Abstract
Four new quinazolinone alkaloids, namely, aniquinazolines A–D (1–4), were isolated and identified from the culture of Aspergillus nidulans MA-143, an endophytic fungus obtained from the leaves of marine mangrove plant Rhizophora stylosa. The structures of the new compounds were elucidated by spectroscopic analysis, and their absolute configurations were determined on the basis of chiral HPLC analysis of the acidic hydrolysates. The structure for 1 was confirmed by single-crystal X-ray diffraction analysis. All these compounds were examined for antibacterial and cytotoxic activity as well as brine shrimp (Artemia salina) lethality.
Keywords: mangrove, endophytic fungus, Aspergillus nidulans, secondary metabolites, bioactivity
1. Introduction
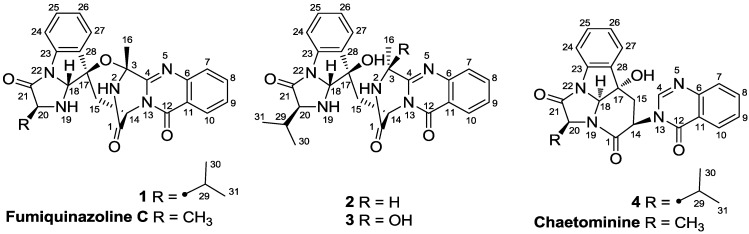
Quinazolinone derivatives generally refer to the compounds possessing a building block of quinazolin-4-one. More than 160 naturally occurring quinazolinones have been isolated from different organisms including plants, animals and microorganisms [1]. This family of alkaloids has attracted great attention due to their diversified structures and potent biological activities such as cytotoxicity, anti-feedant, and anti-microbial activities [2,3,4,5,6,7,8,9]. It is notable that some quinazolinone derivatives biogenetically synthesized by nonribosomal peptide synthesis (NRPS) [10] were isolated from a diverse group of fungi including Aspergillus fumigatus, A. flavipes, A. versicolor, Chaetomium sp., and Penicillium thymicola. During our ongoing investigation for the bioactive secondary metabolites of marine-derived fungi [11,12,13,14,15], four new quinazolinone alkaloids, namely, aniquinazolines A–D (1–4) (Figure 1), were isolated and elucidated from the culture of the fungus Aspergillus nidulans MA-143, which was obtained from the leaves of a mangrove plant Rhizophora stylosa. Details of the isolation, structure elucidation, and biological activity of compounds 1–4 are reported herein.
Figure 1.
Structures of the isolated compounds 1–4 and reference compounds fumiquinazoline C and chaetominine.
2. Results and Discussion
2.1. Structure Elucidation of the New Compounds 1–4
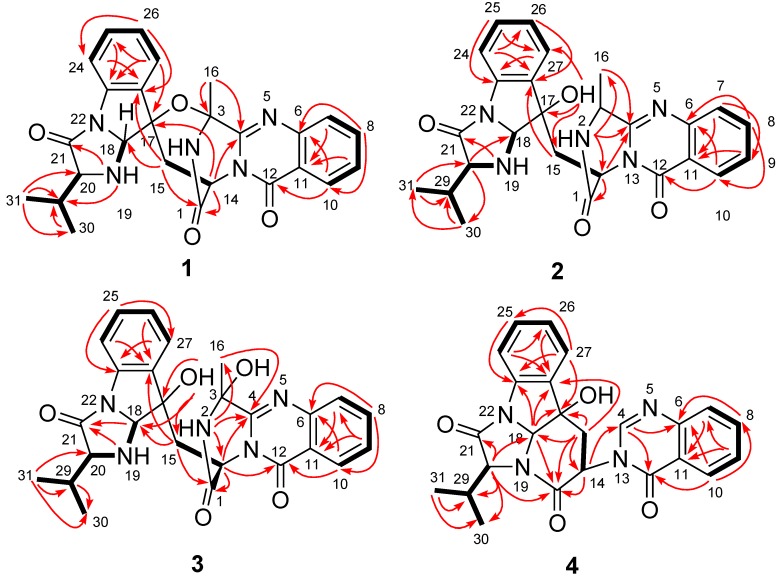
Compound 1 was obtained as a colorless crystal. Its molecular formula was determined as C26H25N5O4 on the basis of HRESIMS, with 17 degrees of unsaturation. Analysis of the 1H NMR spectrum of 1 revealed the resonances of four triple-doublets at δH 7.88 (td dd, J = 7.9, 1.3 Hz, H-8), 7.61 (td, J = 7.9, 0.9 Hz, H-9), 7.31 (td, J = 7.7, 0.9 Hz, H-25) and 7.04 (td, J = 7.7, 0.9 Hz, H-26), two double-doublets at δH 7.75 (dd, J = 7.9, 0.9 Hz, H-7) and 8.18 (dd, J = 7.9, 1.3 Hz, H-10), and two doublets at δH 7.38 (d, J = 7.7 Hz, H-24) and 7.25 (d, J = 7.7 Hz, H-27) (Table 1), which indicated the presence of two 1,2-disubstituted phenyl groups (C-6 to C-11 and C-23 to C-28) in 1. Its 13C and DEPT NMR spectra showed signals for three methyls, one methylene, eight sp2 and four sp3 methines, as well as ten quaternary carbons including two oxygenated sp3 carbons and three ester/amide carbonyl carbons (Table 2). A detailed NMR data comparison with those reported for fumiquinazoline C, a quinazolinone alkaloid identified from a fungal strain of Aspergillus fumigatus originally derived from the marine fish Pseudolabrus japonicus [2], revealed that the two compounds had the same carbon skeleton. Compound 1 differed from fumiquinazoline C mainly at the presence of an isopropyl group (δH 2.09/δC 31.4, CH-29; δH 1.07/δC 17.9, CH3-30; and δH 1.07/δC 18.5, CH3-31) connected at C-20 in 1, as it was a methyl group (δH 1.06/δC 18.7, CH3) in that of fumiquinazoline C. This deduction was further verified by the 1H–1H COSY correlations from H-29 to H-20 and H3-30/H3-31 as well as by the observed HMBC correlations from H3-31 to C-20, C-29, and C-30 and from NH-19 to C-29 as shown in Figure 2.
