Abstract
We describe outcomes after allogeneic transplantation in 34 patients with dyskeratosis congenita transplanted between 1981 and 2009. The median age at transplantation was 13 years (range 2 – 35). Approximately 50% of transplants were from related donors. Bone marrow was the predominant source of stem cells (n=24/34). The day-28 probability of neutrophil recovery was 73% and the day-100 platelet recovery was 72%. The day-100 probability of grade II-IV acute GVHD and the 3-year probability of chronic GVHD were 24% and 37%, respectively. The 10-year probability of survival was 30%; 14 patients were alive at last follow-up. Ten deaths occurred within 4 months from transplantation due to graft failure (n=6) or other transplant-related complications; 9 of these patients had been transplanted from mismatched related or from unrelated donors. Another 10 deaths occurred after 4 months; 6 of them occurred more than 5 years from transplantation, 4 of these were attributed to pulmonary failure. Transplant-regimen intensity and transplants from mismatched related or unrelated donors were associated with early mortality. Transplantation of grafts from HLA-matched siblings with cyclophosphamide-containing non-radiation regimens was associated with early low toxicity. Late mortality was attributed mainly to pulmonary complications and likely related to the underlying disease.
INTRODUCTION
Dyskeratosis congenita (DC) is a rare, inherited, heterogeneous, multisystem disorder of bone marrow failure and cancer susceptibility. Classical DC is characterized by the clinical diagnostic triad of nail dystrophy, lacy reticular pigmentation of the neck and upper chest, and oral leukoplakia(1). While the mucocutaneous triad may be subtle, hematologic abnormalities are common, affecting approximately 80-90% of patients by 30 years of age. Mutations in DC causative genes (TERT and TERC) have been detected in subsets of patients with apparently acquired aplastic anemia or myelodysplastic syndrome (1). Bone marrow failure (BMF) is a common cause of premature death in DC; other causes of include obstructive and interstitial pulmonary complications and malignancies(2,3). Patients with DC have extremely short telomeres (<1st percentile for their age) due to germline defects in telomere biology (4). A germline mutation in a key telomere biology gene is identified in approximately two-thirds of DC families(1,5, 6). Telomeres consist of long TTAGGG nucleotide repeats and a protein complex termed “shelterin” at chromosome ends; they are essential for maintaining chromosomal stability(7). Telomere length measurement by flow cytometry with fluorescent in situ hybridization (flow-FISH) in leukocyte subsets is highly sensitive and specific for diagnosing DC(4). BMF in patients with DC does not respond to immunosuppressive therapy(8). Hematopoietic cell transplantation (HCT) is currently the only modality with curative potential for the bone marrow defect. No standard protocols are available for HCT in DC patients but recent data suggest successful engraftment and lower toxicity with reduced intensity protocols resulting in better overall short-term survival (6,9-12). In this review, we used data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) to describe outcomes after HCT in a larger cohort of patients with DC.
Methods
Data source
The CIBMTR is a voluntary working group of more than 450 transplant centers worldwide that contribute patient, disease, transplant, and outcome information on allogeneic and autologus transplantations. Participating centers report consecutive transplants. Data are reported to a Statistical Center at the Medical College of Wisconsin or the Data Coordinating Center, National Marrow Donor Program, Minneapolis. Thirty-four transplants for DC were reported by 26 transplant centers from 1981 to 2009 (32 transplants occurred after 1989). The diagnosis of DC was assigned by the transplant center and BMF, the indication for transplantation. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
End points
The primary outcome was overall survival; death from any cause was considered as an event and surviving patients censored at last follow-up. Other assessed outcomes were 1) neutrophil recovery, defined as achieving an absolute neutrophil count ≥ 0.5 ×109/L for 3 consecutive days; 2) platelet recovery, defined as achieving a platelet count ≥20 × 109/L independent of transfusions for 7 consecutive days; 3) acute (grade II-IV, and III-IV graft versus host disease (GVHD) and 4) chronic GVHD. Acute and chronic GVHD were defined according to standard criteria(13, 14).
