Abstract
Objective:
To develop a prediction model that quantifies the risk of being overweight at 10 years of age.
Subjects/Methods:
In total, 3121 participants from the GINIplus (German Infant Nutritional Intervention plus environmental and genetic influences on allergy development) and LISAplus (Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood plus Air Pollution and Genetics) German birth cohorts were recruited. We predicted standardized body mass index (BMI) at 10 years of age using standardized BMIs from birth to 5 years. Parental education, family income and maternal smoking during pregnancy were considered as covariates. Linear and logistic regression models were used to evaluate the impact of risk factors on BMI and on being overweight at 10 years of age, respectively.
Results:
Birth weight, standardized BMI at 5 years (60–64 months) (β=0.77; 95% confidence interval (CI): 0.73–0.81) and maternal smoking during pregnancy were positively associated with standardized BMI at 10 years of age. Standardized BMI and overweight at 5 years were strongest predictors of being overweight at 10 years. Conversely, high parental education conferred a protective effect (β=−0.15; 95% CI: −0.29 to −0.01). Being overweight at 5 years (60–64 months) increased the risk of being overweight at 10 years of age with odds ratios above 10. Among children who were predicted to be overweight at 10 years, cross-validation results showed that 76.8% of female subjects and 68.1% of male subjects would be overweight at 10 years of age.
Conclusion:
BMI and being overweight at 5 years of age are strong predictors of being overweight at 10 years of age. The effectiveness of targeted interventions in children who are overweight at 5 years of age should be explored.
Keywords: overweight, obesity, children, risk factors, prediction
Introduction
Obesity is a major public health challenge. It is associated with numerous illnesses and often persists into adulthood.1 Being overweight during childhood and adolescence adversely affects the onset of many diseases later in life.2 Identifying children at risk of being overweight early on in life is essential for effective prevention and intervention.3
Shankaran et al.4 reported that children who are overweight at younger ages have a higher risk of being overweight at 11 years of age. Moreover, it was noted that measurements taken closer to 11 years of age had greater predictive power. Similar results are found in other studies.5, 6 Several studies have also shown that birth weight and maternal smoking during pregnancy are associated with childhood obesity.7, 8, 9, 10 Familial factors can explain up to 27% of the variance in a child's weight status.11 There are also many other factors that increase the likelihood of being overweight later in life, such as rapid growth during the first two years of life, short duration of breastfeeding, obesity in infancy, short sleep duration, <30 min of daily physical activity and consumption of sugar-sweetened beverages, and so on.12, 13 Prediction models for being overweight and obese have previously been developed based on some of these determinants, but those utilizing information on body mass indices (BMIs) collected during very early life (<5 years) are scarce.
In this study, we established an overweight prediction model using standardized deviation scores of anthropometric parameters collected during the first 5 years of life. Considering the availability of covariates and the aim of developing a concise and easily applicable prediction model, we only included parental education, socioeconomic status and maternal smoking during pregnancy as covariates. Cross-validation was used to test the validity of our prediction model.
Methods
Study population
Data from two ongoing German birth cohorts of healthy full-term neonates were used in this study. GINIplus (German Infant Nutritional Intervention plus environmental and genetic influences on allergy development) is an ongoing birth cohort study initiated to investigate prospectively the influence of a nutritional intervention during infancy, air pollution and genetics on allergy development. GINIplus participants (N=5991 newborns) were recruited from obstetric clinics in Munich, Leipzig, Wesel and Bad Honnef between September 1995 and July 1998. Details of the study design are described elsewhere.14, 15 A total of 2252 newborns with atopic heredity participated in a hydrolyzed protein infant formula intervention study for the first 4 months of life.14 This randomized controlled trial showed marginally different BMIs across formula groups during the first year of life, but this effect did not persist to 6 and 10 years of age.15 A sensitivity analysis in which formula type was entered into the models as a confounder did not substantially change the results in this study (results not shown). LISAplus (Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood plus Air Pollution and Genetics) is an ongoing birth cohort examining the impact of lifestyle-related factors, air pollution and genetics on immune system and childhood allergy development. In total, 3097 neonates were recruited from 14 obstetrical clinics in Munich, Leipzig, Wesel and Bad Honnef between November 1997 and January 1999. Two participants removed their consent to participate at the 6-year follow-up, and are not included in the analysis. A detailed description of the study's screening and recruitment has been described previously.16, 17 Detailed information on allergy and traffic emissions was collected at the 6-year follow-up, and since this time, the term ‘plus‘ was added to the previous names of the cohorts. This analysis is limited to the 3121 children who participated in the physical examination at the 10-year follow-up (1921 from GINIplus and 1200 from LISAplus).
Approval by the respective local ethics committees (Bavarian General Medical Council, University of Leipzig, Medical Council of North-Rhine-Westphalia) and written consent from all participating families were obtained for both studies.
Outcome and covariates
Anthropometric measurements of weight and height were collected by pediatricians during physical examinations at birth, at weeks 4–6, at months 3–4, 6–7, 10–12, 21–24, 46–48 and 60–64 and at 10 years of age. Data from birth to age 60–64 months were obtained from administrative records and that at 10 years from a physical examination conducted as part of the 10-year follow-up of the GINIplus and LISAplus studies.
BMI values were calculated from these weight and height measurements and transformed to standardized deviation scores (z-scores) according to the sex- and age-specific ‘WHO-Child-Growth-Standards' for children.18, 19 This standardization allows for comparisons across studies.