Table 1.
1H NMR (500MHz) data ofcompounds 1–4 in DMSO-d6 (δ in ppm).
| Position | 1 (J in Hz) | 2 (J in Hz) | 3 (J in Hz) | 4 (J in Hz) |
|---|---|---|---|---|
| 2-NH | 9.84, br s | 8.61, br s | 9.49, br s | |
| 3 | 4.94, q (6.5) | |||
| 4 | 8.30, s | |||
| 7 | 7.75, dd (7.9, 0.9) | 7.66, d (7.8) | 7.70, m | 7.71, dd (7.9, 0.8) |
| 8 | 7.88, td (7.9, 1.3) | 7.82, t (7.8) | 7.86, td (7.8, 1.2) | 7.87, td (7.9, 1.3) |
| 9 | 7.61, td (7.9, 0.9) | 7.53, t (7.8) | 7.58, td (7.8, 1.2) | 7.59, td (7.9, 0.8) |
| 10 | 8.18, dd (7.9, 1.3) | 8.12, d (7.8) | 8.16, dd (7.8, 1.2) | 8.18, dd (7.9, 1.3) |
| 14 | 5.36, dd (5.5, 1.5) | 5.58, dd (9.6, 4.3) | 5.44, dd (7.4, 6.5) | 4.86, dd (8.1, 5.3) |
| 15 | 2.98, dd (14.0, 5.5) 1.92,dd (14.0, 1.5) | 2.59, dd (14.8, 9.6) 1.83, dd (14.8, 4.3) | 2.61, dd (14.5, 7.4) 2.20, dd (14.5, 6.5) | 3.01, dd (14.0, 8.1) 2.77, dd (14.0, 5.3) |
| 16 | 1.91, s | 1.59, d (6.5) | 1.76, s | |
| 17-OH | 5.67, br s | 5.67, br s | 5.45, br s * | |
| 18 | 5.70, dd (6.0, 1.5) | 5.26, d (5.8) | 5.31, dd (7.3, 1.5) | 5.82, s |
| 19-NH | 2.67, d (5.5) | 3.51, m | 3.38, dd (7.3, 2.8) | |
| 20 | 3.59, br d (5.5) | 3.55, br s | 3.53, br s | 4.21, d (9.3) |
| 24 | 7.38, d (7.7) | 7.35, d (7.8) | 7.34, d (7.6) | 7.47, d (7.4) |
| 25 | 7.31, td (7.7, 0.9) | 7.26, t (7.8) | 7.27, td (7.6, 1.0) | 7.43, td (7.4, 1.2) |
| 26 | 7.04, td (7.7, 0.9) | 7.09, t (7.8) | 7.15, td (7.6, 1.0) | 7.26, td (7.4, 1.2) |
| 27 | 7.25, d (7.7) | 7.82, d (7.8) | 7.70, m | 7.54, d (7.4) |
| 29 | 2.09, dq (13.4, 6.8) | 1.99, m | 1.98, m | 2.45, m |
| 30 | 1.07, d (6.8) | 0.93, d (6.8) | 0.91, d (6.6) | 1.15, d (6.7) |
| 31 | 1.07, d (6.8) | 0.96, d (6.8) | 0.92, d (6.6) | 1.12, d (6.7) |
* This exchangeable proton was detected in acetone-d6.
Table 2.
13C NMR (125MHz) data of compounds 1–4 in DMSO-d6 (δ in ppm).