Statistical analysis
The probability of overall survival was calculated using the Kaplan-Meier estimator(15). Patients were followed from transplantation until death or last contact for surviving patients. The probabilities of neutrophil recovery, platelet recovery, acute and chronic GVHD were calculated using the cumulative incidence estimator with death as the competing risk(16). Ninety-five percent confidence intervals (CI) were generated using log transformation. Analyses were performed using SAS version 9.3 (Carey, NC).
Results
Patient, disease and transplant characteristics are shown in Tables 1, 2A and 2B. The median age at transplantation was 13 years (range 2 – 35). For over half of transplantations the interval between transplantation and diagnosis of DC was greater than 3 years. Pre-transplant co-morbidities were reported for about 60% of transplants; cytomegalovirus (CMV) infection was the most frequently reported co-morbidity. Approximately 50% of transplants utilized grafts from a related donor. Bone marrow was the predominant source of stem cells. Various transplant conditioning regimens were used. Nine patients received total body irradiation (TBI) for 200 cGy (n=5), 400 cGy (n=1), 450 cGy (n=1) or 500 cGy (n=1); the dose was unknown in one patient. When cyclophosphamide was used with TBI, the dose was 200 mg/kg except for one patient who received low dose TBI (200 cGy) and 50 mg/kg of cyclophosphamide. The remaining patients received alkylating agents with or without fludarabine (10 patients received cyclophosphamide alone at 200 mg/kg; the donors were HLA-matched siblings in 8 and unrelated individuals in 2). When cyclophosphamide was used with fludarabine the dose was 120 mg/kg and with busulfan, 120 mg/kg or 200 mg/kg. In the 4 patients who received bususlfan or melphalan combined with fludarabine the busulfan dose was < 6 mg/kg, and the melphalan dose < 150 mg/m2. The median follow-up of surviving patients was 46 (range 3 – 116) months.
Table 1.
Patient, disease and transplant characteristics
| Variable | N (%) |
|---|---|
| Number of patients | 34 |
| Age at transplant, years | |
| 2-9 | 14 (41) |
| 10-19 | 11 (32) |
| 20-29 | 7 (21) |
| 30-35 | 2 (6) |
| Sex | |
| Male | 28(82) |
| Female | 6 (18) |
| Performance score | |
| <90% | 10 (29) |
| 90 - 100% | 18 (53) |
| Not reported | 6 (18) |
| Comorbid diseases | |
| CMV infection+pulmonary disease +liver disease | 1 ( 3) |
| CMV infection+pulmonary disease | 1 ( 3) |
| CMV infection only | 14 (41) |
| Comorbid not specified* | 4 (12) |
| No comorbidity | 14 (41) |
| Interval from DC diagnosis to transplant, months | |
| Median (range) | 38 (4-214) |
| <12 | 10 (29) |
| 12-23 | 2 ( 6) |
| 24-35 | 3 ( 9) |
| 36-60 | 7 (21) |
| >60 | 12 (35) |
| Conditioning regimens | |
| Cyclophosphamide + **TBI ≤500 cGy | 6 (17) |
| TBI 200 cGy + fludarabine | 3 ( 9) |
| Melphalan*** + fludarabine | 3 ( 9) |
| Busulfan**** + fludarabine | 1 ( 3) |
| Cyclophosphamide + fludarabine | 4 (12) |
| Cyclophosphamide + busulfan | 6 (18) |
| Cyclophosphamide only | 10 (29) |
| Not reported | 1 ( 3) |
| Graft type | |
| Bone marrow | 24 (71) |
| Peripheral blood | 7 (21) |
| Cord blood | 3 ( 9) |
| Type of donor | |
| HLA-identical sibling | 16 (47) |
| Other relative | 2 ( 6) |
| Matched unrelated donor | 9 (26) |
| Mismatched unrelated donor | 7 (21) |
| Graft-versus-host disease prophylaxis | |
| Ex vivo T-cell depletion | 1 ( 3) |
| CD34 selection | 1 ( 3) |
| Tacrolimus-containing | 5 (15) |
| Cyclosporine-containing | 26 (76) |
| Not reported | 1 ( 3) |
| In vivo T-cell depletion | |
| Anti-thymocyte globulin | 10 (29) |
| Alemtuzumab | 3 ( 9) |
| None | 15 (44) |
| Not reported | 6 (18) |
| Follow-up, surviving patients | 46 (3-116) |
Abbreviations: TBI = total body irradiation
Other comorbid includes: organ impairment (n=1), external otitis, peumocystis carinii pneumonia (PCP) infection (n=1), and not specified (n=2)
Doses: Cyclophosphamide +TBI ≤ 500 cGy: TBI dose 200 cGy (n=3), 400 cGy (n=1), 450 cGy (n=1) and 500 cGy (n=1)
Melphalan dose: 138 mg/m2, 72 mg/m2, 140 mg/m2
Busulfan dose: 3mg/kg (n=1)
Table 2A.
