Abstract
This study describes a novel spatial memory paradigm for monkeys and reports the effects of neonatal damage to the hippocampus on performance in adulthood. Monkeys were trained to forage in eight boxes hung on the walls of a large enclosure. Each box contained a different food item that varied in its intrinsic reward value, as determined from food preference testing. Monkeys were trained on a spatial and a cued version of the task. In the spatial task, the boxes looked identical and remained fixed in location whereas in the cued task, the boxes were individuated with colored plaques and changed location on each trial. Ten adult Rhesus macaques (5 neonatal sham-operated and 5 with neonatal neurotoxic hippocampal lesions) were allowed to forage once daily until they preferentially visited boxes containing preferred foods. The data suggest that all monkeys learned to discriminate preferred from nonpreferred food locations, but that monkeys with neonatal hippocampal damage committed significantly more working memory errors than controls in both tasks. Furthermore, following selective satiation, controls altered their foraging pattern to avoid the satiated food, whereas lesioned animals did not, suggesting that neonatal hippocampal lesions prohibit learning of specific food-place associations. We conclude that whereas an intact hippocampus is necessary to form specific item-in-place associations, in its absence, cortical areas may support more broad distinctions between food types that allow monkeys to discriminate places containing highly preferred foods.
Keywords: monkey, hippocampus, spatial, memory, episodic
Results from several decades of research suggest that animals can remember the location of items of biological significance (e.g., food, shelter) in their environment (Kessels, de Haan, Kappelle, & Postma, 2001; King, Trinkler, Hartley, Vargha-Khadem, & Burgess, 2004; Lopez et al., 2001; O’Keefe & Nadel, 1978). It is the encoding and use of the spatial information needed to remember such important locations for which an intact hippocampus is believed to be especially critical. Numerous studies from both the animal and human literature demonstrate that damage to the hippocampus in animals or amnesic patients produces deficits in spatial memory, and electrophysiological recording in animals and imaging in humans show selective activity in the hippocampal region during spatial navigation tasks (Holscher, 2003; Hough & Bingman, 2004; Iaria, Petrides, Dagher, Pike, & Bohbot, 2003; Maguire, Frith, Burgess, Donnett, & O’Keefe, 1998; Morris, Garrud, Rawlins, & O’Keefe, 1982; Muller, Kubie, & Ranck, 1987; O’Keefe & Dostrovsky, 1971; Vargha-Khadem et al., 1997).
Similar studies in primates are relatively few, and their findings remain inconclusive. Spared and impaired performance have been reported in tasks requiring the monkey to use a nonmatching-to-location rule, depending upon the number of locations, and/or the length of the delay (Alvarado, Wright, & Bachevalier, 2002; Angeli et al., 1993; Beason-Held, Rosene, Killiany, & Moss, 1999; Parkinson, Murray, & Mishkin, 1988; but see Murray & Mishkin, 1998, for negative result). Similarly equivocal findings were observed in tasks requiring the animal to associate object and place (Gaffan, 1994; Gaffan & Harrison, 1989; Murray, Baxter, & Gaffan, 1998; but see Málková & Mishkin, 2003, for negative result), or detect spatial changes of objects within the environment (Bachevalier & Nemanic, 2008; Gaffan & Harrison, 1989; Parker & Gaffan, 1997; Poucet, 1989). Monkeys with neurotoxic lesions of the hippocampus are also impaired on tasks that require object discrimination reversals and spatial scene learning, but not on place discrimination and reversals (Murray et al., 1998; although aspiration lesions do impair place reversals; Mahut, 1971; Mahut & Zola, 1973) or serial spatial reversals (Ridley et al., 1997). In a recent review, Lavenex and Lavenex (2009) suggested that the above tasks predominantly rely on egocentric (rather than allocentric) encoding and as such are unlikely to require the intact hippocampus.
By extension, foraging tasks in which the subject is freely moving offer a distinct advantage because the monkey is required to navigate through an environment to search for food, making the tasks more consistent with natural tendencies, and increasing the likelihood that spatial processing is necessary to solve the task rather than an alternative, nonspatial strategy. To date, only a few groups have used such freely moving foraging tasks to investigate the role of the hippocampus in spatial memory. For example, Hampton, Hampstead, and Murray (2004) used an open field to test the effects of adult hippocampal lesions on spatial memory and found that an intact hippocampus is critical for remembering goal locations for longer than a few minutes, although only three locations were used in this task. Lavenex, Amaral, and Lavenex (2006) and Lavenex, Lavanex, and Amaral (2007) used a similar task with adult and neonatal hippocampectomized monkeys. This design utilized 18 inverted cups arranged in concentric hexagons in an open field. Monkeys were required to learn two patterns of possible baited cups using spatial or cued locations. They found that adult lesions of the hippocampus prevented spatial relational learning (Lavenex et al., 2006), but that neonatal lesions did not (Lavenex et al., 2007). A drawback to their design, however, is that the process of foraging at a potentially baited site permanently reveals that site as visited, by means of an overturned or otherwise displaced cup. This renders the task free from the need for working memory and eliminates the possibility of measuring within-trial repetition errors. Furthermore, although relational in nature (i.e., the rewarded cups are positioned in a particular relationship with the maze and with each other), all possible rewarded locations are associated with the corners of the hexagons, a powerful internal cue, and thus on the basis of a single selection, it is possible to eliminate nonpossible choices.
In an open field variant of the radial-arm maze, Rapp, Kansky, and Roberts (1997) utilized an open platform octagonal maze with baited wells covered by automatically closing hinged doors at the perimeter. Using a working memory design to compare age-related cognitive deficits, tethered monkeys were required to return to a central start point between choices and delays were interposed after the first four choices. Aged monkeys showed delay-dependent impairments on spatial working memory. A point of particular interest in this study was that the aged monkeys were able to learn the task using a response-based strategy (picking the adjacent boxes in order) that yielded adequate performance, but did not require the use of spatial cues (Rapp et al., 1997). They also showed delay-dependent decline in accuracy for foraging unvisited wells. The advantage of Rapp’s platform design includes the hidden nature of the reward, combined with an absence of visible change once that well is visited. Potential disadvantages are that the animals are not free to explore and forage at will, the investigator cannot limit access to particular wells at will, and the design is limited to the specific pattern of eight locations.
Building on these earlier tasks, we designed a novel foraging paradigm that incorporated the advantages of movable, remote-controlled, lockable foraging stations, an open field in which the stations could be distributed both in two and three dimensions (see also Haley et al., 2012; Ludvig, Tang, Eichenbaum, & Gohil, 2003), and multiple entry points to reduce specific foraging habits. Food rewards were concealed in eight identical foraging boxes behind lockable swinging doors so that there was no visible cue indicating whether a box had been visited (allowing for repetition errors). Because the locking mechanism is radio-controlled, the boxes can easily be placed at any position along the walls, allowing for a variety of spatial arrays. We compared performance on this spatial task with a nonspatial, cued version in which the front panel of each foraging box had a unique color cue to differentiate it from the other seven, and each box changed position on every trial.
In the present study, we used this maze in combination with a food-preference paradigm to further test monkeys’ memory capacity to form food-place associations; specifically, to test the degree to which monkeys could remember the specific locations of seven different food items (both preferred and nonpreferred) and one unbaited location in a large enclosure containing orienting visual cues. Foraging in the eight goal locations required the monkey to move through the environment and learn which locations contained preferred foods. Optimal foraging required them to visit preferred food locations once, while avoiding nonpreferred locations and the unbaited box, and remember which locations had been visited during the trial to avoid repetition. We were particularly interested to assess whether the monkeys had learned the absolute location (or color cue) of each of their preferred food items within the cage.
We anticipated that monkeys would develop a pattern of foraging consistent with the relative food preferences, and would avoid nonpreferred or unbaited locations. Therefore, once each task was learned, we tested their knowledge of food locations using a reinforcer-devaluation paradigm (Málková, Gaffan, & Murray, 1997) to assess whether a monkey’s foraging pattern would change after being satiated on its preferred food. Given the choice, both adult-operated (Chudasama, Wright, & Murray, 2008; Machado & Bachevalier, 2007) and neonatal-operated (Kazama, Glavis-Bloom, & Bachevalier, 2008) normal and hippocampectomized monkeys will avoid selecting a food that they have just eaten to satiety. Thus, if the monkey formed accurate food/place associations, then it should avoid foraging at the location of a preferred food item that it has consumed to satiety just prior to the probe trial.