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 168.6, C | 168.9, C | 169.7, C | 164.8, C |
| 3 | 84.2, C | 48.5, CH | 84.4, C | |
| 4 | 151.5, C | 153.3, C | 150.3, C | 146.7, CH |
| 6 | 146.6, C | 146.6, C | 146.0, C | 147.5, C |
| 7 | 127.8, CH | 126.9, CH | 127.3, CH | 127.1, CH |
| 8 | 134.6, CH | 134.4, CH | 134.6, CH | 134.6, CH |
| 9 | 127.5, CH | 126.7, CH | 127.4, CH | 127.3, CH |
| 10 | 126.4, CH | 126.4, CH | 126.4, CH | 126.1, CH |
| 11 | 120.5, C | 120.0, C | 120.1, C | 121.5, C |
| 12 | 158.8, C | 160.2, C | 160.5, C | 159.7, C |
| 14 | 52.7, CH | 51.8, CH | 52.6, CH | 55.6, CH |
| 15 | 33.7, CH2 | 35.5, CH2 | 39.1, CH2 | 34.1, CH2 |
| 16 | 23.7, CH3 | 16.5, CH3 | 20.3, CH3 | |
| 17 | 86.3, C | 80.1, C | 80.3, C | 73.8, C |
| 18 | 88.4, CH | 88.1, CH | 88.1, CH | 83.7, CH |
| 20 | 69.0, CH | 69.1, CH | 69.0, CH | 69.3, CH |
| 21 | 171.5, C | 172.1, C | 172.6, C | 173.8, C |
| 23 | 136.6, C | 137.2, C | 137.7, C | 140.2, C |
| 24 | 114.3, CH | 115.1, CH | 115.2, CH | 114.2, CH |
| 25 | 129.7, CH | 128.8, CH | 128.6, CH | 130.0, CH |
| 26 | 125.1, CH | 124.6, CH | 124.6, CH | 125.1, CH |
| 27 | 126.2, CH | 125.4, CH | 125.2, CH | 124.5, CH |
| 28 | 137.4, C | 138.7, C | 138.8, C | 134.9, C |
| 29 | 31.4, CH | 31.0, CH | 31.0, CH | 28.2, CH |
| 30 | 17.9, CH3 | 17.6, CH3 | 17.4, CH3 | 18.6, CH3 |
| 31 | 18.5, CH3 | 18.6, CH3 | 18.5, CH3 | 20.1, CH3 |
Figure 2.
Key 1H–1H COSY (bold lines) and HMBC (red arrows) correlations of compounds 1–4.
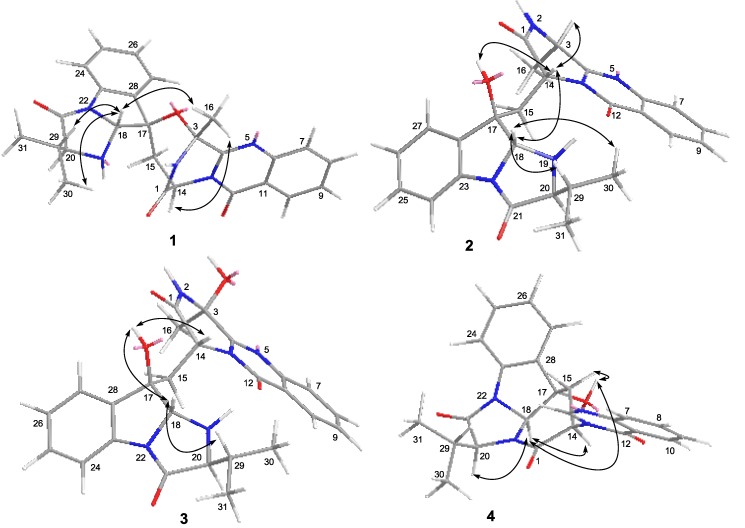
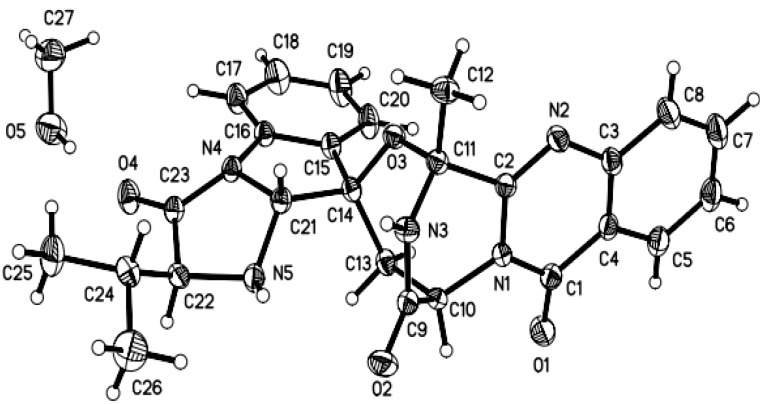
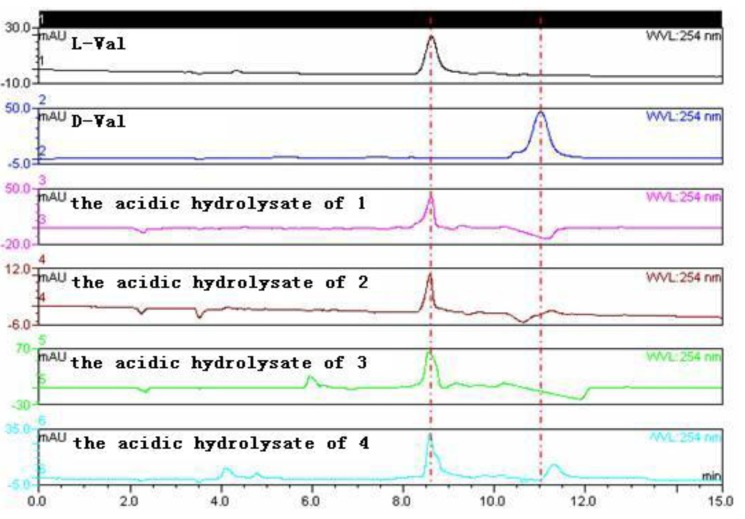
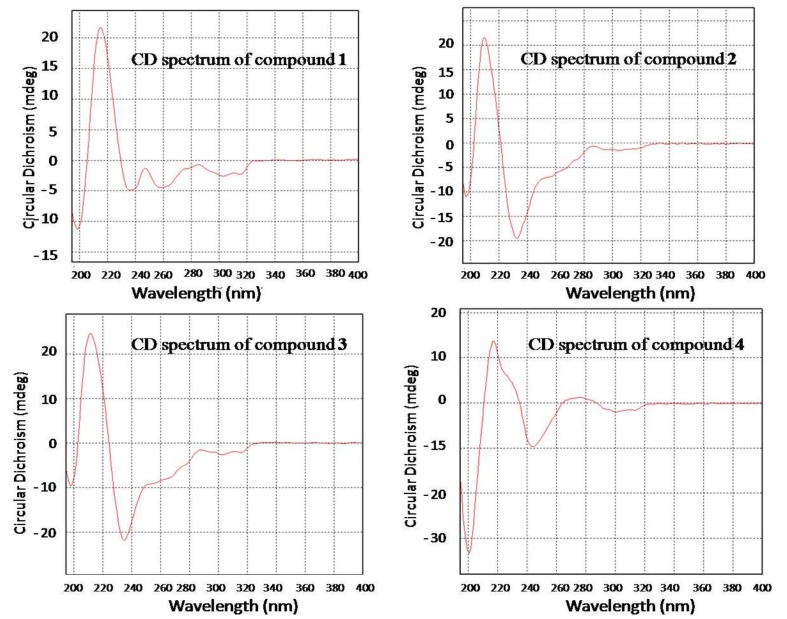
The relative configuration of compound 1 was determined by NOESY spectrum and by the X-ray experiment. The NOE correlations from H-16 to H-14 and H-18 as well as from H-18 to H-29 indicated the same orientation of these protons (Figure 3). The structure and relative configuration of compound 1 were confirmed by the single crystal X-ray diffraction analysis (Figure 4). The absolute configuration of 1 was deduced by chiral HPLC analysis of the degradation products of the acidic hydrolysates. The HPLC profile of the hydrolysates was compared with that of authentic standard to establish the configuration of the amino acid-derived unit as l-valine (Figure 5), corresponding to the S-configuration for C-20. The absolute configuration of compound 1 was therefore assigned to be 3R, 14R, 17R, 18R, and 20S, which was identical to that of fumiquinazoline C [2]. The ECD (Electronic Circular Dichroism) spectrum of 1 showed negative Cotton Effect (CE) at 199, 238, 258, and 303 nm, and positive CE at 216 nm (Figure 6). The structure of 1 was thus elucidated and it was named as aniquinazoline A.
Figure 3.
NOESY correlations of compounds 1–4.
Figure 4.
X-ray structure of compound 1 (Note: A different numbering system is used for the structure in the text).
Figure 5.
Chiral HPLC analysis of the amino acid derivatives of compounds 1–4.
Figure 6.
Electronic Circular Dichroism (ECD) spectra of compounds 1–4.