Characteristics of patients who are alive after transplantation
| Age at diagnosis |
Age at transplant |
Year of transplant |
Donor source |
Conditioning regimen |
GVHD | Time to last contact |
Status |
|---|---|---|---|---|---|---|---|
| 7.7 years | 18.0 years | 1992 | HLA-matched sibling |
Busulfan + cyclophosphamide |
Chronic | 114.3 months |
Alive |
| 15.0 years | 18.5 years | 1997 | HLA-matched sibling |
Cyclophosphamide | Chronic | 73 months |
Alive |
| 11.1 years | 17.6 years | 2000 | HLA-matched sibling |
Cyclophosphamide | None | 82.2 months |
Alive |
| 19.4 years | 21.7 years | 2001 | HLA-matched sibling |
Cyclophosphamide | None | 115.7 months |
Alive |
| 9.0 years | 13.3 years | 2006 | Matched unrelated donor* |
Melphalan + fludarabine |
None | 59.6 months |
Alive |
| 6.5 years | 6.8 years | 2007 | HLA-matched sibling |
Cyclophosphamide | Chronic | 49.1 months |
Alive |
| 24.7 years | 35.1 years | 2007 | Matched unrelated donor* |
Busulfan + fludarabine |
Chronic | 60.4 months |
Alive |
| 6.6 years | 13.7 years | 2008 | HLA-matched sibling* |
Cyclophosphamide | None | 3.32 months |
Alive |
| 1.1 years | 3.2 years | 2008 | Matched unrelated donor |
TBI 200 cGy + fludarabine |
None | 29.2 months |
Alive |
| 4.3 years | 4.7 years | 2008 | Matched unrelated donor |
Cyclophosphamide fludarabine |
None | 38.7 months |
Alive |
| 4.1 years | 9.4 years | 2008 | Matched unrelated donor |
TBI 200 cGy + fludarabine |
Chronic | 39.8 months |
Alive |
| 1.7 years | 2.3 years | 2008 | Matched unrelated donor |
Cyclophosphamide | Chronic | 43.2 months |
Alive |
| 19.4 years | 19.9 years | 2009 | HLA-matched sibling* |
Cyclophosphamide + fludarabine |
Acute | 12.5 months |
Alive |
| 4.4 years | 4.8 years | 2009 | HLA-matched sibling |
Unknown | None | 37.4 months |
Alive |
peripheral blood progenitor cells; others received bone marrow
Table 2B.