At every time point, the standard deviation (s.d.) of our population's standardized BMI measurements was calculated. If a child's respective standardized BMI was greater than this s.d., the child was identified as being overweight at this time point, the methodology is consistent with WHO standards.20
The set of covariates examined were maternal smoking during pregnancy, family income and parental education level. As the distribution of family income was skewed, this information was grouped into three categories based on the quartiles of monthly average income per person in the household (low for <25%, medium for 25–75% and high for >75%). Parental education levels were defined based on the highest number of years either parent attended school (low <10 years, medium=10 years and high >10 years).
Statistical analysis
As male and female subjects have different developmental tracks for obesity,21 analyses were conducted pooled and stratified by sex.
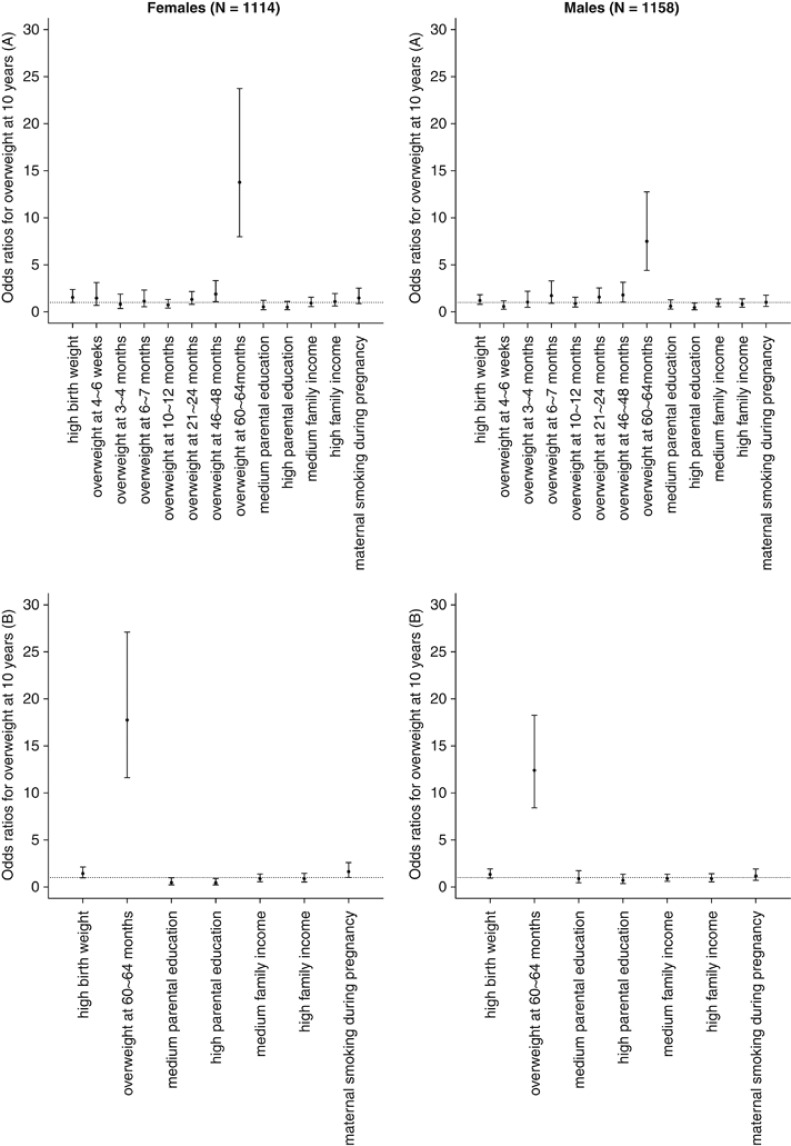
Linear regression was used to analyze the effects of birth weight, standardized BMI measurements during the first 5 years of life and covariates on standardized BMI measurements at the age of 10 years. Logistic regression was used to assess the risk of being overweight at 10 years and to verify the results obtained from the linear regression model. All models were adjusted for parental education, family income and maternal smoking during pregnancy. Multivariate analyses indicated that only birth weight and standardized BMI at the age of 60–64 months were significantly associated with the outcome of interest. Sensitivity analyses excluding variables at 60–64 months (for example, only including standardized BMIs up to the age of 46–48 months) and including variables at a later age (for example, also including standardized BMI at the age of 6 years) were conducted. On the basis of the results from these models, birth weight and standardized BMI recorded at the age of 60–64 months appeared to be strong predictors of 10-year standardized BMI or being overweight at 10 years of age, and these two variables were selected in the main models (Figure 1, Model B).
Figure 1.
ORs for the association between risk factors and being overweight at 10 years. Models included (A) all categorized standardized BMIs during the first 5 years of life and all covariates and (B) only select variables. Variables were selected based on multivariate models, in which only birth weight and standardized BMI at 60–64 months were constantly associated with the outcome of interest. Parental education, family income and maternal smoking during pregnancy were included as covariates. The reference populations used in the models for being overweight at different ages were the corresponding non-overweight participants. The lowest scales of parental education and family income were used as the reference categories for these variables. Maternal non-smokers were used as the reference population for maternal smoking during pregnancy.
Among the available study population (N=3121), 2272 children had complete information on all variables included in the linear model. To test the validity of the linear model, two-thirds (N=1515) of the complete cases were randomly divided into a training data set, and the remaining third (N=757) into a validation data set. The training data set was used to fit the linear regression model. The validation data set was used to compare predicted overweight status at 10 years of age with the true overweight status obtained from the physical examinations. The mean-square prediction error, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were obtained. In this study, sensitivity provides a measure for the percentage of correctly predicted overweight children out of those who were overweight and specificity provides a measure for the percentage of correctly predicted non-overweight children out of those who were not overweight. PPV reflects the probability of being overweight at 10 years if a child was predicted to be overweight using our model, whereas NPV reflects the probability of not being overweight at 10 years if a child was predicted as non-overweight. This procedure was repeated 1000 times and the mean and percentiles of these indices were calculated.
