelopathy. We report a patient who developed superficial siderosis following neurosurgery for syringomyelia and who had an improvement in his hearing and mobility following treatment with a new iron chelation therapy that can penetrate the blood–brain barrier. It provides an intriguing insight into a therapy that could potentially modify the course of this rare neurodegenerative disorder. Further studies are required to assess the clinical efficacy of deferiprone in superficial siderosis.
Background
A 65 year old patient was diagnosed with superficial siderosis after presenting to our hospital with an insidious onset of deafness and ataxia.
Our article includes an MRI of the brain of the patient that illustrates the classical radiological appearances of this disease.
There is currently no available treatment for superficial siderosis but our patient had an improvement in his ataxia and a transient improvement in his hearing with the iron chelation agent deferiprone.
Case presentation
A male patient presented in May 2006, aged 65 years, with an 8-week history of progressive bilateral lower limb numbness, ataxia, headaches, intermittent vertigo and left-sided hearing impairment with pulsatile tinnitus. He had a history of syringomyelia, treated by a syringosubarachnoid shunt in 1994 and shunt removal and extensive laminectomy in 1996, leaving him with mild allodynia in his arms but no other deficit. On examination his gait was markedly ataxic, and he was unable to tandem walk. He had left-sided sensorineural hearing loss. His long-standing upper limb allodynia was unchanged. He had a mild (4–4+) spastic paraparesis with brisk lower limb reflexes and extensor plantar responses. He had heel-shin ataxia bilaterally. Sensory examination of the lower limbs was normal and Romberg's sign was negative.
His ataxia progressed over the next 4 years with frequent falls, and he became dependent on a rollator. His sensorineural hearing loss worsened and became bilateral, so that he became dependent on lip-reading and beyond the reach of hearing aids. His headaches persisted and he developed somnolence, anosmia, fatigue, urinary urgency and incontinence.
Investigations
MRI of the brain revealed striking hypointensity on T2-weighted sequences along the brain surface, most markedly in the brainstem and vermis, consistent with superficial siderosis (see figure 1). MRI of the spine showed long-standing postoperative appearances with some residual syrinx from C7 to T2. Cerebrospinal fluid (CSF) testing demonstrated elevated protein of 1.2 g/L, 19 000 red cells/cm3 and xanthchromia. Two separate four-vessel angiograms of his brain and spinal cord did not identify any abnormal vessels.
Figure 1.
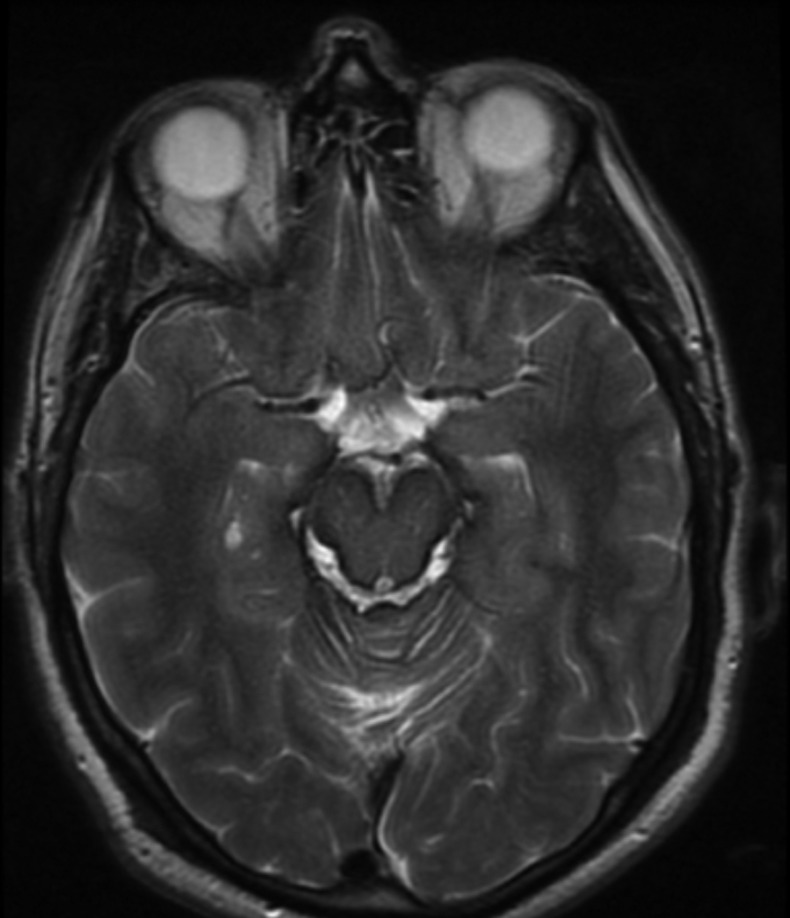
MRI of the brain demonstrates hypointensity on T2-weighted sequences along the brain surface, most markedly in the brainstem and vermis, consistent with superficial siderosis.