Characteristics of patients who are died after transplantation
| Age at diagnosis |
Age at transplant |
Year of transplant |
Donor source |
Conditioning regimen |
GVHD | Time to last |
Cause of death |
|---|---|---|---|---|---|---|---|
| 4.1 years | 8.0 years | 1981 | HLA- matched sibling |
TBI 450 cGy+ cyclophosphamide |
Chronic | 71.1 months |
GVHD |
| 17.5 years | 26.0 years | 1984 | Mismatched relative |
TBI 500 cGy + cyclophosphamide |
None | 2.6 months |
Graft failure |
| 3.8 years | 5.0 years | 1990 | HLA- matched sibling |
Cyclophosphamide | Acute & Chronic |
111.3 months |
Pulmonary failure |
| 1.7 years | 2.1 years | 1992 | Mismatched unrelated donor |
Busulfan + cyclophosphamide |
None | 1.3 months |
Graft failure |
| 8.8 years | 16.3 years | 1993 | HLA- matched sibling |
Cyclophosphamide | None | 144.0 months |
Pulmonary failure |
| 4.7 years | 10.0 years | 1993 | Mismatched relative |
Busulfan + fuldarabine |
Acute & Chronic |
16.8 months |
Infection |
| 4.14 years | 7.6 years | 1994 | HLA- matched sibling |
TBI + cyclophosphamide |
Acute & Chronic |
3.8 months |
ARDS |
| 9.6 years | 13.6 years | 1994 | HLA- matched sibling |
Busulfan + fludarabine |
Acute | 27.5 months |
Pulmonary failure |
| 5.0 years | 8.0 years | 1996 | Mismatched unrelated donor§ |
Busulfan + cyclophosphamide |
Acute | 2.9 months |
Graft failure |
| 11.8 years | 25.8 years | 1998 | Mismatched unrelated donor |
TBI 400 cGy+ cyclophosphamide |
None | 3.3 months |
Graft failure |
| 5.1 years | 8.0 years | 1998 | HLA- matched sibling |
Cyclophosphamide | None | 120.0 months |
Pulmonary failure |
| 5.5 years | 7.5 years | 2000 | HLA- matched sibling |
Cyclophosphamide + fludarabine |
None | 16.1 months |
Graft failure |
| 4.5 years | 13.0 years | 2001 | HLA- matched sibling* |
Busulfan + fludarabine |
Acute | 70.5 months | Not reported |
| 10.0 years | 13.2 years | 2003 | Mismatched unrelated donor* |
Cyclophosphamide + fludarabine |
None | 2.0 months |
Graft failure |
| 32.1 years | 33.0 years | 2003 | Mismatched unrelated donor§ |
Melphalan + fludarabine |
None | 1.6 months |
Hemorrhage |
| at birth | 17.8 years | 2004 | Matched unrelated donor* |
TBI 200 cGy + fludarabine |
Acute | 87.5 months |
Pulmonary failure |
| 21.2 years | 22.0 years | 2005 | Mismatched unrelated donor |
Melphalan + fludarabine |
Chronic | 16.2 months |
Graft failure |
| 23.0 years | 24.0 years | 2005 | Mismatched unrelated donor§ |
TBI 200 cGy + cyclophosphamide |
None | 2.1 months |
Graft failure |
| 11.9 years | 29.4 years | 2008 | Matched unrelated donor |
Cyclophosphamide | None | 1.8 months |
Encephalopathy |
| 22.0 years | 22.9 years | 2009 | Matched unrelated donor |
TBI 200 cGy | None | 0.6 months |
Infection |
peripheral blood progenitor cells
umbilical cord blood; others received bone marrow
indicate additional drugs.
Abbreviations: TBI, total body irradiation; ARDS, acute respiratory distress syndrome
Hematopoietic recovery, GVHD, and post-transplant malignancies
Thirty patients achieved neutrophil recovery, with a cumulative incidence of 73% (95% CI 53 – 85) by day 28. Four patients with primary graft failure died, two of them after a second transplant attempt. Among the 30 patients with neutrophil recovery, six (20%) developed secondary graft failure (four died, and 2 are surviving after second transplants at two and four months after the first transplant, respectively). All but one graft failure occurred in recipients of mismatched related or unrelated donor transplants; the transplant conditioning regimens for these patients is shown in Table 2B The day-100 probability of platelet recovery in the 25 evaluable patients was 72% (95% CI 49 – 86).
Eight patients developed acute GVHD (4 grade II, 1 grade III, and 3 grade IV). The day-100 probability of acute GVHD grades II-IV was 24% (95% CI 11 – 39). Eleven patients developed chronic GVHD (4 limited, 6 extensive, and 1 unknown). Three of the 11 patients had a prior history of acute GVHD. The 3-year probability of chronic GVHD was 37% (95% CI 19 – 54).