Compound 2 was obtained as yellowish solid. The HRESIMS experiment established its molecular formula C26H27N5O4 (16 degrees of unsaturation), with two more H-atom than that of 1. The 1H and 13C NMR data of 2 were very similar to those of 1 (Table 1, Table 2), indicating that they shared the same carbon skeleton. However, the oxygenated sp3 quaternary carbon at δC 84.2 (C-3) in the 13C NMR spectrum of 1 disappeared in that of 2. Instead, signals for a methine group (δH 4.94/δC 48.5, CH-3) were observed in the NMR spectra of 2 (Table 1, Table 2). Accordingly, the singlet methyl signal at δH 1.91 (H3-16) in the 1H NMR spectrum of 1 was replaced by a doublet methyl group at δH 1.59 (J = 6.5 Hz) in that of 2, and an exchangeable OH proton at δH 5.67 (br s, 17-OH) was observed (Table 1). This OH group was attached at C-17 on the basis of HMBC correlations from the OH proton to C-15 (δC 35.5, CH2), C-17 (δC 80.1, C), and C-27 (δC 125.4, CH) (Figure 2). The 1H–1H COSY and HMBC correlations shown in Figure 2 confirmed the planar structure of 2. The relative configuration of compound 2 was determined by NOESY spectrum as well as by comparing the 1H-NMR J-values with that of fumiquinazolines A and B [2]. The observed NOE correlations from H-18 to H-14 and H-29 and from H-14 to H-3 and 17-OH, indicated them on the same face of the molecule (Figure 3). In contrast to compound 1, no NOE correlations could be observed from H-16 to H-14 and H-18. Instead, NOE correlation from H-14 to H-3 was observed in the NOESY spectrum of 2 (see Figure 3 as well as Figure S12b in the Supplementary Materials). In the reference report, a large coupling constant (J = 4.9 Hz) for H-3 and NH-2 was observed in fumiquinazoline B, whereas its C-3 epimer, fumiquinazoline A, showed a very small coupling constant (J = 0.3 Hz) [2]. In the case of compound 2, the proton NH-2 was observed as a broad singlet (Table 1), which means the coupling constant between H-3 and NH-2 was very small, thus suggesting the configuration at C-3 is close to that of funiquinazoline A. The above data suggested that the C3–O–C17 ether ring in 1 was opening in that of 2, and the configuration of C-3 changed during the ring-opening process. To substantiate this, compound 1 was reduced with NaBH4 in THF at room temperature for 40 min and the resulting product was revealed to be identical to 2 by co-TLC analysis. The 1H-NMR and 1H–1H COSY as well as NOESY spectra also showed that the reduced product of 1 was the same as that of compound 2 (see Supplementary Material). However, it was described in the reference that reduction of fumiquinazoline C yielded fumiquinazoline A without C-3 configuration change [2], and this differs from our observation as discussed above. Unfortunately, no NOESY experiment was performed and described for the reducing product of fumiquinazoline C [2], which would have helped give solid evidence to clarify the problem. Based on the above data, the relative configurations between C-3 and C-14 of fumiquinazoline A [2] should be reconsidered.