Last, despite the limited evidence for a role of the monkey hippocampus in spatial foraging tasks, one would predict an effect of hippocampal damage on the spatial, but not on a cued version of this task. Furthermore, the evidence of a role for the hippocampus in “what-where” associations (see Hampton & Schwartz, 2004, for review) would also suggest an impairment in learning specific food/place associations. Because a primary focus of our research program is the development of hippocampal function, we tested the above proposition comparing adult monkeys who had sustained neonatal ibotenic acid injections to the hippocampus with their cohort controls to evaluate their ability to learn specific food/place associations. Given that these animals with neonatal hippocampal lesions had already shown impaired performance in an object-in-place VPC (visual paired comparison) task when tested in adulthood (Blue, Kazama, & Bachevalier, 2009), we predicted that they would also show poorer learning of spatial associations and would commit more errors relative to controls.
Method
All experimental procedures were approved by the Institutional Animal Care and Use Committees at University of Texas at Houston and Emory University.
Subjects
Animals were 10 adult rhesus macaques (Macaca mulatta) of both sexes (6 male, 4 female), weighing 5 to 8 kg, and ranging between 3 and 4 years of age at the beginning of testing. Five received a sham surgery (Group Neo-C), and five received MRI-guided neurotoxic lesions of the hippocampus (Group Neo-Hibo), between 8 and 12 days of age. All monkeys in this study were surrogate-nursery reared at University of Texas at Houston and moved to Yerkes National Primate Research Center at 1.5–3 years of age. They have all had the same extensive cognitive and behavioral testing history. At the time of this study, all monkeys were housed individually and maintained on a diet of monkey chow (Lab Diet #5037, PMI Nutrition International Inc., Brent-wood, MO) supplemented with fresh fruits and vegetables. Animal housing rooms were maintained on a 12:12 hour light–dark cycle, and water was available ad libitum. During the present study, the food ration was given once daily following testing, and adjusted individually to ensure that the monkeys were motivated to perform, and maintaining the monkeys at 85% or above of their free-feeding weight.
Neuroimaging
Neuroimaging procedures for MRI-guided stereotaxic surgery and postsurgical lesion confirmation have been described in detail elsewhere (Heuer & Bachevalier, 2011; Zeamer, Heuer, & Bachevalier, 2010). Briefly, immediately prior to surgery, each infant monkey was sedated and anesthetized using isoflurane (3.0% vol/vol to effect) and secured in a nonferromagnetic stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). The monkey was placed in the scanner (GE Signa 1.5T, GE Medical Systems Milwaukee, WI), and a 5-cm surface coil was placed over the monkey’s head. Following a brief localizer, monkeys received a 3D T1-weighted fast spin gradient echo (FSPGR; used to determine injection coordinates), and a fluid-attenuated inversion recovery (FLAIR) scan. Throughout the entire 45- to 60-min MRI scanning procedure, the monkey’s heart rate, body temperature, and SPO2 were monitored. After completion of the neuroimaging procedures, the monkey was kept anesthetized in the stereotaxic apparatus and immediately brought to the surgical suite. These two scanning procedures were repeated 6–8 days after surgery in monkeys of Group Neo-Hibo.
Surgical Procedures
The surgical procedures have been described in detail elsewhere (Heuer & Bachevalier, 2011; Zeamer et al., 2010). Briefly, upon arrival to the surgical suite, monkeys were maintained under isoflurane anesthesia (1.0%–2.0% vol/vol, to effect) and, using aseptic techniques, the scalp was opened in anatomical layers, and a craniotomy was made in the skull above the injection sites. Bone wax (Ethicon, Inc., Somerville, NJ; 2.5-g size) was applied to the edges of the craniotomy to stop any bleeding from the bone. The exposed dura was opened over the injection sites to allow insertion of the microsyringe needles. For monkeys in Group Neo-C, the surgical procedure was stopped at this point and no needle penetration was performed. For monkeys in Group Neo-Hibo, the needles from two 10-µl Hamilton syringes filled with the neurotoxin, ibotenic acid (Biosearch Technologies, Novato, CA; 10 mg/ml in PBS, pH 7.4), were simultaneously lowered to each injection site of the hippocampus in each hemisphere. At each site, 0.4–0.8 µl of ibotenic acid was injected at a rate of 0.4 µl/min. Monkeys received 7–08 such injections along the length of each hippocampus, separated by 2 mm, for an average of 5 µl per hippocampus. For all monkeys, the incision was closed in anatomical layers, and the monkey was removed from the isoflurane gas anesthesia and recovered in the surgical facility until it regained consciousness.
Beginning 12 hr before surgery, and maintained until postsurgical day 7, all monkeys received dexamethazone sodium phosphate (.4 mg/kg, sc) to control swelling, and Cephalexin (25 mg/kg, po) to minimize the risk of infection. A topical antibiotic ointment was applied to the incision daily, and acetaminophen (10 mg/kg, po) was given four times a day for 3 days after surgery to reduce pain.
MRI-Lesion Evaluation
Because all subjects are still undergoing behavioral testing, histological evaluations have not been made. Rather, the extent of hippocampal damage was assessed using the postsurgical FLAIR images and the T1-weighted images obtained 1-year postsurgery using methods described by Málková, Lex, Mishkin, and Saunders (2001), and Nemanic, Alvarado, Price, Jackson, and Bachevalier (2002). The FLAIR images were used to identify areas of hyper-signal (indicative of brain edema caused by cell death) and were compared with the FSPGR images to accurately identify the borders of the brain structures showing hypersignal. The hypersignal obtained from postsurgical FLAIR images taken at 1-mm intervals was mapped onto matched coronal sections on the brain atlas of a normal infant rhesus macaque (J. Bachevalier, unpublished data). These drawings were then imported into ImageJ (available at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) to measure the cross-sectional area (in square pixels) of damage to intended and unintended brain areas. The total volume of damage for a given area in each hemisphere was determined by summing the area damaged on each section, and multiplying this sum by slice thickness (1 mm). The volume of damage was then divided by the normal volume of this area, estimated from the brain template, to estimate a percentage of the total volume damaged in a given brain area.
Apparatus
Food preference task
Food preference was assessed using a standard Wisconsin General Testing Apparatus (WGTA). The WGTA was equipped with a testing tray containing three food wells (2 cm in diameter and 1 cm deep, 16 cm away from the monkey transfer cage), one in the center of the tray and the other two located 13 cm on either side of the center well. Only the two lateral wells of the tray were used.
Food items used in the task were cut to be of similar size and included: (a) plain, blue M&M candy (Mars Candies, Hack- ettstown, NJ), (b) unsalted peanut, (c) raw carrot, (d) raisin, (e) banana-flavored pellet (P.J. Noyes, Inc., Lancaster, NH, 1 g size), (f) raw radish, (g) garlic clove, (h) dog chow, (i) Altoid (curiously strong breath mints), and (g) Cheerio (bland oat cereal).