Three patients developed post-transplant malignancies: n = 1 EBV associated lymphoproliferative disease (1 month post-HCT at age 5 years; conditioning regimen: cyclophosphamide and fludarabine with in vivo T-cell depletion); n = 1 squamous cell carcinoma of the skin (4 months post-HCT at age 22 years; conditioning regimen: melphalan and fludarabine; chronic GVHD onset: 4 months); n = 1 basal cell carcinoma of the skin (2 months post-HCT at age 35 years; conditioning regimen: busulfan and fludarabine; chronic GVHD onset 10 months). Although both patients with skin cancer reported chronic GVHD, the onset of chronic GVHD coincided or was after the onset of skin cancer. Neither patient reported a history of acute GVHD.
Overall Survival
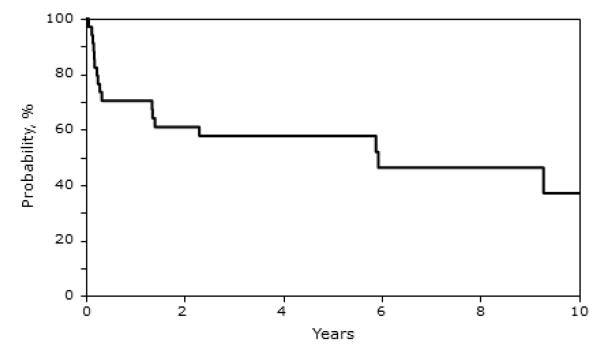
Twenty of the 34 patients (59%) died and 10 of these deaths occurred during the first 4 months after transplantation). Not unsurprisingly, the 5-year probabilities of overall survival were higher for the 21 patients transplanted between 2000 and 2009 (65%, 95% CI 40-82) compared to the 11 patients transplanted prior to 2000 (46%, 95% CI 19 – 70). Tables 2A and 2B summarize the transplant characteristics of patients who are alive and deceased, respectively. The most common causes of death were graft failure and pulmonary failure/complications. Early deaths (n=10) were attributed mainly to primary or secondary graft failure (n=6). Eight early deaths occurred after unrelated donor transplants, one after a mismatched related donor transplant, and one after an HLA-matched sibling donor transplant. Deaths beyond 4 months (n=10; 6 occurred beyond 5 years) were attributed to pulmonary failure/complications (n=5), GVHD (n=1), infection (n=1), graft failure (n=2); in one patient the cause of death was not reported. None of the patients reported to have died from pulmonary failure were reported to have had pulmonary disease at transplantation. Notably, the use of cyclophosphamide alone for conditioning was associated with the longest survival. It resulted in one early death at 1.8 months post-transplant after an unrelated donor transplant, and 3 additional deaths attributed to pulmonary complications occurred at 9, 10 and 12 years after transplantation, respectively. These very late events occurred in recipients of HLA-matched sibling transplants. The 1-, 5-, 10- and 12-year probabilities of overall survival were 70%, 57%, 30%, and 15%, respectively (Figure 1).
Figure 1.
Probalility of Overall Survival
DISCUSSION
In this retrospective study, we present a systematic evaluation of HCT-related outcomes in patients with DC reported to a transplant registry. Published case reports suggested high transplant-related mortality and organ toxicity in patients with DC(2). Patients in the present study had a slightly inferior survival compared to that reported by Dietz and colleagues, 57% vs. 64% at 5-years (9). This may be explained in part by the fact that our study population underwent HCT over a 20-year period and changes in transplantation strategies including donor selection and supportive care may have influenced survival. In our population transplanted between 2000 and 2009, we also observed a 5-year survival rate of 65% albeit in twenty-one patients. There was a predominance of male patients in our study, which is likely due to the over-representation of the X-linked form of the disease and the fact that about 20% of classic DC patients have a germline mutation in the X-linked gene, DKC1. In the past decade autosomal dominant (TINF2, TERC, TERT, and RTEL1) and autosomal recessive (NOP10, NHP2, WRAP53, TERT, RTEL1 and CTC1) genes leading to DC have been recognized (1,5,6). It is possible that in this historical cohort, DC females were under-diagnosed.