The absolute configuration of C-20 in compound 2 was also assigned as S based on the chiral HPLC analysis of its acidic hydrolysates (Figure 5) and the absolute configurations of other chiral centers in 2 were therefore assigned as 3R, 14R, 17R, and 18R. The structure of 2 was thus elucidated and named as aniquinazoline B.
Compound 3 was obtained as a yellowish solid. Its molecular formula was determined to be C26H27N5O5 (16 degrees of unsaturation) on the basis of HRESIMS data, with one more O-atom than that of 2. The assignments of the NMR data of 3 (Table 1, Table 2) matched well with that of the corresponding signals for 2, except for the presence of an OH group at C-3 in 3, which was consistent with the difference in molecular formula. Accordingly, the non-oxygenated CH signals resonated at δH 4.94/δC 48.5 (CH-3) in the NMR spectra of 2 and disappeared in that of 3. Instead, an oxygenated quaternary carbon at δC 84.4 (C-3) was observed in the 13C NMR spectrum of 3 (Table 2). Correspondingly, the methyl doublet signal at δH 1.59 (J = 6.5 Hz, H3-16) in the 1H NMR spectrum of 2 was replaced by a downfield singlet at δH 1.76 (H3-16) in that of 3 (Table 1). The 1H–1H COSY and HMBC correlations shown in Figure 2 confirmed the planar structure of 3.
The relative configuration of compound 3 was determined by NOESY spectrum. The NOE correlations from H-18 to H-29 and 17-OH and from 17-OH to H-14 revealed these protons were on the same face of the molecule (Figure 3). The relative configuration at C-3 was assigned as being the same as that of 2 from the biogenetic point of view. The absolute configuration of C-20 was also determined as S by the chiral HPLC analysis of the acidic hydrolysates of 3 (Figure 5). Consequently, the absolute configuration of compound 3 was assigned as 3S, 14R, 17R, 18R, and 20S. The very similar ECD spectra for compounds 2 and 3 (Figure 6) supported the above assignment. The structure of compound 3 was thus determined and was named aniquinazoline C.
Compound 4 was isolated as a yellowish solid. Its molecular formula was determined as C24H22N4O4 by HRESIMS data. Detailed comparison of NMR data of compound 4 (Table 1, Table 2) with those of chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015 originally isolated from the apparently healthy Adenophora axilliflora leaves [5], suggested that they shared the same carbon skeleton. The main difference was that the methyl group at C-20 in chaetominine was replaced by an isopropyl group in 4. This deduction was verified by 1H–1H COSY and HMBC correlations as shown in Figure 2. The relative configuration of 4 was assigned by NOESY spectrum. The correlations from H-18 to H-14, 17-OH and H-20 revealed their same orientation on the molecule (Figure 3). The absolute configuration of C-20 was assigned as S on the basis of the chiral HPLC analysis of the acidic degradation product of 4 (Figure 5). Thus the absolute configuration of compound 4 was determined as 14R, 17S, 18S, and 20S, and it was named as aniquinazoline D.
2.2. Biological Activities of the Isolated Compounds
The isolated compounds 1–4 were examined for brine shrimp toxicity, antitumor, and antibacterial activities. Compounds 1–4 showed potent lethality against brine shrimp with LD50 values of 1.27, 2.11, 4.95 and 3.42 μΜ, respectively, which were stronger than that of the positive control colchicine (with LD50 value of 88.4 μΜ). Compounds 1–4 were also evaluated for antitumor and antibacterial activity, but none of them displayed inhibitory activity against four cell lines (BEL-7402, MDA-MB-231, HL-60, and K562) and two bacteria (Escherichia coli and Staphyloccocus aureus).