Foraging tasks
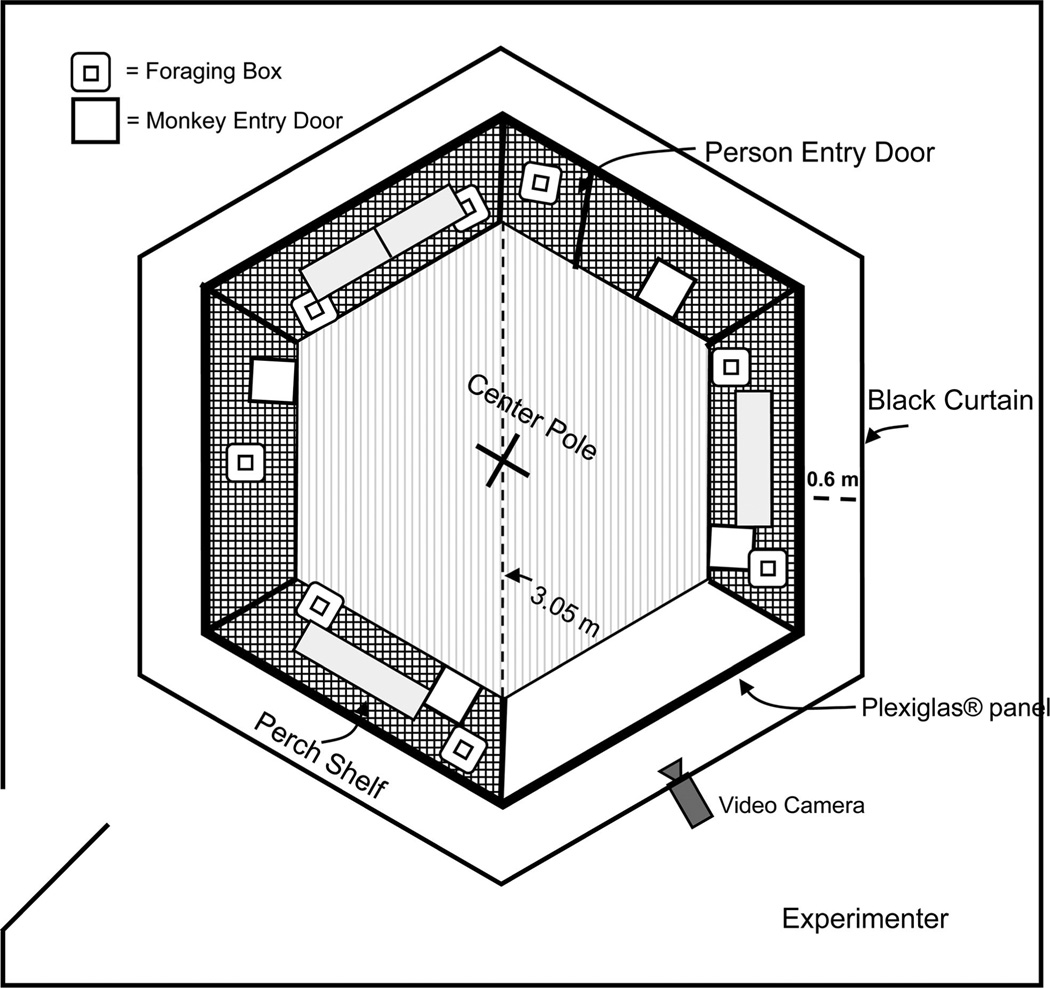
A custom hexagonal primate cage (Britz-Heidbrink, Wheatland, WY; 3.05 m diagonally) made of stainless steel served as a testing environment for the foraging tasks. Each wall (1.52 m wide and 2.13 m tall) of the cage was made of stainless steel, 2.5 cm wire mesh, except for one wall that was made of clear Plexiglas (2 cm thick) to permit optimal video recording of the monkeys. As shown in Figure 1, four shelves were located on three walls of the cage, and a vertical stainless steel pole with five perches extending from it was positioned in its center. Four vertically sliding doors on four walls enabled the monkeys to enter and exit the cage. The entire cage was surrounded by a black curtain hung from a frame made of 1 [1/2] inch PVC pipe, at a distance of 0.6 m from the cage walls. Visual cues were attached to the curtain and served as external cues for spatial orientation. Opaque plastic transport boxes (51 cm long × 33 cm wide × 38 cm tall) were used to transfer the monkeys from the housing area to the foraging cage. Experimenters remained outside of the curtain during formal testing and, through a small opening in the curtain, a stationary video camera positioned in front of the Plexiglas wall recorded the monkey’s foraging pattern during all phases of this experiment.
Figure 1.
Schematic of foraging cage, viewed from above.
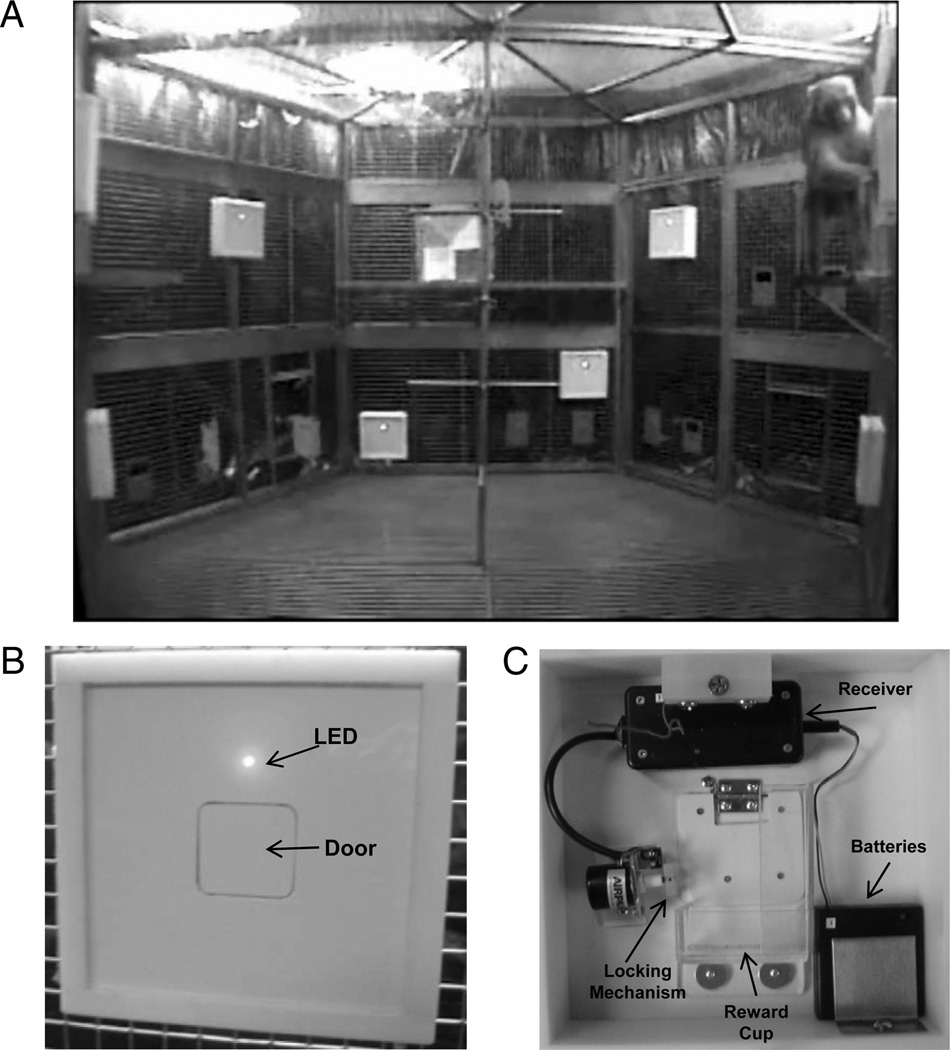
Eight identical foraging boxes (26 cm wide × 26 cm tall × 7 cm deep) were constructed with 2-cm thick Teflon and were used to hide food from the monkey’s view while it was in the cage. As shown in Figure 2, each box consisted of a uniform white panel with a door in the center that could be pushed open by the monkeys to retrieve food rewards contained in a small bin attached behind the panel (for the cued task, the front panel of each box was overlaid with one of seven different color panels (one remained white) to distinguish each box from the others). A radio-controlled locking system behind each door allowed the experimenter to control access to the reward bin of each foraging box. A red LED located above each door indicated the accessibility of the box. When the LED was illuminated, the door of that foraging box was unlocked and foraging was permitted. The foraging boxes were hung on the inside of the social cage so that there were four boxes in the upper half, and four boxes near the floor, and they were distributed around the five stainless steel walls of the cage as illustrated in Figures 1 and 2.
Figure 2.
(A) Subject Neo-Hibo-2 (right) foraging in an upper location during a training session. (B) Foraging boxes: Front of box showing foraging door and illuminated LED indicating the door is unlocked. (C) Rear view of box showing radio controlled locking mechanism.
Behavioral Testing
Forced-choice food preference task
Using 10 food items of varying palatability, monkey’s ranked food preferences were assessed over the course of 5 days in the WGTA using procedures similar to those described by Murray, Gaffan, and Flint (1996). In each trial, two different foods were paired. The experimenter began each trial by placing one food item in each of the lateral wells of the testing tray according to a pseudorandom schedule. The monkey was allowed up to 15 s to choose one food item, and if neither food item was selected by the monkey, the trial was discarded. For each of 4 testing days, monkeys received all 45 possible pairings of food items, counterbalancing left/right permutations between test days. The fifth day was a repeat of the first day of testing so that each food was presented a total of 45 times across all 5 days of testing. Only the fourth and fifth days of testing were used to rank the subject’s food preferences.
Foraging Tasks
Shaping
The monkeys were shaped to reach into the foraging boxes to retrieve treats in their home cages over 2 days, on average. When the monkey reliably reached through the flap door into the box, they began the next phase of training.
The monkeys were then familiarized with the experimental setting over several consecutive days. They were brought into the cage daily, using one of the four doors, and were permitted to move freely to become familiar with the surroundings of the cage, the foraging boxes, and the black curtain. During this phase, the experimenter first remained inside the black curtain and offered treats to the monkey for 10 min, and then moved and remained quietly behind the curtain for an additional 5 min to adapt the monkey to remaining in the cage alone. This procedure was terminated when the monkey decreased instances of stress responses, such as freezing, screams, coos, and pacing, and required approximately 5 days.