Despite improvement in transplant outcomes in many disorders, patients with DC still face significant challenges; the 5-year post-transplant survival probability was only 57%. Our data suggest that DC patients receiving low intensity conditioning have fewer early adverse events, but continue to suffer from late severe outcomes (mainly pulmonary toxicity, including fibrosis). On the other hand, high-dose conditioning regimens were associated with severe organ toxicity and death consistent with previously published case reports (2). The use of reduced intensity regimens has resulted in successful engraftment and lower toxicity in several recent studies (9-12) and our observations are consistent with these reports. But pulmonary fibrosis may develop in these patients at any time in the course of the disease as part of disease pathogenesis. Germline mutations in TERT or TERC can cause both apparently isolated aplastic anemia and pulmonary fibrosis. However, further evaluation of family history often reveals features of a DC-related telomere biology disorder. HCT is an effective treatment for DC related bone marrow aplasia, but doesn’t correct abnormalities related to the underlying genetic defects in DC. In agreement with earlier reports (2, 17), the high proportion of death due to pulmonary causes in the current report suggests that pulmonary complications may indeed be accelerated after HCT. Pulmonary fibrosis in DC patients may reflect cell apoptosis resulting from critically short telomeres in rapidly dividing lung cells. Therefore, our data suggest that the optimal preparative regimen should be one that minimizes pulmonary toxicity. It may also be prudent to perform regular pulmonary function tests as a screening tool for early diagnosis of pulmonary failure after HCT. Of note, successful lung transplantation has been performed in a patient with DC but additional studies are required to ascertain long-term outcomes.
In agreement with other reports (18, 19), DC patients who were transplanted from HLA-identical siblings had better overall survival. However, it is important to note that due to significant disease heterogeneity and the presence of silent carriers of disease-causing mutations, all potential related donors must be carefully evaluated for DC. We recommend all potential related donors should have mutation testing for the causative gene identified in the index patient. If the causative gene is unknown, telomere length measurement by flow-FISH should be carried out to rule-out occult DC in the potential related donor. Needless to say, the presence of the causative gene and/or short telomere length in apparently unaffected family members, bar these individuals from serving as donors for patients with DC. An earlier case report(20) described a complicated post-transplant course (delayed engraftment with long-lasting neutropenia and death from sepsis approximately 6 month after transplantation) in an adult patient who received HCT for an apparent acquired severe aplastic anemia that was subsequently recognized as DC. The apparently healthy matched sibling was a clinically silent carrier of the same germline mutation; the sibling short telomeres and TERT mutation were identified several years later (20).
Ten of the 34 patients in the present study did not engraft or experienced late graft failure after primary engraftment. Impaired engraftment in patients with DC might be explained by altered cytokine expression and bone marrow stromal cell function defects associated with telomere dysfunction (21). Late graft failure after primary engraftment has been reported in association with short telomeres in 2 reported cases (22).
Three patients developed post-transplant malignancies, a complication that is not rare in transplant recipients (23). However, it is important to note that the 2 solid cancers (squamous cell carcinoma of the skin and basal cell carcinoma) reported here occurred very early after transplantation, which is less common. Our data support early surveillance for skin cancer in DC patients following HCT. The occurrence of skin cancer may be explained by the known high cancer risk in DC patients (reported to be 11-fold higher than in the general population). This risk is greatest for cancers of the tongue, acute myeloid leukemia, cancer of the cervix, non-Hodgkin lymphoma, and basal cell carcinoma of the skin (18). It is noteworthy that both patients with skin cancer were older, in their third and fourth decade and the occurrence of cancer may be a direct consequence of the underlying diagnosis of DC rather than the transplantation procedure. That said the high cancer risk and pulmonary complications in DC patients call for investigation of HCT regimens customized specifically for DC; an experience that proved successful in HCT for Fanconi anemia patients (24). Our data suggest that when the intensity of the transplant-conditioning regimen is low, early mortality is low but these patients develop pulmonary complications several years later leading to their demise.