3. Experimental Section
3.1. General
The melting point was determined with an SGW X-4 micro-melting-point apparatus. The optical rotations were measured on an Optical Activity A-55 polarimeter. UV data were obtained on a Lengguang Gold S54 spectrophotometer. The 1H, 13C, and 2D NMR spectroscopic data were acquired on Bruker Advance 500 spectrometers. Mass spectra were measured on a VG Autospec 3000 mass spectrometer. HPLC analysis was performed on a Dionex HPLC System equipped with a P680 pump, an ASI-100 automated sample injector, a TCC-100 column oven, a UV-DAD 340U detector, and a Dionex Acclaim ODS column (4.6 × 250 mm, 5 μm). Semi-preparative HPLC was operated on a Dionex UltiMate U3000 system using an Agilent Prep RP-18 column (21.2 × 250 mm, 10 μm) with UV detection. A Phenomenex-Chirex 3126 N,S-dioctyl-(d)-penicillamine column (250 × 4.60 mm, 5 μm) was used for chiral HPLC analysis. Column chromatography (CC) was performed with silica gel (200–300 mesh, Qingdao Marine Chemical Factory), Lobar LiChroprep RP-18 (40–63 μm; Merck), and Sephadex LH-20 (18–110 μm, Merck).
3.2. Fungal Material
The fungus Aspergillus nidulans MA-143 was isolated from the leaves of marine mangrove plant Rhizophora stylosa. The fungus grew slowly on potato dextrose agar (PDA) plate and turned from white to green mycelia within 5 days. The fungus was identified by sequence analysis of the ITS region of its rDNA as described previously [16] and the sequence data obtained from the fungus have been deposited in GenBank with accession number JQ839285. The strain is preserved at the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences.
3.3. Fermentation
The fermentation was statically carried out in liquid potato-dextrose broth medium (PDB) (1000 mL seawater, 20 g glucose, 5 g peptone, 3 g yeast extract, pH 6.5–7.0, liquid medium/flask = 300 mL) in 1 L Erlenmeyer flasks for 30 days at room temperature.
3.4. Extraction and Isolation
The fermented whole broth (100 flasks) was filtered through cheesecloth to separate the culture broth and mycelia, which were extracted exhaustively with EtOAc and MeOH, respectively. Since the chemical profiles of the two extracts were almost identical, they were combined and concentrated to afford the crude extract (31.0 g) for further separation. The crude extract was subjected to silica gel vacuum liquid chromatography (VLC) eluting with mixed solvents of increasing polarity (petroleum ether-EtOAc, 5:1 to 1:1, and then CHCl3–MeOH, 20:1 to 0:1) to yield 7 fractions (Fr.1 to Fr.7). Fraction 2 (11.0 g) was further separated to seven subfractions (Fr.2.1–2.7) by CC on silica gel eluted with CHCl3–MeOH (100:1 to 5:1). Fr.2.1 (7.0 g) was subjected to CC on Lobar LiChroprep C18 eluting with MeOH-H2O gradient (1:9 to 1:0) and then further purified by semi-preparative HPLC (MeOH–H2O 7:3, 16 mL/min) to afford 2 (tR = 14.6 min, 5.2 mg). Fr.2.2 (3.0 g) was subjected to CC on silica gel eluted with CHCl3–MeOH (100:1 to 5:1) and Sephadex LH-20 (petroleum ether–CHCl3–MeOH, 5:5:1), and then further purified by semi-preparative HPLC (MeOH–H2O 7:3, 16 mL/min) to obtain 1 (tR = 15.8 min, 20.0 mg), 3 (tR = 13.5 min, 5.5 mg), and 4 (tR = 10.5 min, 7.7 mg).
Aniquinazoline A (1): colorless crystal; mp 243–245 °C; [α]20D: −13 (c 0.30, MeOH); UV (MeOH) λmax (log ε) 200 (4.41), 218 (4.26), 245 (3.97), 270 (3.82), 297 (3.32), 305 (3.37) nm; CD λmax (Δε) 199 (−11.33), 216 (+21.60), 238 (−4.92), 258 (−4.52), 303 (−2.63) nm; 1H and 13C NMR data, see Table 1, Table 2; ESIMS m/z 472 [M + H]+; HRESIMS m/z 472.1982 [M + H]+ (calcd for C26H26N5O4+, 472.1985, Δ 0.3 ppm).
Aniquinazoline B (2): yellowish solid; [α]20D: −118 (c 0.28, MeOH); UV (MeOH) λmax (log ε) 202 (4.46), 225 (4.33), 243 (4.03), 304 (3.35) nm; CD λmax (Δε) 197 (−10.99), 210 (+21.55), 233 (−19.63), 304 (−1.59) nm; 1H and 13C NMR data, see Table 1, Table 2; ESIMS m/z 474 [M + H]+; HRESIMS m/z 474.2143 [M + H]+ (calcd for C26H28N5O4+, 474.2141, Δ 0.2 ppm).
Aniquinazoline C (3): yellowish solid; [α]20D: −19 (c 0.28, MeOH); UV (MeOH) λmax (log ε) 200 (4.63), 214 (4.51), 246 (4.14), 271 (4.01), 290 (3.70), 301 (3.61) nm; CD λmax (Δε) 199 (−9.37), 212 (+24.53), 235 (−21.94), 303 (−2.65) nm; 1H and 13C NMR data, see Table 1, Table 2; ESIMS m/z 490 [M + H]+; HRESIMS m/z 490.2084 [M + H]+ (calcd for C26H28N5O5+, 490.2090, Δ 0.6 ppm).