Treatment
The patient was started on iron chelation therapy with deferiprone 1 g three times a day in December 2011. He experienced nausea for 3 days on initiation of deferiprone but had otherwise no side effects; haematological and biochemical monitoring was normal.
Outcome and follow-up
On assessment in March 2012 his ataxia had improved; he had only one fall in the preceding 4 months and was able to tandem walk for the first time since presentation. His hearing also improved and repeat pure tone audiometry (averaged across speech frequencies 0.25–8kHz) in March 2012 revealed a 12 dB improvement in hearing in his right ear and a 10 dB improvement in his left ear when compared with audiometry performed prior to the treatment in September 2011.
Six months later, his walking remained improved and his headaches had fully resolved. Unfortunately, however, the improvement in his hearing was not fully sustained; pure tone audiometry 6 months later showed deterioration back to pretreatment levels, although he maintained the left responses in highest frequencies. Despite the patient's partial clinical improvement, repeat MRI of the brain performed 1 year after the initiation of deferiprone revealed no discernible change in haemosiderin deposition.
Discussion
Superficial siderosis is caused by recurrent haemorrhage into the subarachnoid space leading to haemosiderin deposition in the subpial layers of the cerebellum, brainstem, cranial nerves and spinal cord. It typically causes the triad of ataxia, deafness and myelopathy.1 Sources of this subarachnoid bleeding includes trauma, previous neurosurgical procedures, vascular lesions, tumours and dural abnormalities. Our patient had posterior fossa surgery performed to treat syringomyelia. In line with other reports in the literature, we postulate that this surgery may have contributed to episodic or chronic bleeding into the CSF.2 However, often the source of the haemorrhage is unclear despite thorough investigations. The interval between presumed onset of subarachnoid bleeding and manifestation of clinical symptoms of the disease can range between 8 and 37 years.3 In cases where the source of the bleeding has been identified, attempts have been made to surgically correct it. However, the disease often progresses despite this, due to the irreversible nature of the neural tissue damage caused by haemosiderin deposition.4
Deferiprone was approved for the treatment of patients with thalassaemia who had iron overload due to blood transfusions in the US Federal Drug Administration in October 2011. Uniquely among iron chelators, deferiprone can cross the blood–brain barrier to chelate haemosiderin in the central nervous system.5 Levy et al6 conducted a pilot safety trial in 10 participants with superficial siderosis treated with deferiprone and found that four patients showed a decrease in haemosiderin deposition on MRI after 90 days and that it had a satisfactory safety profile. Further studies are required to assess the clinical efficacy of deferiprone in superficial siderosis.
Learning points.
The differential diagnosis for ataxia and deafness is comparatively narrow and should alert the physician to the possibility of superficial siderosis.
MRI is the investigation of choice for diagnosis of superficial siderosis.
The classical MRI appearance shows hypo intensity along the leptomeninges in T2-weighted sequences.
While there is currently no evidence-based treatment available for superficial siderosis, further trials with the iron chelating agent deferiprone is warranted as it has proven safe in a pilot study, and there is anecdotal evidence of its effectiveness in certain patients.
Footnotes
Contributors: GC and GL drafted the manuscript. GC and DB were involved in the acquisition of data and they both critically revised the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fearnley JM, Stevens JM, Rudge P. Superficial siderosis of the central nervous system. Brain 1995;2013:1051–66 [DOI] [PubMed] [Google Scholar]
- 2.McCarron MO, Flynn PA, Owens C, et al. Superficial siderosis of the central nervous system many years after neurosurgical procedures. J Neurol Neurosurg Psychiatry 2003;2013:1326–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson NE, Sheffield S, Hope JK. Superficial siderosis of the central nervous system: a late complication of cerebellar tumors. Neurology 1999;2013:163–9 [DOI] [PubMed] [Google Scholar]
- 4.Egawa S, Yoshii T, Sakati K, et al. Dural closure for the treatment of superficial siderosis. J Neurosurg Spine 2013;2013:388–93 [DOI] [PubMed] [Google Scholar]
- 5.Freedenburg AM, Sethi RK, Allen DD, et al. The pharmacokinetics and blood-brain barrier permeation of the chelators 1,2 dimethly-, 1,2 diethyl- and 1-[ethan-1′ol]-2-methyl-3-hydroxypyridin-4-one in the rat. Toxicology 1996;2013:191–9 [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Llinas RH. Pilot safety trial of deferiprone in 10 subjects with superficial siderosis. Stroke 2012;2013:120–4 [DOI] [PubMed] [Google Scholar]