Our study is the largest to-date and provides the first quantitative assessment of HCT outcomes in a sizable cohort of DC patients. However, it is limited by the lack of power to evaluate predictors of outcomes due to limited sample size, a variety of transplant conditioning regimens and the biological heterogeneity of DC. Furthermore, under-representation of DC patients with atypical clinical features is expected. A larger collaborative study with well-defined clinical phenotypes and laboratory characterizations is needed to validate and expand on our observations. In the meantime, for DC patients with BMF, allogeneic HCT with a suitably matched related or unrelated donor remains an acceptable treatment option. The choice of transplant conditioning regimen is important, with regimens of lesser intensity being favored. The data suggest long-term surveillance is important particularly for cancers and pulmonary complications both of which are likely to be secondary to the genetic defect that is not corrected by HCT. This is invaluable information when counseling patients and families. It is also crucial to incorporate telomere length measurements in the pre-transplant evaluation of patients with apparently acquired severe aplastic anemia to identify individuals with unrecognized DC and treat them accordingly.
ACKNOWLEDGEMENT
Supported by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases, Heath Resources and Services Administration (HHSH234200637015C) and the intramural program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (SMG, BPA, NG, PSR and SAS). The opinions, findings, and conclusions or recommendations expressed herein are those of the authors and do not reflect the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest
REFERENCES
- 1.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr Transplant. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 3.Giri N, Lee R, Faro A, et al. Lung transplantation for pulmonary fibrosis in dykeratosis congenita: case report and systematic literature review. BMC Blood Disord. 2011;11:3. doi: 10.1186/1471-2326-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walne AJ, Bhagat T, Kirwan M, et al. Mutation in the telomere capping complex in bone marrow failure. Haematologica. 2013;98:334–338. doi: 10.3324/haematol.2012.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballew BJ, Yaeger M, Jacobs K, et al. Germline mutations of regulator of telomere elongation helicase1, RTEL1 in dyskeratosis congenita. Hum Genet. 2013;132:473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mech Ageing Dev. 2008;129:35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Rahawan MM, Giri N, Alter BP. Intensive immunosuppression therapy for aplastic anemia associated with dyskeratosis congenita. Int J Hematol. 2006;83:275–276. doi: 10.1532/IJH97.06030. [DOI] [PubMed] [Google Scholar]
- 9.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishio N, Takahashi Y, Ohashi H, et al. Reduced-intensity conditioning for alternative donor hematopoietic stem cell transplantation in patients with dyskeratosis congenita. Pediatr Transplant. 2011;15:161–166. doi: 10.1111/j.1399-3046.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 11.Vuong LG, Hemmati PG, Neuburger S, et al. Reduced-intensity conditioning using fludarabine and antithymocyte globulin alone allows stable engraftment in a patient with dyskeratosis congenita. Acta Haematol. 2010;124:200–203. doi: 10.1159/000318721. [DOI] [PubMed] [Google Scholar]
- 12.Ayas M, Nassar A, Hamideh AA, et al. Reduced intensity conditioning is effective for hematopoietic SCT in dyskeratosis congenital-related BM failure. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.35. (DOI:10:1038/bmt2013.35) [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 16.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representation of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Rocha V, Devergie A, Socie G, et al. Unusual complications after bone marrow transplantation for dyskeratosis congenita. Br J Haematol. 1998;103:243–248. doi: 10.1046/j.1365-2141.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 18.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw PH, Haut PR, Olszewski M, Kletzel M. Hematopoietic stem-cell transplantation using unrelated cord-blood versus matched sibling marrow in pediatric bone marrow failure syndrome: one center’s experience. Pediatr Transplant. 1999;3:315–321. doi: 10.1034/j.1399-3046.1999.00062.x. [DOI] [PubMed] [Google Scholar]
- 20.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 21.Ju Z, Jiang H, Jaworski M, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 22.Awaya N, Baerlocher GM, Manley TJ, et al. Telomere shortening in hematopoietic stem cell transplantation: a potential mechanism for late graft failure? Biol Blood Marrow Transplant. 2002;8:597–600. doi: 10.1053/bbmt.2002.v8.abbmt080597. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia - when and how? Br J Haematol. 2010;149:14–21. doi: 10.1111/j.1365-2141.2010.08078.x. [DOI] [PubMed] [Google Scholar]