In the final shaping phase, the monkey was trained that the unlocking of the foraging boxes was contingent upon its touching or climbing onto the center pole. First, each of the eight foraging boxes was baited with one Froot Loop. The experimenter transported the monkey from the living quarters, released it into the cage, and then moved behind the curtain. The monkey was free to move about the cage, but the foraging boxes remained locked (LED lights off) until it reached the center pole. The LED lights were then turned on, indicating to the monkey that it could forage freely until all boxes had been visited once, or until 10 min had elapsed since the boxes were unlocked. If the monkey had not foraged in all locations, a correction procedure was implemented. The experimenter entered the curtain and directed the monkey’s attention to the foraging locations it did not visit, allowed the monkey to forage in these locations, and then removed the monkey from the cage. Monkeys received one trial per day until the monkey foraged in all eight locations within 5 min from their first visit for 2 consecutive days, thus assuring the monkey was willing to forage in all locations.
Spatial task
In the experimental phase, the monkeys had to learn the locations of four preferred foods, three nonpreferred foods, and an empty box. With the eight foraging boxes in static locations, the seven food items selected from the food preference task for each monkey (the four most preferred and the three least preferred) were used to bait each of seven boxes, while the eighth remained empty. The foods were distributed in a pseudorandom order across all boxes, with two preferred and two nonpreferred foods in the boxes on the upper walls, and the same under the shelves (we included the empty box in the nonpreferred category). Once the location for each food was determined for a subject, it was placed in that location for that subject on subsequent trials; however, the location of a given food (e.g., peanut) differed across subjects. For each trial, the monkey entered the cage from one of four predetermined locations, which varied daily. Monkeys were free to move about the cage, but the boxes were only unlocked after it contacted or climbed onto the center pole. At this point, the monkey was permitted to forage freely until all boxes had been visited at least once, or until 2 min had elapsed since foraging in a previously not visited box, whichever came first. At the end of the trial, the boxes were locked and the monkey was removed from the cage. Monkeys received one trial per day, and each was trained at the same time each day to control for motivational factors relating to circadian rhythm.
Monkeys were trained to a criterion of visiting three preferred food locations within their first four choices for two consecutive days (demonstrating that they knew the locations of their preferred foods). The session following this criterion day was a probe trial that tested whether the monkey had formed specific food/place associations.
Reinforcer devaluation probe trials
To determine whether monkeys had learned which specific box contained their most preferred food, we performed a reinforcer devaluation of that food by allowing the monkey to consume the specific food item to satiety just prior to the probe trial.
The devaluation procedure was adapted from Málková and colleagues (1997). Immediately prior to testing, the monkey was given 200 g of their favorite food in their living quarters and allowed to eat freely for 30 min. The experimenter gave the monkey an additional 100 g of the same food every 15 min until 5 min had passed without any further ingestion. Measures recorded included the amount of food (in grams) consumed and the total time (in seconds) required to become sated.
After the devaluation, the monkey was immediately transported to the spatial cage and was allowed to forage using the same procedures as those described in the learning phase. Measures taken included the order in which all boxes were visited, and the number of box selections made before selecting the box containing the devalued food. Immediately following the devaluation trial, each monkey was presented with a choice of the devalued food or another preferred food to confirm the level of satiation. The devaluation probe trial was repeated twice per monkey, with subjects reachieving criterion performance between probes.
Cued task
All monkeys were trained on the cued task after learning the spatial task because we did not want to reinforce attention to local cues, which might negatively impact learning of the spatial task. In addition, there was a substantial gap between training on the two tasks (from 9 months to 1 year) to reduce possible carryover of inappropriate strategies or habits associated with learning the spatial task and during which other age-critical planned behavioral testing took place (visual recognition memory; spatial memory span; self-ordered tasks; serial-order tasks: Heuer & Bachevalier, 2011; Heuer & Bachevalier, in press). Training on the cued task was identical to the spatial task except that each foraging box was made unique with panels of differing colors adhered to the box fronts, and the cued-boxes changed position each trial so that location was not a viable strategy for solving the task. Each monkey’s preferred and nonpreferred foods were the same; however, the empty box was also baited with a food from the nonpreferred list that had not been used before. All other procedures were identical to the previous task, including the rein-forcer devaluation probe trial.
Behavioral measures and data analysis
For the foraging tasks, all sessions were scored live as well as videotaped through the clear cage wall for later evaluation. Measures recorded during training included number of forages (taking food out of a box), balks (opening a box, but leaving the food inside), errors (returning to a previously visited box), total choices (forages, balks, and errors combined), how many preferred foods were found within the first four choices, and order of foraging choices (whether preferred foods were chosen before nonpreferred foods).
Performance measures for the learning phase of both tasks were as follows: for Acquisition, the number of trials, choices, and errors before attaining training criterion, and errors rates by task and food type; for Criterion, total choices, number of choices to first errors and error rates by Task and Food type during the 2 days of criterion.
For the Devaluation phase, the effects of satiation were represented by a single score that took into account the number of choices made prior to visiting the devalued food box and converting that measure into a percent of the total choices made prior to foraging in the devalued food box. For example, picking the devalued food first will result in a score of 0%. If, however, the monkey visited the devalued box on its 4th choice and stopped, it would score 75% (3 out of 4 total choices prior to devalued box). In the sessions following the devaluation probe trial, the monkeys were retrained to criterion, and then received a second devaluation for a different preferred food. The two devaluation scores were averaged and compared with the averaged performance on the two criterion days preceding devaluation days.
Because this is a novel task, performance of Group Neo-C was analyzed independently to establish the behavioral norms for each phase of the task, and they are presented separately at the beginning of each phase. For Acquisition and Criterion, behavioral measures were analyzed using a two-way analysis of variance (ANOVA), with tasks (spatial vs. cued) and food type (preferred vs. nonpreferred) as repeated-measure factors, and for devaluation, percent scores were analyzed using a two-way ANOVA with tasks (spatial vs. cued) and days (criterion vs. devaluation) as repeated-measure factors.
The effects of neonatal hippocampal damage were compared using two- or three-way ANOVAs. For acquisition (trials, choices, and errors) and criterion (total choices and choices to first error) phases, behavioral measures were analyzed with Group (Neo-C vs. Neo-Hibo) as the between factor and task (spatial vs. cued) as the within-subjects factors with repeated measures. Also, for both phases, errors by food type were analyzed with a Group × Tasks × Food types (preferred vs. nonpreferred) with repeated-measures for the last two factors. For devaluation, percent choices prior to devalued food was analyzed with group (Neo-C vs. Neo-Hibo) as the between factor and tasks (spatial vs. cued) and days (criterion vs. devaluation) as within-subjects factors with repeated-measures. Significant interactions were further explored using Generalized Linear Model (GLM) multivariate analyses, simple effects, or planned comparisons.
Results
Lesion Extent
The experimental group is still undergoing evaluation, and therefore the percentage of damage to the hippocampal formation (intended) and to adjacent cortical regions (unintended) was estimated from the postsurgical FLAIR MR images. Detailed descriptions of each subject as well as MR images of representative cases have been previously published (Zeamer et al., 2010; Heuer & Bachevalier, 2011) and are briefly summarized below. Estimates of intended and unintended damage are shown in Table 1. The weighted average (Hodos & Bobko, 1984) provides an excellent estimate of whether a lesion is mild to moderate and highly unilateral (W% < 25%) or extensive and symmetrical (W% > 50%).
Table 1.
Intended and Unintended Damage in Group Neo-Hibo
| Intended damage |
Unintended damage |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hippocampal formation |
TH/TF |
|||||||
| Subjects | L% | R% | X% | W% | L% | R% | X% | W% |
| Neo-Hibo-1 | 63.8 | 2.9 | 33.2 | 1.9 | 3.1 | 0.5 | 1.8 | 0 |
| Neo-Hibo-2 | 54.4 | 80.9 | 67.6 | 44 | 21.4 | 2.7 | 12.1 | 0.6 |
| Neo-Hibo-3 | 78.5 | 96.3 | 87.4 | 75.6 | 6.1 | 5.5 | 5.8 | 0.3 |
| Neo-Hibo-4 | 20.3 | 67.3 | 43.8 | 13.7 | 15.3 | 0 | 7.6 | 0 |
| Neo-Hibo-5 | 20.7 | 84 | 52.6 | 17.4 | 6.1 | 4 | 5.1 | 0.2 |
| M | 47.5 | 66.3 | 56.9 | 30.5 | 10.4 | 2.5 | 6.5 | 0.2 |
Note. L% = percent damage to the left hemisphere; R% = percent damage to the right hemisphere; X% = average damage to both hemispheres; W% = weighted average damage to both hemispheres, W% = (L% × R%)/100; TH/TF = cytoarchitectonic fields of the parahippocam-pal gyrus as defined by von Bonin and Bailey (1947). Percent damage to the hippocampal formation for the five animals in Group Neo-Hibo. Numbers in bold indicate they are an average of the numbers above.