Differences between male and female subjects, as well as between participants (N=3121) and non-participants (N=5965), of the 10-year physical examination were tested using Pearson's χ2 test for categorical variables or one-way analysis of variance for continuous variables. Study characteristics are described using means, s.d.'s or percentages. Model results are presented as linear regression coefficients (β) for standardized BMIs and as odds ratios (OR) for being overweight (yes/no), with corresponding 95% confidence intervals (CI). The cross-validation results are presented using means and percentiles for each measure; 2.5th and 97.5 percentiles refer to a 95% CI. P-values below 0.05 are considered statistically significant. All analyses were performed using the statistical software package R, version 2.14.1.22
Results
Pooled and sex-specific characteristics of the participants of the 10-year physical examination are summarized in Table 1. The mean birth weight was 3.40 kg (s.d.=0.44) for female subjects and 3.55 kg (s.d.=0.46) for male subjects. The means of the standardized BMIs for examinations conducted during the first 6 months of life were consistently negative for both genders. Those for the standardized BMIs obtained from physical examinations conducted after 1 year of age tended to be positive, with the exception of that calculated for months 60–64 (−0.03 and −0.01 for female subjects and male subjects, respectively). The s.d.'s were all approximately 1.0, as expected.23 A mean standardized BMI <0 indicates that the entire distribution is shifted downward, which suggests that the majority of the study population have lower BMIs than the reference population. The mean standardized BMI for male subjects (0.24) at 10 years of age was statistically higher than for female subjects (0.13). Overweight rates in male subjects were slightly higher than in female subjects, but were not statistically significant.
Table 1. Characteristics of participants of the physical examination at 10 years of age.
| Mean (s.d.) or % (n/N) | ||
|---|---|---|
| |
Girls (n=1533) |
Boys (n=1588) |
| Study cohort | ||
| GINIplus | 62.9 (964/1533) | 60.3 (957/1588) |
| LISAplus | 37.1 (569/1533) | 39.7 (631/1588) |
| Study center | ||
| Munich | 53.6 (822/1533) | 54.4 (863/1588) |
| Wesel | 30.4 (466/1533) | 29.8 (473/1588) |
| Leipzig | 10.7 (164/1533) | 11.0 (174/1588) |
| Bad Honnef | 5.3 (81/1533) | 4.9 (78/1588) |
| Parental educationa | ||
| Low | 4.8 (73/1527) | 5.9 (93/1582) |
| Medium | 26.6 (406/1527) | 26.9 (425/1582) |
| High | 68.6 (1048/1527) | 67.3 (1064/1582) |
| Family incomeb | ||
| Low | 20.5 (288/1403) | 23.3 (337/1446) |
| Medium | 48.8 (685/1403) | 46.7 (675/1446) |
| High | 30.7 (430/1403) | 30.0 (434/1446) |
| Maternal smoking during pregnancy | 13.9 (194/1398) | 11.8 (173/1469) |
| Birth weightc (kg) | 3.40 (0.44) | 3.55 (0.46) |
| Standardized BMId | ||
| 4–6 weeks | −0.41 (0.93) | −0.36 (1.04) |
| 3-4 months | −0.52 (0.98) | −0.45 (1.06) |
| 6-7 months | −0.33 (0.99) | −0.33 (1.08) |
| 10–12 months | 0.03 (0.95) | 0.05 (1.05) |
| 21–24 months | 0.26 (0.97) | 0.29 (1.06) |
| 46–48 months | 0.05 (0.87) | 0.12 (0.95) |
| 60–64 months | −0.03 (0.87) | −0.01 (0.97) |
| 10 yearsc | 0.13 (1.01) | 0.24 (1.10) |
| Overweighte | ||
| 4–6 weeks | 6.8 (100/1467) | 8.1 (123/1515) |
| 3-4 months | 6.3 (92/1456) | 7.3 (110/1503) |
| 6–7 months | 9.1 (132/1445) | 9.2 (138/1500) |
| 10–12 months | 16.5 (227/1373) | 16.3 (232/1424) |
| 21–24 months | 21.8 (318/1458) | 23.8 (358/1506) |
| 46–48 months | 15.1 (219/1452) | 15.7 (236/1499) |
| 60–64 months | 13.1 (180/1377) | 12.8 (180/1411) |
| 10 years | 20.3 (311/1529) | 21.1 (335/1587) |
Abbreviations: BMI, body mass index; GINIplus, German Infant Nutritional Intervention plus environmental and genetic influences on allergy development; LISAplus, Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood plus Air Pollution and Genetics; WHO, World Health Organization.
Categorized according to the highest number of years either parent attended school: low <10 years, medium=10 years and high >10 years.
Defined according to quartiles of monthly average income: low <25%, medium=25–75% and high >75%.
Significant difference between female and male subjects.
According to WHO definition.20
The baseline characteristics of participants and non-participants of the 10-year follow-up examination are compared in Table 2. Non-participants were more likely to have parents with low-to-medium levels of education, live in families with low income, have mothers who smoked during pregnancy and to be overweight at the age of 3–4, 46–48 and 60–64 months.