Aniquinazoline D (4): yellowish solid; [α]20D: −33 (c 0.37, MeOH); UV (MeOH) λmax (log ε) 200 (6.06), 218 (5.91), 246 (5.61), 268 (5.52), 298 (4.98), 312 (4.92) nm; CD λmax (Δε) 201 (−33.36), 218 (+13.62), 244 (−9.78), 276 (+1.17), 301 (−2.08) nm; 1H and 13C NMR data, see Table 1, Table 2; ESIMS m/z 431 [M + H]+; HRESIMS m/z 431.1710 [M + H]+ (calcd for C24H23N4O4+, 431.1719, Δ 0.9 ppm).
3.5. X-ray Crystallographic Analysis of Compounds 1
All crystallographic data [17] were collected on a Bruker Smart-1000 CCD diffractometer equipped with a graphite-monochromatic Mo-Kα radiation (λ = 0.71073 Å) at 298(2) K. The data were corrected for absorption by using the program SADABS [18]. The structure was solved by direct methods with the SHELXTL software package [19]. All non-hydrogen atoms were refined anisotropically. The H atoms were located by geometrical calculations, and their positions and thermal parameters were fixed during the structure refinement. The structure was refined by full-matrix least-squares techniques [20].
Crystal data for compound 1: C27H29N5O5, F.W. = 503.55, one molecule containing a CH3OH solvent molecule in the unit, monoclinic space group P2(1), unit cell dimensions a = 11.3860(5) Å, b = 7.8898(3) Å, c = 13.6963(5) Å, V = 1230.18(8) Å3, α = γ = 90°, β = 91.044(3)°, Z = 2, dcalcd = 1.359 mg/m3, crystal dimensions 0.45 × 0.40 × 0.32 mm, μ = 0.096 mm−1, F(000) = 532. The 7506 measurements yielded 4172 independent reflections after equivalent data were averaged, and Lorentz and polarization corrections were applied. The final refinement gave R1 = 0.0332 and wR2 = 0.0778[I > 2σ(I)].
3.6. Amino Acid Analysis
Compounds 1 (1.3 mg), 2 (0.9 mg), 3 (0.8 mg), and 4 (1.1 mg) were each solved in 10 mL 6 N HCl and heated at 110 °C for 24 h in sealed tubes. The solutions were then evaporated to dryness under reduced pressure. Each sample, including the standard amino acid l-val and d-val, was solved in 1 mL of eluting solvent (2 mM CuSO4·5H2O, with 5 mL CH3CN in every 100 mL solvent) and was concentrated at a speed of 12,000 rpm. Chiral HPLC analysis was carried out using a Phenomenex-Chirex-3126 column at 254 nm with flow rate 1.0 mL/min at 40 °C. The HPLC analysis showed that the products of acidic hydrolysis of 1–4 contained the identical amino acid, which have the same retention time as that of the standard l-val. The results established the chiral center of the valine moiety in the structure of the four compounds to be S-configuration (Figure 5).
3.7. Reduction of Compound 1
Compound 1 (6.8 mg) was dissolved in 1.0 mL THF (tetrahydrofuran), and NaBH4 (5.0 mg) was added to the solution. The mixture was stirred at room temperature for 40 min and then concentrated under reduced pressure. The residue was diluted with water, extracted by CH2Cl2 and then evaporated. The reducing product (1.5 mg) was obtained from the extract by preparative TLC (CHCl3–MeOH, 30:1). Compound 2 and the reducing product were evidenced to be identical on the basis of co-TLC and NMR data analysis (see Supplementary Material, Figures S28–S30).
3.8. Brine Shrimp Toxicity
Brine shrimp (Artemia salina) toxicity of the isolated compounds was determined as described previously [21]. Colchicine was used as a positive control.
3.9. Cytotoxicity Assay
The cytotoxic activity against BEL-7402 (human hepatocellular carcinoma), MDA-MB-231 (human breast carcinoma), HL-60 (human promyelocytic leukemia cells), and K562 (human acute myelocytic leukemia cell line) cell lines were determined according to previously reported methods [22].
3.10. Antibacterial Assay
Antibacterial assay against E. coli and S. aureus was carried out using the well diffusion method [23]. Chloromycetin was used as a positive control.
4. Conclusions
Four new quinazolinone alkaloids (1–4) were isolated from the mangrove-derived endophytic fungus Aspergillus nidulans MA-143. The structures and relative configurations were determined on the interpretation of the NMR data and the absolute configuration of all compounds was determined by chiral HPLC analysis of the acid hydrolysates. Compounds 1–4 exhibited potent brine shrimp toxicity with LD50 values of 1.27, 2.11, 4.95 and 3.42 μΜ, respectively.
Acknowledgments
This work was financially supported by Ministry of Science and Technology (2013AA092901 and 2010CB833802) and by National Natural Science Foundation of China (31270403).