As described in Table 1, for two monkeys (Neo-Hibo-2 and Neo-Hibo-3) the hippocampal lesion was extensive and bilateral (from 54% to 96.3%). For the three remaining monkeys (Neo-Hibo-1, –4, and –5), the hippocampal damage was asymmetrical with more damage on one side (64%, 67.3%, and 84%, respectively) than on the other side (2.9%, 20.3%, and 20.7%, respectively).
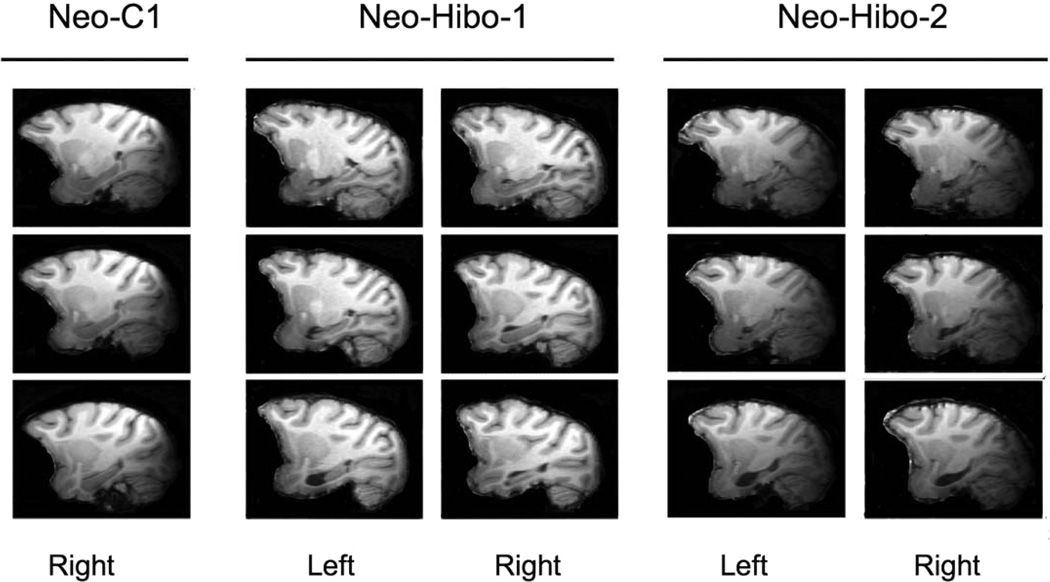
Unintended damage to adjacent structures was mild for all cases and was mostly located in areas TH/TF. In addition, minor unilateral damage was sustained to the amygdala in four cases: Neo-Hibo-1, 14% left; Neo-Hibo-3, 1.7% left; Neo-Hibo-4, 4.7% right; Neo-Hibo-5, 4.9% right. An example of the damage in two subjects is illustrated in Figure 3. Case Neo-Hibo-2 is an example of bilateral damage, whereas case Neo-Hibo-1 sustained asymmetrical damage. Magnetic resonance images through three sagittal levels through the left and right hippocampus for these two subjects and equivalent sections through a normal control. These scans were taken in adulthood, at 8–9 years of age.
Figure 3.
Magnetic resonance images (T1-weighted), taken in adulthood at 8 to 9 years of age, of one control and two subjects from Group Neo-Hibo at three sagittal levels through the left and right hippocampus. Subject Neo-Hibo-1 has a largely unilateral lesion with more sparing of the right hippocampus (Table 3), whereas subject Neo-Hibo-2 has a bilateral lesion with minimal sparing.
Acquisition
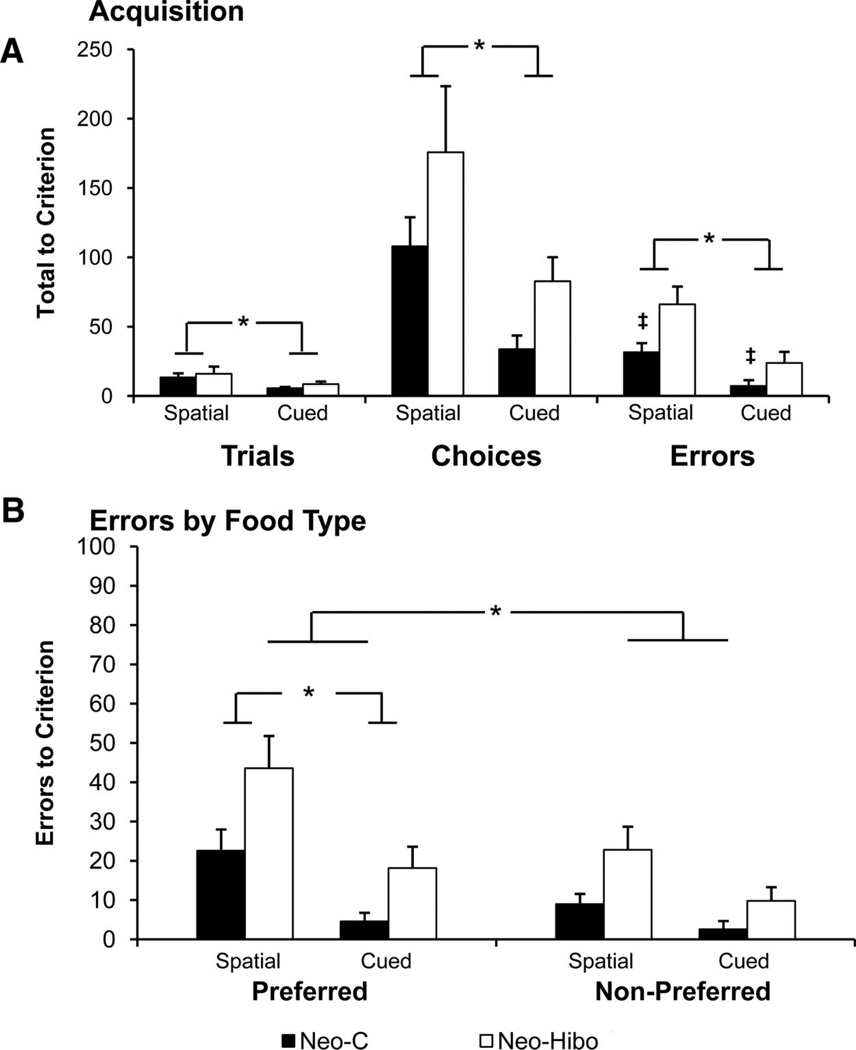
Table 2 and Figure 4A display the number of trials, choices, and errors needed to reach the learning criterion for each monkey of both groups. In addition, to better assess the nature of foraging behavior on each task, we examined whether the errors (repeated visits to previously foraged locations) were equally distributed across preferred and nonpreferred food locations during the learning phase (Table 2 and Figure 4B).
Table 2.
Acquisition
| Trials |
Choices |
Errors |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spatial | Cued | Spatial | Cued | Spatial |
Cued |
|||||
| Subjects | Pref | N-Pref | Total | Pref | N-Pref | Total | ||||
| Neo-C-1 | 11 | 5 | 113 | 29 | 18 | 9 | 27 | 4 | 2 | 6 |
| Neo-C-2 | 20 | 5 | 155 | 24 | 31 | 8 | 39 | 2 | 0 | 2 |
| Neo-C-3 | 12 | 6 | 116 | 19 | 21 | 18 | 39 | 0 | 0 | 0 |
| Neo-C-4 | 4 | 3 | 30 | 24 | 6 | 2 | 8 | 4 | 0 | 4 |
| Neo-C-6 | 20 | 9 | 126 | 73 | 37 | 8 | 45 | 13 | 11 | 24 |
| Average | 13.4 | 5.6 | 108 | 33.8 | 22.6 | 9 | 31.6 | 4.6 | 2.6 | 7.2 |
| Neo-Hibo-1 | 17 | 4 | 195 | 29 | 65 | 20 | 85 | 1 | 0 | 3 |
| Neo-Hibo-2 | 36 | 7 | 352 | 80 | 62 | 44 | 105 | 12 | 9 | 24 |
| Neo-Hibo-3 | 7 | 7 | 104 | 111 | 32 | 15 | 47 | 32 | 15 | 22 |
| Neo-Hibo-4 | 13 | 15 | 137 | 131 | 33 | 25 | 58 | 24 | 20 | 42 |
| Neo-Hibo-5 | 7 | 10 | 91 | 63 | 26 | 10 | 36 | 22 | 3 | 28 |
| Average | 16 | 8.6 | 175.8 | 82.8 | 43.6 | 22.8 | 66.2 | 18.2 | 9.8 | 23.8 |
Note. Total number of training trials, choices, and errors accumulated to reach criterion for each group for the spatial and cued tasks. Total errors during the learning phase are listed by whether they were made to locations of preferred (Pref) or nonpreferred (N-Pref) foods. Numbers in bold represent an average of the numbers in the column.