Table 2. Differences between participants and non-participants of the 10-year physical examination.
| Mean (s.d.) or % (n/N) | |||
|---|---|---|---|
| |
Participants (n=3121) |
Non-participants (n=5965) |
Total (n=9086) |
| Gender male | 50.9 (1588/3121) | 51.5 (2988/5804) | 51.3 (4576/8925) |
| Study cohorta | |||
| GINIplus | 61.6 (1921/3121) | 68.2 (4070/5965) | 65.9 (5991/9086) |
| LISAplus | 38.4 (1200/3121) | 31.8 (1895/5965) | 34.1 (3095/9086) |
| Study centera | |||
| Munich | 54.0 (1685/3121) | 45.7 (2729/5965) | 48.6 (4414/9086) |
| Wesel | 30.1 (939/3121) | 41.1 (2451/5965) | 37.3 (3390/9086) |
| Leipzig | 10.8 (338/3121) | 10.7 (638/5965) | 10.7 (976/9086) |
| Bad Honnef | 5.1 (159/3121) | 2.5 (147/5965) | 3.4 (306/9086) |
| Parental educationa,b | |||
| Low | 5.3 (166/3109) | 13.4 (788/5881) | 10.6 (954/8990) |
| Medium | 26.7 (831/3109) | 31.0 (1825/5881) | 29.5 (2656/8990) |
| High | 67.9 (2112/3109) | 55.6 (3268/5881) | 59.8 (5380/8990) |
| Family incomea,c | |||
| Low | 21.9 (625/2849) | 25.3 (421/1666) | 23.2 (1046/4515) |
| Medium | 47.7 (1360/2849) | 47.8 (797/1666) | 47.8 (2157/4515) |
| High | 30.3 (864/2849) | 26.9 (448/1666) | 29.1 (1312/4515) |
| Maternal smoking during pregnancya | 12.8 (367/2867) | 20.8 (1112/5358) | 18.0 (1479/8225) |
| Birth weight (kg) | 3.47 (0.46) | 3.47 (0.46) | 3.47 (0.46) |
| Standardized BMId | |||
| 4–6 weeks | −0.38 (0.99) | −0.36 (0.99) | −0.37 (0.99) |
| 3–4 monthsa | −0.48 (1.02) | −0.40 (1.04) | −0.44 (1.03) |
| 6–7 monthsa | −0.33 (1.04) | −0.28 (1.05) | −0.30 (1.05) |
| 10–12 months | 0.04 (1.00) | 0.07 (1.02) | 0.06 (1.01) |
| 21–4 months | 0.27 (1.00) | 0.25 (1.03) | 0.26 (1.02) |
| 46–48 months | 0.09 (0.91) | 0.09 (1.00) | 0.09 (0.96) |
| 60–64 months | −0.02 (0.92) | 0.00 (0.99) | −0.01 (0.95) |
| 10 years | 0.19 (1.06) | — | 0.19 (1.06) |
| Overweighte | |||
| 4–6 weeks | 7.5 (223/2982) | 7.9 (342/4333) | 7.7 (565/7315) |
| 3–4 monthsa | 6.8 (202/2959) | 8.0 (343/4288) | 7.5 (545/7247) |
| 6–7 months | 9.2 (270/2945) | 10.2 (427/4200) | 9.8 (697/7145) |
| 10–12 months | 16.4 (459/2797) | 17.6 (709/4032) | 17.1 (1168/6829) |
| 21–24 months | 22.8 (676/2964) | 22.3 (853/3822) | 22.5 (1529/6786) |
| 46–48 monthsa | 15.4 (455/2951) | 17.1 (551/3214) | 16.3 (1006/6165) |
| 60–64 monthsa | 12.9 (360/2788) | 14.8 (397/2692) | 13.8 (757/5480) |
| 10 years | 20.7 (646/3116) | — | 20.7 (646/3116) |
Abbreviations: BMI, body mass index; GINIplus, German Infant Nutritional Intervention plus environmental and genetic influences on allergy development; LISAplus, Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood plus Air Pollution and Genetics; WHO, World Health Organization.
Significant difference between participants and non-participants.
Categorized according to the highest number of years either parent attended school: low <10 years, medium=10 years and high >10 years.
Defined according to quartiles of monthly average income: low <25%, medium=25–75% and high >75%.
According to WHO definition.20
The associations between the investigated covariates and standardized BMI at 10 years are presented in Table 3. When considering the entire study population, standardized BMI at 10 years was significantly associated with birth weight (β=0.11; 95% CI: 0.03–0.19), standardized BMI at 60–64 months of age (β=0.77; 95% CI: 0.73–0.81), high parental education (β=−0.15; 95% CI: −0.29 to −0.01) and maternal smoking during pregnancy (β=0.13; 95% CI: 0.03–0.22), but neither with high nor medium family income (β=−0.01; 95% CI: −0.11 to 0.09 and β=−0.04; 95% CI: −0.12 to 0.04, respectively). As shown in Table 3, a 1-unit increase in standardized BMI at 60–64 months of age increased the standardized BMI at 10 years of age by 0.78 in female subjects and 0.77 in male subjects. Maternal smoking during pregnancy had a positive effect on standardized BMI at 10 years of age among female subjects and male subjects (β=0.15 and 0.11, respectively), but was only significant among female subjects (P=0.02).