Supplementary Files
Supplementary Information (PDF, 1503 KB)
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Mhaske S.B., Argade N.P. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron. 2006;62:9787–9826. doi: 10.1016/j.tet.2006.07.098. [DOI] [Google Scholar]
- 2.Takahashi C., Matsushita T., Doi M., Minoura K., Shingu T., Kumeda Y., Numata A. Fumiquinazolines A–G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J. Chem. Soc. Perkin. Trans. I. 1995:2345–2353. doi: 10.1039/P19950002345. [DOI] [Google Scholar]
- 3.Larsen T.O., Frydenvang K., Frisvad J.C., Christophersen C. UV-guided isolation of alantrypinone, a novel Penicillium alkaloid. J. Nat. Prod. 1998;61:1154–1157. doi: 10.1021/np980056v. [DOI] [PubMed] [Google Scholar]
- 4.Numata A., Takahashi C., Matsushita T., Miyamoto T., Kawai K., Usami Y., Matsumura E., Inoue M., Ohishi H., Shingu T. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992;33:1621–1624. doi: 10.1016/S0040-4039(00)91690-3. [DOI] [Google Scholar]
- 5.Jiao R.H., Xu S., Liu J.Y., Ge H.M., Ding H., Xu C., Zhu H.L., Tan R.X. Chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015. Org. Lett. 2006;8:5709–5712. doi: 10.1021/ol062257t. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang Y.B., Teng X.C., Wang Y., Liu P.P., Li G.Q., Zhu W.M. New quinazolinone alkaloids within rare amino acid residue from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Org. Lett. 2011;13:1130–1133. doi: 10.1021/ol103164n. [DOI] [PubMed] [Google Scholar]
- 7.Fremlin L.J., Piggott A.M., Lacey E., Capon R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009;72:666–670. doi: 10.1021/np800777f. [DOI] [PubMed] [Google Scholar]
- 8.Barrow C.J., Sun H.H. Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J. Nat. Prod. 1994;57:471–476. doi: 10.1021/np50106a005. [DOI] [PubMed] [Google Scholar]
- 9.Li X.J., Zhang Q., Zhang A.L., Gao J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic Activities. J. Agric. Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 10.Ames B.D., Haynes S.W., Gao X., Evans B.S., Kelleher N.L., Tang Y., Walsh C.T. Complexity generation in fungal peptidyl alkaloid biosynthesis: Oxidation of fumiquinazoline A to the heptacyclic hemiaminal fumiquinazoline C by the flavoenzyme Af12070 from Aspergillus fumigatus. Biochemistry. 2011;50:8756–8769. doi: 10.1021/bi201302w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H.F., Li X.M., Meng L., Cui C.M., Gao S.S., Li C.S., Huang C.G., Wang B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012;75:148–152. doi: 10.1021/np2006742. [DOI] [PubMed] [Google Scholar]
- 12.Du F.Y., Li X.M., Li C.S., Shang Z., Wang B.G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus Eurotium cristatum EN-220. Bioorg. Med. Chem. Lett. 2012;22:4650–4653. doi: 10.1016/j.bmcl.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 13.Li C.S., An C.Y., Li X.M., Gao S.S., Cui C.M., Sun H.F., Wang B.G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011;74:1331–1334. doi: 10.1021/np200037z. [DOI] [PubMed] [Google Scholar]
- 14.Gao S.S., Li X.M., Du F.Y., Li C.S., Proksch P., Wang B.G. Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Mar. Drugs. 2011;9:59–70. doi: 10.3390/md9010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D., Li X.M., Meng L., Li C.S., Gao S.S., Shang Z., Proksch P., Huang C.G., Wang B.G. Nigerapyrones A–H, α-pyrone derivatives from the marine mangrove-derived endophytic fungus Aspergillus niger MA-132. J. Nat. Prod. 2011;74:1787–1791. doi: 10.1021/np200381u. [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Li X.M., Teuscher F., Li D.L., Diesel A., Ebel R., Proksch P., Wang B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006;69:1622–1625. doi: 10.1021/np060248n. [DOI] [PubMed] [Google Scholar]
- 17.Cambridge Crystallographic Data Centre (CCDC) [(accessed on 5 March 2013)]. Available online: http://www.ccdc.cam.ac.uk/data_request/cif.
- 18.Sheldrick G.M. SADABS, Software for Empirical Absorption Correction. University of Göttingen; Göttingen, Germany: 1996. [Google Scholar]
- 19.Sheldrick G.M. SHELXTL, Structure Determination Software Programs. Bruker Analytical X-ray System Inc.; Madison, WI, USA: 1997. [Google Scholar]
- 20.Sheldrick G.M. SHELXL-97 and SHELXS-97, Program for X-ray Crystal Structure Solution and Refinement. University of Göttingen; Göttingen, Germany: 1997. [Google Scholar]
- 21.Gerwick W.H., Proteau P.J., Nagle D.G., Hamel E., Blokhin A., Slate D.L. Structure of curacin A, a novel antimitotic, antiproliferative, and brine shrimp toxic natural product from the marine Cyanobacterium Lyngbya majuscula. J. Org. Chem. 1994;59:1243–1245. [Google Scholar]
- 22.Bergeron R.J., Cavanaugh P.F., Jr., Kline S.J., Hughes R.G., Jr., Elliott G.T., Porter C.W. Antineoplastic and antiherpetic activity of spermidine catecholamide iron chelators. Biochem. Biophys. Res. Commun. 1984;121:848–854. doi: 10.1016/0006-291X(84)90755-1. [DOI] [PubMed] [Google Scholar]
- 23.Al-Burtamani S.K.S., Fatope M.O., Marwah R.G., Onifade A.K., Al-Saidi S.H. Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol. 2005;96:107–112. doi: 10.1016/j.jep.2004.08.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information (PDF, 1503 KB)