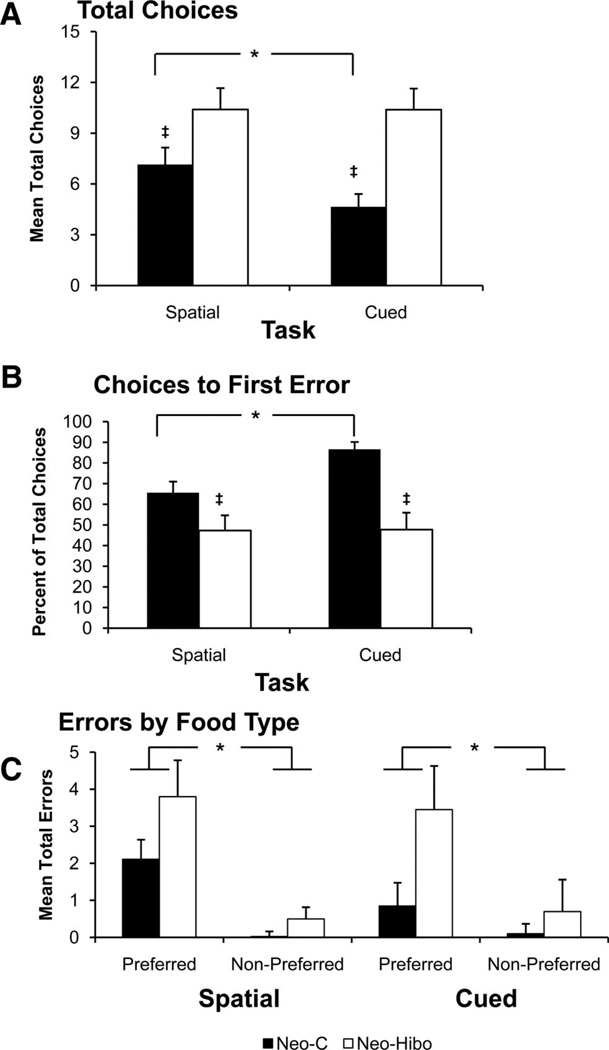
Figure 4.
Acquisition. (A) Trials, Choices and Errors to criterion during acquisition. Dark bars Group Neo-C, white bars Group Neo-Hibo. (B) Errors by food type (preferred or nonpreferred). Asterisk indicates significant Group differences, and the ‡ symbol indicates significant within-group differences (see text). Error bars indicate ±SEM.
Group Neo-C
Control monkeys learned the cued task much faster than the spatial task, requiring significantly fewer trials, task effect: F(1, 4) = 10.6, p = .03, or choices, task effect: F(1, 4) = 12.3, p = .03, and committing significantly fewer errors to criterion, task effect: F(1, 4) = 14.7, p = .02, in the cued task compared with the spatial task. It is interesting to note that monkeys in Group Neo-C committed approximately twice as many errors to preferred food locations as to nonpreferred, food effect: F(1, 4) = 8.64, p = .04 (Figure 4B), suggesting that they were concentrating their foraging in locations containing preferred foods. Although this difference was greater for the spatial than the cued tasks, the interaction between tasks and food types failed to reach significance, F(1, 4) = 4.85,p = .09.
Group Neo-Hibo
Although monkeys in Group Neo-Hibo required the same number of trials as Group Neo-C to reach the training criterion for the spatial and cued tasks, group effect: F < 1; Figure 4A, they made more choices, group effect, F(1, 8) = 4.82, p = .059, than Group Neo-C on both tasks. However, both groups reached criterion in the cued task faster than in the spatial task, requiring fewer trials or choices, task effect: F(1, 8) = 5.38, p = .049; F(1, 8) = 8.52, p = .02, respectively. None of the interactions reached significance, F < 1. In addition to group and task differences, errors to criterion were also evaluated with respect to food type. For this measure, Group Neo-Hibo (Figure 4B) committed more errors to criterion, group effect: F(1, 8), = 15.67, p = .004, than Group C, and both groups committed more errors to preferred than nonpreferred food boxes, food type effect: F(1, 8) = 33.4, p = .001, reflecting the learned tendency to forage in preferred-food boxes. Both groups committed more errors on the spatial than the cued task, task effect: F(1, 8) = 10.135, p = .013, and both groups committed more errors to preferred-food boxes in the spatial task than in the cued task, task × food type: F(1, 8) = 5.51, p = .047. No other interactions were reliable (all ps > .05).
Criterion
Table 3 and Figure 5 list individual scores and illustrate group performance, choices (A), choices to first error (B), errors by food type (C), respectively, averaged across the 2 criterion days.
Table 3.
Criterion
| Choices |
% Choices to error |
Errors |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spatia | Cued | Spatial | Cued | Spatial |
Cued |
|||||
| Subjects | Pref | N-Pref | Total | Pref | N-Pref | Total | ||||
| Neo-C-1 | 10.8 | 7.25 | 48.96 | 80.4 | 4.0 | 0.75 | 4.8 | 1.0 | 0.0 | 1.0 |
| Neo-C-2 | 6.0 | 3.0 | 80.1 | 100 | 1.25 | 0.25 | 1.5 | 0.0 | 0.0 | 0.0 |
| Neo-C-3 | 6.3 | 3.25 | 72.9 | 83.8 | 1.0 | 0.25 | 1.3 | 0.5 | 0.0 | 0.5 |
| Neo-C-4 | 7.8 | 4.75 | 66.8 | 87.5 | 2.3 | 0.00 | 2.3 | 1.25 | 0.0 | 1.25 |
| Neo-C-6 | 5.0 | 5.0 | 59.2 | 81.4 | 2.0 | 0.00 | 2.0 | 1.5 | 0.5 | 1.5 |
| Average | 7.18 | 4.65 | 65.6 | 86.59 | 2.11 | 0.25 | 2.35 | 0.85 | 0.1 | 0.95 |
| Neo-Hibo-1 | 8.3 | 10.0 | 40.8 | 60.1 | 3.0 | 0.0 | 3.0 | 2.0 | 0.75 | 2.75 |
| Neo-Hibo-2 | 10.8 | 11.2 | 52.1 | 44.7 | 3.0 | 1.0 | 3.0 | 3.0 | 0.5 | 3.5 |
| Neo-Hibo-3 | 14.0 | 14.0 | 28.6 | 28.6 | 6.53 | 1.5 | 6.5 | 6.5 | 1.5 | 8.0 |
| Neo-Hibo-4 | 7.0 | 6.25 | 72.6 | 72.2 | 1.0 | 0.0 | 1.0 | 1.75 | 0.25 | 2.0 |
| Neo-Hibo-5 | 12.0 | 10.5 | 42.7 | 33.2 | 5.5 | 0.0 | 5.5 | 4.0 | 0.5 | 4.5 |
| Average | 10.4 | 10.39 | 47.34 | 47.76 | 3.81 | 0.5 | 4.3 | 3.45 | 0.7 | 4.15 |
Note. Average choices and errors across the 2 criterion days. Errors include repeated visits to locations of preferred foods or nonpreferred foods after those locations had been foraged and are expressed as a percentage of total choices in each trial. % choices to error = percentage of total choices completed prior to committing the first error averaged across the two criterion sessions. All conventions as for Table 2. Numbers in bold indicate that those numbers are the average of the numbers in the column.
Figure 5.
Performance during the Criterion Phase. (A) Total Choices during criterion. (B) Choices to first error (percentage of total choices completed prior to an error during the trial). (C) Errors to preferred vs. nonpreferred boxes. All conventions as in Figure 4.