Table 3. Linear regression coefficients for the association between potential risk factors and standardized BMIs at 10 years.
| Risk factorsa | Females (N=1114) | Males (N=1158) | Total (N=2272) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| βb | 95% CI | P-value | β | 95% CI | P-value | β | 95% CI | P-value | ||||
| Birth weight | 0.09 | −0.01, 0.19 | 0.08 | 0.11 | 0.01, 0.21 | 0.03 | 0.11 | 0.03, 0.19 | 0.002 | |||
| Standardized BMIc at 60–64 months | 0.78 | 0.72, 0.84 | <0.001 | 0.77 | 0.73, 0.81 | <0.001 | 0.77 | 0.73, 0.81 | <0.001 | |||
| Family incomed | ||||||||||||
| Low | Ref. | Ref. | Ref. | |||||||||
| Medium | 0.00 | −0.12, 0.12 | 0.93 | −0.07 | −0.19, 0.05 | 0.23 | −0.04 | −0.12, 0.04 | 0.37 | |||
| High | 0.04 | −0.10, 0.18 | 0.50 | −0.06 | −0.20, 0.08 | 0.35 | −0.01 | −0.11, 0.09 | 0.76 | |||
| Parental educatione | ||||||||||||
| Low | Ref. | Ref. | Ref. | |||||||||
| Medium | −0.12 | −0.34, 0.10 | 0.27 | −0.06 | −0.26, 0.14 | 0.53 | −0.10 | −0.26, 0.06 | 0.19 | |||
| High | −0.15 | −0.37, 0.07 | 0.15 | −0.15 | −0.35, 0.05 | 0.14 | −0.15 | −0.29, −0.01 | 0.03 | |||
| Maternal smoking during pregnancy | 0.15 | 0.01, 0.29 | 0.02 | 0.11 | −0.05, 0.27 | 0.16 | 0.13 | 0.03, 0.22 | 0.01 | |||
Abbreviations: CI, confidence interval; Ref., reference; WHO, World Health Organization.
Only risk factors that were associated with standardized BMI at 10 years were included in our prediction model.
Estimated change in 10-year standardized BMI.
Calculated using WHO macros.(18, 19).
Defined according to quartiles of monthly average income: low <25%, medium=25–75% and high >75%.
Categorized according to the highest number of years either parent attended school: low <10 years, medium=10 years and high >10 years.
Being overweight at the age of 60–64 months was a strong risk factor for being overweight at 10 years of age (Figure 1). Children who were overweight at the age of 60–64 months were approximately 15 times more likely to be overweight at the 10-year examination compared with children who were not overweight at the age of 60–64 months. This effect was strongest among female subjects (OR=17.75; 95% CI: 11.62–27.10 vs OR=12.40; 95% CI: 8.41–18.26 for female subjects and male subjects, respectively). The association between high parental education and being overweight at 10 years of age was also statistically significant among female subjects (OR=0.44; 95% CI: 0.22–0.89 vs OR=0.70; 95% CI: 0.36–1.35 for female subjects and male subjects, respectively). Birth weight and maternal smoking during pregnancy were significant in the prediction model for the entire population. In the stratified analysis, maternal smoking during pregnancy (OR=1.63; 95% CI: 1.02–2.59 vs OR=1.17; 95% CI: 0.71–1.91 for female subjects and male subjects, respectively) and high parental education (OR=0.44; 95% CI: 0.22–0.89 vs OR=0.70; 95% CI: 0.36–1.35 for female subjects and male subjects, respectively) were more strongly associated with being overweight at 10 years of age among female subjects compared with male subjects.
The cross-validation results of the linear prediction model are presented in Table 4. In all, 37.0% of female subjects and 38.0% of male subjects who were overweight at 10 years of age were predicted as being overweight; 97.2% of female subjects and 95.5% male subjects who were not overweight at 10 years of age were predicted as being not overweight. Among children who were predicted to be overweight at 10 years, 76.8% of female subjects and 68.1% of male subjects were actually overweight at 10 years of age. Finally, among children who were predicted to be not overweight at 10 years, 86.2% of female subjects and 85.8% of male subjects were actually not overweight at 10 years of age.
Table 4. Cross-validation results (n=1000 iterations) for the prediction modela.
| Girls (N=1114) | Boys (N=1158) | Total (N=2272) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Mean |
2.5% Quantile |
97.5% Quantile |
Mean |
2.5% Quantile |
97.5% Quantile |
Mean |
2.5% Quantile |
97.5% Quantile |
| MSE | <0.001 | <0.001 | 0.003 | 0.002 | <0.001 | 0.005 | <0.001 | <0.001 | 0.002 |
| Sensitivity (%) | 37.0 | 27.2 | 47.1 | 38.0 | 28.8 | 48.3 | 37.1 | 30.8 | 44.1 |
| Specificity (%) | 97.2 | 95.3 | 98.9 | 95.5 | 93.1 | 97.6 | 96.5 | 95.1 | 97.8 |
| PPV (%) | 76.8 | 63.3 | 88.9 | 68.1 | 56.0 | 80.5 | 72.5 | 64.0 | 81.1 |
| NPV (%) | 86.2 | 82.8 | 89.5 | 85.8 | 82.7 | 89.0 | 86.1 | 83.9 | 88.3 |
Abbreviations: BMI, body mass index; MSE, mean-square prediction error; PPV, positive predictive value; NPV, negative predictive value.
Based on the linear prediction model. Being overweight was defined using standard deviations of standardized BMIs at 10 years of age (girls: >1.01; boys: >1.10; total: >1.06).
Discussion
We developed a model that uses anthropometric parameters during the first 5 years of life to predict whether a child will be overweight at 10 years of age. Birth weight, standardized BMI at the age of 60–64 months, parental education, family income and maternal smoking during pregnancy were included in the final prediction model. High birth weight, high standardized BMI at approximately 5 years of age (60–64 months) and maternal smoking during pregnancy increased the risk of being overweight at 10 years. Conversely, children of parents with high parental education were less likely to be overweight at 10 years of age. The sensitivity of our model was low, but the specificity was high. The PPV was higher in the model for female subjects; the NPV was similar across genders.
Previous studies have consistently shown that birth weight is positively associated with being overweight or obese later in life.4, 24 This association is linear in some studies25 and U-shaped in others.26 In our study, birth weight was positively and linearly associated with standardized BMI at 10 years, but this effect was only significant among male subjects. High birth weight was associated with being overweight at 10 years of age, although the association was not statistically significant. One possible reason for the linear relationship between birth weight and BMI later in life is that only full-term neonates with a birth weight >2.5 kg were included in our study. Children with low birth weight tend to show catch-up growth, which might be associated with being overweight later in life.
In our study, we considered the effect of standardized BMIs calculated at different time periods throughout early life and childhood on standardized BMI and being overweight at 10 years of age. Only the standardized BMI at 60–64 months of age was significantly associated with being overweight at the age of 10 years in all models. BMIs up to the age of 4 years do not appear to be closely related to BMI at age of 10 years. These results support the concept that the time and slope of the adiposity rebound, that is, the age at and degree of increase in BMI from the age of approximately 5 years onwards, is a key predictor of later obesity risk.27, 28, 29, 30 This hypothesis is in line with the recent observation that preschool and primary school age are the most critical periods for determining obesity later in life.31 The important impact of the adiposity rebound on later body size may explain why BMI at the approximate age of the adiposity rebound was closely related to BMI at 10 years of age, and BMIs at earlier ages were not. Children who already have a high BMI at the age of 60–64 months are expected to have an earlier age at and a steeper slope of the adiposity rebound. In line with our observation, Angbratt et al.5 also reported that BMI at 5 years was closely associated with BMI at 15 years.
We did several additional sensitivity analyses to confirm the observed high ORs at 60–64 months of age. First, we included being overweight restricted up to the age of 46–48 months in the models, but excluded being overweight at 60–64 months of age. In these models, being overweight at 4 years (46–48 months) was significantly associated with being overweight at 10 years. The magnitude of the 4-year effect decreased substantially when variables at 60–64 months were reintroduced into the models. Second, when we added being overweight at 6 years to the models, being overweight at 5 years remained significantly associated with being overweight at 10 years, whereas the estimate associated with 46–48 months was no longer significant. In summary, BMI/overweight at 5 years (60–64 months) is a stronger predictor of body weight status at 10 years than any other earlier ages we examined. However, these models also imply that being overweight closer to age 10 years may better predict being overweight at 10 years of age. This hypothesis is in line with another longitudinal study based on 907 children, followed from birth to age 11 years, which concluded measurements closer to 11 years of age better predicted being overweight at 11 years of age.4 As the numbers of subjects with measured BMI at 6 years in this study were only half of the total population available, and as our results already revealed that from age 5 years onwards, being overweight was predictive of being overweight at 10 years of age, we did not include information collected at 6 years in the main models.
Several studies have shown that maternal smoking during pregnancy increases the risk of being overweight or obese among children.9, 32, 33 In our study, we found a significant association only among female subjects. Further studies are needed to replicate this result and investigate whether and why gender-specific effects exist. One hypothesis may be that tobacco smoke exposure impacts the metabolism in female subjects differently than that in male subjects. Because second-hand smoking is also a risk factor for being overweight or obese,34 we also studied the effect of this covariate on standardized BMI at 10 years, but found no significant associations.
In previous works, parental education, family household income and occupational status were combined into one socioeconomic status variable (SES).35, 36 Parental education is an independent risk factor for being overweight,37 and there are complex patterns that govern the associations between family income and being overweight, which can also differ by race and gender.38 To try to disentangle these effects, we studied the effect of parental education and family income on the risk of being overweight at 10 years using two separate variables.
A recent Dutch study reported that changes in BMI s.d. scores in each 1-year-interval from birth up to the age of 8 years were all significantly associated with being overweight at 8 years of age. The results of this study suggest that there is no specific critical time period during development that is notably associated with being overweight later in childhood.39 In contrast, our results show that BMI at approximately 5 years of age, which might be the time of the adiposity rebound, is a strong predictor of being overweight later in life. There are important differences between this study and the recent Dutch study, which may explain the differing results. First, self-reported and measured anthropometric data were combined in the Dutch study, whereas only anthropometric data collected during physical examinations was used in our study. Thus, reporting bias and consequently misclassification of being overweight is likely lower in this study. Second, the previous Dutch paper had a larger sample size than this study (3963 vs 2272), but had fewer children with complete information on all variables (489 vs 2272). Missing data were imputed in the Dutch study, whereas we limited our analysis to only those children who provided complete information. Imputing values may introduce more information bias and a greater uncertainty in the results, which could offset the benefits of a greater sample size. Third, confounding variables were taken into account in our study, whereas models were not adjusted for potential confounders in the Dutch study. Finally, information before 2 years of age was available at more time points in our study (at birth, at weeks 4–6, at months 3–4, 6–7, 10–12 in our study compared to at birth, at 3 months, at 1 year of age for the Dutch study).
Nonetheless, our study is not without limitations. Loss to follow-up is inevitable in cohort studies. In our study, 5965 (65.7%) subjects originally recruited at birth did not participate in the 10-year physical examination. Non-participants were more likely to be overweight, come from a family with a low household income and low parental education, and their mothers were more likely to have smoked during pregnancy. A second limitation of this study is that we did not consider all known causes of childhood obesity, such as genetic variation, epigenetics, endocrine disease, diet, physical activity and sleep.12 These risk factors are not available in both the GINIplus and LISAplus study. Furthermore, including a large number of covariates into the prediction model would have hindered our goal of developing a concise and simple model for application.