Group Neo-C
During criterion days, control monkeys were better able to restrict their forages to locations of preferred foods on the basis of local cues, rather than spatial locations as they made significantly more choices on the spatial than the cued tasks. task effect: F(1, 4) = 15.6,p = .02 (Figure 5A). Although there was no task difference with respect to the number of total errors committed, task effect: F(1, 4) = 4.95, p = .09 (Table 3), control subjects completed fewer choices prior to committing their first error in the spatial than in the cued tasks, task effect: F(1, 4) = 41.38, p = .003. Further confirming their ability to discriminate preferred food locations, control monkeys committed the majority of errors to preferred food locations, food type effect: F(1, 4) = 16.68,p = .015 (Figure 5C), and this difference was greater in the spatial task than the cued task, Task × Food interaction: F(1, 4) = 11.92,p = .026.
Group Neo-Hibo
Despite having learned the task in the same number of trials as controls, Group Neo-Hibo made more choices per trial than Group Neo-C during the criterion phase, group effect: F(1, 8) = 9.21, p = .02 (Figure 5A) on both the spatial and cued tasks. In addition, unlike controls that made fewer choices on the cued than the spatial tasks, monkeys in Group Neo-Hibo made the same number of choices on both tasks, Group × Task interaction: F(1, 8) = 8.89,p = .018. Similarly, Group Neo-Hibo made fewer choices prior to committing their first error than Group Neo-C in both tasks (Figure 5B), Group effect: F(1, 8) = 11.3, p = .01. Thus, whereas choices to first error varied significantly between tasks for control monkeys, they were fewer and did not vary across tasks for monkeys in Group Neo-Hibo, Group × Task interaction: F(1, 8) = 11.64,p = .009.
Errors to criterion were compared using a Group × Task × Food type ANOVA with repeated measures that revealed that Group Neo-Hibo committed more errors than controls, although this group difference fell just short of significance, F(1, 8) = 4.82, p = .059. However, the effect of food type was reliable, F(1, 8) = 29.48, p = .001, and the interaction between group and food type approached significance, F(1, 8) = 4.66, p = .063, reflecting the greater number of preferred food errors for Group Neo-Hibo than controls. Similarly, the interaction between task and food type was reliable, F(1, 8) = 6.87,p = .031, reflecting significantly more preferred food errors in the spatial task for both groups.
Reinforcer Devaluation
Individual devaluation scores are listed in Table 4, and group averages are illustrated in Figure 6. For all subjects in both groups, satiation of the devalued food was deemed successful because all monkeys (a) refused to consume the devalued food if that box was visited during the probe trial (balk), and (b) when given a free choice at the end of the probe trial between the devalued and another food, the devalued food was never chosen by any monkey.
Table 4.
Devaluation
| Percentage of choices before devalued food |
||||
|---|---|---|---|---|
| Spatial |
Cued |
|||
| Subjects | Criterion | Devaluation | Criterion | Devaluation |
| Neo-C-1 | 16.7 | 55.0 | 7.2 | 80 |
| Neo-C-2 | 0.0 | 75.0 | 29.2 | 100 |
| Neo-C-3 | 0.0 | 75.0 | 25.0 | 100 |
| Neo-C-4 | 13.4 | 55.6 | 5.0 | 100 |
| Neo-C-6 | 18.35 | 50.0 | 25.0 | 100 |
| Average | 9.68 | 62.11 | 18.26 | 96 |
| Neo-Hibo-1 | 9.1 | 4.6 | 7.2 | 100 |
| Neo-Hibo-2 | 0.0 | 15.5 | 9.6 | 14.6 |
| Neo-Hibo-3 | 3.6 | 28.6 | 5.6 | 71.5 |
| Neo-Hibo-4 | 5.6 | 50.0 | 29.2 | 66.7 |
| Neo-Hibo-5 | 4.2 | 100 | 9.1 | 20.0 |
| Average | 4.5 | 39.7 | 12.1 | 54.5 |
Note. Percentage of total choices completed prior to visiting the location of the devalued food on the last criterion day compared with the trial following reinforcer devaluation. Low numbers indicate choice occurred at the beginning of the trial, higher numbers indicate the choice occurred later. 100% indicates the box was not visited. Numbers in bold indicate that those numbers are the average of the numbers in the column.
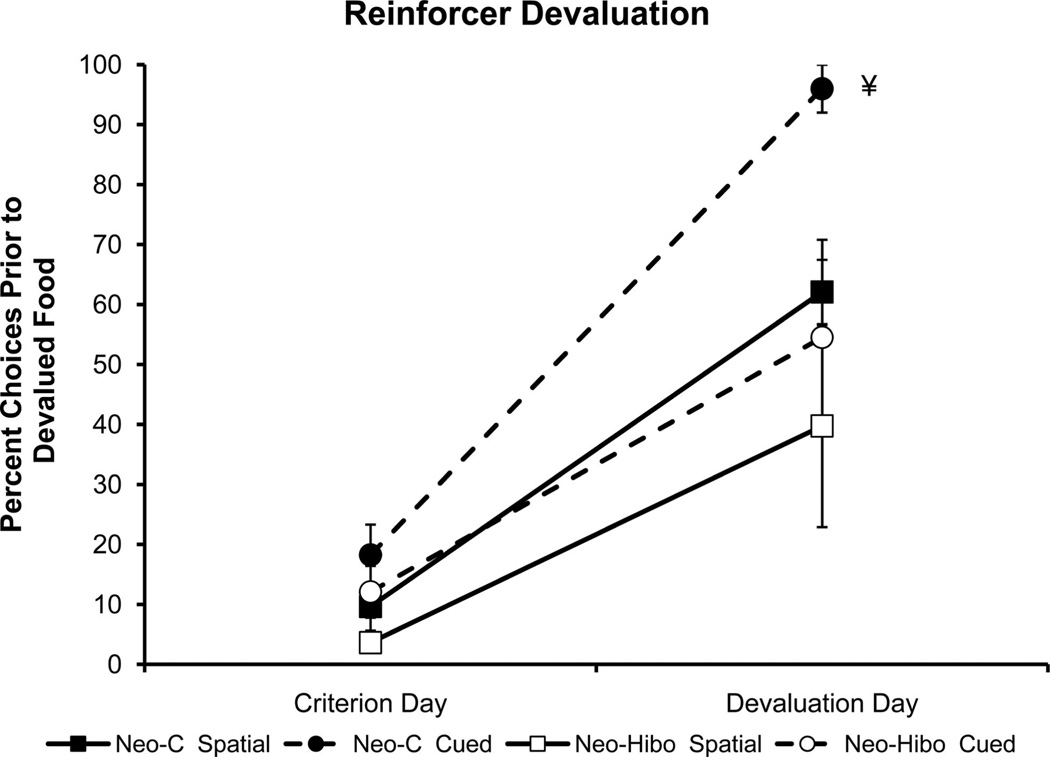
Figure 6.
Effects of devaluation of preferred food on foraging pattern, compared with the criterion day, 1 trial prior to devaluation. ¥: In addition to the main effects of Group, Task and Trial, a Group × Trial interaction was also reliable, indicating the effect of devaluation was greater in magnitude for controls than for Group Neo-Hibo. Group Neo-C, filled symbols; Group Neo-Hibo, open symbols; circles/dashed lines indicate cued task, squares/solid lines indicate spatial task. All other conventions as in Figure 4.
Group Neo-c
For the sham-operated controls, the to-be-devalued food was chosen within the first two choices for each task on the trial prior to devaluation. After devaluation, however, these same monkeys foraged in that box much later in the trial than the day before, if at all, trial type effect: F(1, 4) = 210.89,p = .001 (Figure 6, filled symbols). This shift was much larger for the cued task than for the spatial task, although the Tasks × Trial type interaction did not reach significance, F(1, 4) = 4.78, p = .09. This pattern of results suggests that control monkeys made a stronger association with the unique identifier and the food in the cued task than with the location in the spatial task.
Group Neo-Hibo
Hippocampal damage did not affect appetite or food preferences, because there were no group differences in amount consumed (F < 1) or time to consume the devalued foods to satiety (F < 1). Similar to Group Neo-C, only one monkey in Group Neo-Hibo failed to shift its foraging pattern after devaluation (Table 4). Nevertheless, during the devaluation probe (Figure 6, open symbols), monkeys in Group Neo-Hibo foraged at the devalued food location much earlier in the probe trial than those in Group Neo-C in both tasks. Thus, the Group × Task × Trial ANOVA with repeated measures across task and trial revealed a main effect of group, F(1, 8) = 13.45, p = .006; of task, F(1, 8) = 5.43, p = .02; and of trial, F(1, 8) = 139.2, p = .001. More important, the Group × Trial interaction was reliable, F(1, 8) = 851, p = .02, indicating greater group difference on the devaluation trial. No other interactions were reliable (Fs <1).