On the basis of our prediction model, we found that high birth weight, high standardized BMI at approximately 5 years of age and maternal smoking during pregnancy increased the risk of being overweight at 10 years of age. Conversely, having parents with a high level of education decreases this risk. The effects of targeted interventions focused on 5-year-old overweight children should be explored. The prediction model we have developed can be used to identify children at high risk of becoming overweight at the age of 10 years.
Acknowledgments
GINIplus study group: Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology I, Munich (JH, HEW, SS, AZ, CMC, MS, PR); Department of Pediatrics, Marien-Hospital, Wesel (DB, AvB, CB, IG); Department of Pediatrics, Ludwig Maximilians University, Munich (SK, DR, SK-E); Department of Pediatrics, Technical University, Munich (CPB, IB, AG, UH); IUF—Institut für Umweltmedizinische Forschung at the Heinrich-Heine-University, Düsseldorf (UK, EL, CC).
LISAplus study group: Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology I, Munich (JH, HEW, SS, CMC, MS); Department of Pediatrics, Municipal Hospital ‘St Georg', Leipzig (MB, UD), Marien-Hospital Wesel, Department of Pediatrics, Wesel (AvB, CB, IG); Pediatric Practice, Bad Honnef (BS); Helmholtz Centre for Environmental Research—UFZ, Department of Environmental Immunology/Core Facility Studies, Leipzig (IL, MB, CG, SR, MS); University of Leipzig, Institute of Hygiene and Environmental Medicine, Leipzig (OH, CD, JM); IUF—Institut für Umweltmedizinische Forschung, Düsseldorf (UK, EL, CC); Technical University Munich, Department of Pediatrics, Munich (CPB, UH); ZAUM—Center for Allergy and Environment, Technical University, Munich (HB, JG, FM).
The GINI study was funded by Helmholtz Zentrum München and grants of the Federal Ministry for Education, Science, Research and Technology 292 (Grant No. 01 EE 9401-4), and the 6- and 10-year follow-up of the GINIplus study was partly funded by the Federal Ministry of Environment (IUF, FKZ 20462296). The LISAplus study was funded by Helmholtz Zentrum München, Munich Center of Health (MC-Health) and partly by grants of the Federal Ministry of Environment (BMU) (for IUF, 298 FKZ 20462296), and Federal Ministry for Education, Science, Research and Technology (Nos. 01 EG 9705/2 and 01 EG 9732).
This work was supported by the Kompetenznetz Adipositas (Competence Network Obesity) funded by the Federal Ministry of Education and Research (FKZ: 01GI1121A).
The authors declare no conflict of interest.
References
- Chiolero A, Lasserre AM, Paccaud F, Bovet P. Childhood obesity: definition, consequences, and prevalence. Rev Med Suisse. 2007;3:1262–1269. [PubMed] [Google Scholar]
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- Nader PR, O'Brien M, Houts R. Identifying risk for obesity in early childhood. Pediatrics. 2006;118:e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Bann C, Das A, Lester B, Bada H, Bauer CR, et al. Risk for obesity in adolescence starts in early childhood. J Perinatol. 2011;31:711–716. doi: 10.1038/jp.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angbratt M, Ekberg J, Walter L, Timpka T. Prediction of obesity from infancy to adolescence. Acta Paediatr. 2011;100:1249–1252. doi: 10.1111/j.1651-2227.2011.02326.x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, et al. Utility of childhood BMI in the prediction of adulthood disease: comparison of national and international references. Obes Res. 2005;13:1106–1115. doi: 10.1038/oby.2005.129. [DOI] [PubMed] [Google Scholar]
- Kelly LA, Lane CJ, Ball GD. Birth weight and body composition in overweight Latino youth: a longitudinal analysis. Obesity (Silver Spring, MD) 2008;16:2524–2528. doi: 10.1038/oby.2008.401. [DOI] [PubMed] [Google Scholar]
- Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring, MD) 2007;15:760–771. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- Gorog K, Pattenden S, Antova T, Niciu E, Rudnai P, Scholtens S, et al. Maternal smoking during pregnancy and childhood obesity: results from the CESAR Study. Matern Child Health J. 2011;15:985–992. doi: 10.1007/s10995-009-0543-5. [DOI] [PubMed] [Google Scholar]
- Toschke AM, Koletzko B, Slikker W, Jr, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur J Pediatr. 2002;161:445–448. doi: 10.1007/s00431-002-0983-z. [DOI] [PubMed] [Google Scholar]
- Moens E, Braet C, Bosmans G, Rosseel Y. Unfavourable family characteristics and their associations with childhood obesity: a cross-sectional study. Eur Eat Disord Rev. 2009;17:315–323. doi: 10.1002/erv.940. [DOI] [PubMed] [Google Scholar]
- Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzko B, Beyer J, Brands B, Demmelmair H, Grote V, Haile G, et al. Early influences of nutrition on postnatal growth. Nutr Mol Endocr Perspect. 2013;71:11–27. doi: 10.1159/000342533. [DOI] [PubMed] [Google Scholar]
- von Berg A, Koletzko S, Filipiak-Pittroff B, Laubereau B, Grubl A, Wichmann HE, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three-year results of the German Infant Nutritional Intervention Study. J Allergy Clin Immunol. 2007;119:718–725. doi: 10.1016/j.jaci.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, von Berg A, Kramer U, et al. Long-term effects of hydrolyzed protein infant formulas on growth—extended follow-up to 10 y of age: results from the German Infant Nutritional Intervention (GINI) study. Am J Clin Nutr. 2011;94 (Suppl:1803S–1807SS. doi: 10.3945/ajcn.110.000679. [DOI] [PubMed] [Google Scholar]
- Chen CM, Rzehak P, Zutavern A, Fahlbusch B, Bischof W, Herbarth O, et al. Longitudinal study on cat allergen exposure and the development of allergy in young children. J Allergy Clin Immunol. 2007;119:1148–1155. doi: 10.1016/j.jaci.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Flexeder C, Thiering E, Brüske I, Koletzko S, Bauer C.-P, Wichmann H.-E, et al. Growth velocity during infancy and onset of asthma in school-aged children. Allergy. 2012;67:257–264. doi: 10.1111/j.1398-9995.2011.02748.x. [DOI] [PubMed] [Google Scholar]
- WHO Anthro and macros version 3.2.2. Available at http://www.who.int/childgrowth/software/en/ (January 2011).
- WHO AnthroPlus software Available at http://www.who.int/growthref/tools/en/ .
- WHO BMI-for-age cut-offs Available at http://www.who.int/growthref/who2007_bmi_for_age/en/index.html .
- Munakata H, Sei M, Ewis AA, Umeno M, Sato Y, Nakano T, et al. Prediction of Japanese children at risk for complications of childhood obesity: gender differences for intervention approaches. J Med Invest. 2010;57:62–68. doi: 10.2152/jmi.57.62. [DOI] [PubMed] [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria, 2010 ISBN 3-900051-07-0. Available at http://www.R-project.org .
- Global Database on Child Growth and Malnutrition Available at http://www.who.int/nutgrowthdb/about/introduction/en/index5.html .
- Gaskins RB, LaGasse LL, Liu J, Shankaran S, Lester BM, Bada HS, et al. Small for gestational age and higher birth weight predict childhood obesity in preterm infants. Am J Perinatol. 2010;27:721–730. doi: 10.1055/s-0030-1253555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen F, Johansson M. The relation of weight, length and ponderal index at birth to body mass index and overweight among 18-year-old males in Sweden. Eur J Epidemiol. 1998;14:373–380. doi: 10.1023/a:1007411613576. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goulding A. Patterns of growth associated with the timing of adiposity rebound. Obesity (Silver Spring, MD) 2009;17:335–341. doi: 10.1038/oby.2008.547. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goulding A. Early adiposity rebound is an important predictor of later obesity. Obesity (Silver Spring, MD) 2009;17:1310. doi: 10.1038/oby.2009.104. [DOI] [PubMed] [Google Scholar]
- Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes (Lond) 2006;30 (Suppl 4:S11–S17. doi: 10.1038/sj.ijo.0803514. [DOI] [PubMed] [Google Scholar]
- Williams SM. Weight and height growth rate and the timing of adiposity rebound. Obes Res. 2005;13:1123–1130. doi: 10.1038/oby.2005.131. [DOI] [PubMed] [Google Scholar]
- von Kries R, Beyerlein A, Müller MJ, Heinrich J, Landsberg B, Bolte G, et al. Different age-specific incidence and remission rates in pre-school and primary school suggest need for targeted obesity prevention in childhood. Int J Obes (Lond) 2012;36:505–510. doi: 10.1038/ijo.2011.251. [DOI] [PubMed] [Google Scholar]
- Koshy G, Delpisheh A, Brabin BJ. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health. 2011;21:286–291. doi: 10.1093/eurpub/ckq173. [DOI] [PubMed] [Google Scholar]
- von Kries R, Toschke AM, Koletzko B, Slikker W. Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156:954–961. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- Raum E, Kupper-Nybelen J, Lamerz A, Hebebrand J, Herpertz-Dahlmann B, Brenner H. Tobacco smoke exposure before, during, and after pregnancy and risk of overweight at age 6. Obesity (Silver Spring, MD) 2011;19:2411–2417. doi: 10.1038/oby.2011.129. [DOI] [PubMed] [Google Scholar]
- Lin SL, Leung GM, Hui LL, Lam TH, Schooling CM. Is informal child care associated with childhood obesity? Evidence from Hong Kong's ‘Children of 1997' birth cohort. Int J Epidemiol. 2011;40:1238–1246. doi: 10.1093/ije/dyr086. [DOI] [PubMed] [Google Scholar]
- Jones-Smith JC, Gordon-Larsen P, Siddiqi A, Popkin BM. Cross-national comparisons of time trends in overweight inequality by socioeconomic status among women using repeated cross-sectional surveys from 37 developing countries, 1989–2007. Am J Epidemiol. 2011;173:667–675. doi: 10.1093/aje/kwq428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta M, Bibi H, Haviv J, Scharf S, Gdalevich M. Parental smoking and education as determinants of overweight in Israeli children. Prev Chronic Dis. 2006;3:A48. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Q. Are American children and adolescents of low socioeconomic status at increased risk of obesity? Changes in the association between overweight and family income between 1971 and 2002. Am J Clin Nutr. 2006;84:707–716. doi: 10.1093/ajcn/84.4.707. [DOI] [PubMed] [Google Scholar]
- Willers SM, Brunekreef B, Smit HA, van der Beek EM, Gehring U, de Jongste C, et al. BMI development of normal weight and overweight children in the PIAMA study. PloS One. 2012;7:e39517. doi: 10.1371/journal.pone.0039517. [DOI] [PMC free article] [PubMed] [Google Scholar]