Discussion
The goals of the present study were twofold: first, to design a spatial foraging task for freely moving monkeys that would allow for flexible layout, options for spatial and nonspatial memory evaluation, and the opportunity to test whether monkeys could form food/place associations; second to determine whether neonatal damage to the hippocampus produced a long-term impairment in spatial memory when tested in adult monkeys. The data suggest that control monkeys were able to (a) learn to forage at preferred food locations and avoid nonpreferred locations, (b) form specific food/place associations, and (c) monitor their within-trial choices to reduce working memory errors. The data further suggest that monkeys with neonatal hippocampal damage (a) learned to discriminate preferred from nonpreferred food locations, (b) did not learn specific food/place associations, and (c) committed significantly more working memory errors than controls.
Foraging Paradigm
We designed a task that required monkeys to move through an environment to obtain a variety of food rewards. Similar to radial arm maze tasks, this paradigm employed eight visually identical foraging boxes that were spatially distributed along the walls of the testing cage, however for the present study, we placed them at two levels, allowing four upper and four lower locations. Monkeys received one trial per day to forage in eight visually identical boxes, where the location of each was associated with a specific preferred or nonpreferred food. A particular advantage of the foraging box design was that there was no visible difference between visited and unvisited containers, and we could restrict access to the boxes by means of a remote-controlled locking mechanism. For the present study, we examined whether monkeys could learn that a particular box (and only that box) contained a specific food. The food item was either highly preferred, or non-preferred, based on pretraining preference tests and each box contained a different food. Furthermore, the location of the food was either determined by the place, or location of the box in the apparatus, when all boxes were otherwise identical (spatial) or by a distinct visual cue placed on each box, and the box changed location every trial (cued). We utilized a reinforcer devaluation paradigm to determine whether the monkeys had knowledge of the location of the specific food, and could demonstrate that knowledge by selectively avoiding the box containing that food, using either spatial location, or visually identifying cues.
Last, a long-standing advantage of radial-arm maze designs has been the ability to distinguish spatial working from reference memory errors (Olton, Becker, & Handelmann, 1979). A failure to learn consistent locations of food can lead to errors in reference memory (i.e., visiting unbaited arms), while a failure to update the choices in the current trial can lead to errors in working memory (i.e., revisiting the same arm within a trial). In the present study, we did not require or manipulate visits to particular locations, although reference memory was indirectly assessed using the devaluation paradigm. However, repeated visits to a food box represent errors in working memory and important advantage of the task design was the ability to measure working memory errors, compared with other foraging tasks where repetition errors are not possible (e.g., Lavenex et al., 2006).
Spatial task
Monkeys in the control group (Neo-C) learned to direct their forages to preferred food locations, in an average of only 13 trials. The rapid rate at which they acquired this task, and accurate manner in which they performed their searches suggests that they could discriminate preferred from nonpreferred boxes based on location, associate those locations with preferred or nonpreferred foods, and develop an efficient strategy with which to search for food, that is, shifting their initial searches to primarily preferred food locations. Because all of the boxes were visually identical, this directed search was dependent upon knowledge of their spatial location relative to cues within the apparatus and perhaps the extramaze visual cues. It is interesting to note that monkeys in Group Neo-Hibo reached criterion just as rapidly as controls, however, they made many more working memory errors on average than controls. Both groups distinguished between preferred and nonpreferred locations, making the bulk of their errors to preferred food locations, but as discussed below, the knowledge of specific food/place pairings differed between the two groups.
Performance during criterion confirmed the tendency for both groups to forage in preferred food locations and the group difference in working memory errors. On the whole, Group Neo-Hibo committed more working memory errors than controls. It is important to note that this increase in working memory errors in Group Neo-Hibo is consistent with the rodent literature in the radial arm maze, in which there are increases in spatial working memory errors, but also with recent reports in which these same monkeys were impaired in monitoring choices in the object self-ordered task (Heuer & Bachevalier, 2011). Because this latter task is typically disrupted by damage to the prefrontal cortex (Petrides, 1995), it is possible that our results reflect not only long-term effects of hippocampal damage, but also suggest a possible disruption in normal dorsolateral prefrontal cortical development as a result of early hippocampal dysfunction (Heuer & Bachevalier, 2011; Saunders, Kolachana, Bachevalier, & Weinberger, 1998).
It is interesting to note that although Group Neo-Hibo also showed more foraging to the preferred than the nonpreferred foods, the results of the reinforcer devaluation suggest that the degree of detailed information about each box learned by each group differed substantially. As previous studies have shown (Machado & Bachevalier, 2007; Málková et al., 1997), satiation of a preferred food reduces the rate at which monkeys will select an object associated with that food and increase the choice rate for objects associated with the less preferred food. In the present study, we utilized this preference switch to measure whether satiation to a preferred food would alter foraging patterns to reduce foraging in that food’s location and hence provide a direct measure of specific food/place associations for each monkey.
Following devaluation, monkeys in Group Neo-C shifted their foraging pattern so that the box containing the devalued food was visited later in the trial, or not at all. The shift in foraging patterns following selective satiation of one preferred food item suggests that they knew not only the general location of “preferred foods,” but that they also knew the location of that specific food item. By contrast, monkeys in Group Neo-Hibo were less impacted by the effects of satiation. That is, not all subjects in this group switched their foraging pattern (i.e., they still visited the devalued food location first) and those that did, foraged in the devalued food’s location earlier in the trial than controls. A possible explanation for this finding is that monkeys with neonatal hippocampal lesions did not satiate as the controls did. This is unlikely though for two reasons. First, at the end of the probe trial, monkeys were offered a choice between the preferred food they were satiated on and another preferred food to assess whether they were willing to take and eat the sated food item. All monkeys refused the devalued preferred food, and ate the alternative preferred food offered, suggesting they were successfully satiated on the devalued food item. Second, the same experimental and control monkeys participated in a reinforcer devaluation task and all showed significant avoidance of selecting objects associated with the devalued food (Kazama et al., 2008).
Thus, it appears that Group Neo-C had learned the locations of each preferred food, whereas Group Neo-Hibo may have distinguished “preferred” from “nonpreferred” locations, but did not associate specific foods with specific places. We did not determine whether the controls also knew the locations of specific nonpre-ferred foods, but there were no task demands that would necessitate the encoding or retention of such information beyond “non-preferred” for those locations. In that sense, the task demands could be decreased to only four specific food locations.
Cued task
Training on the cued task, for which every box-food pair was uniquely visually identified and variably positioned, took place approximately 1 year following training on the spatial task to reduce the transfer of strategies or habits formed when learning the spatial task that might interfere with learning. We also replaced the empty box with one of the monkey’s nonpreferred foods, because we noted that the empty box was actually treated as more neutral than nonpreferred, and most monkeys tended to forage there. For this task, a unique colored and/or patterned panel was attached to the front of each box and each food was associated with a particular cue. However, the cued-boxes themselves were relocated to new positions relative to the cage, and each other, every trial. Thus, no single “place” was consistently associated with any food, forcing the monkeys to rely on the visual cues on the boxes to successfully forage for preferred foods. We expected that the reduced spatial memory requirement and unique visual cues would make the task easier for both groups.
As expected, monkeys in Group Neo-C learned the locations of their preferred foods twice as quickly (6 vs. 14 trials), and with four times fewer errors (7 vs. 32) in the cued task compared with the spatial task. Training order likely accounts for some of the improvement, in that training on the spatial task was learned first and so included learning the “rules of the game.” However, considering that they had to learn 8 new food-cues that moved on every trial, the ease with which they learned the cued version is notable. In addition, the first working memory errors occurred later in the trial for the cued task and were made disproportionately to boxes containing preferred foods.
Performance during the reinforcer devaluation test also confirmed the specificity of knowledge of food-cue pairs as monkeys in Group Neo-C altered the order in which they visited the box marked with the cue associated with the devalued food. In fact, this shift was much greater for the cued task. Furthermore, Group Neo-C visited devalued food boxes much later in the trial (if at all) in the cued task than in the spatial task, reinforcing the idea that they made a stronger association between particular food items and their identifying cues than their particular locations. By contrast, Group Neo-Hibo appeared to use the identical strategy in both the cued and spatial versions of the task, namely, they distinguished between preferred and nonpreferred food boxes but made limited use of specific cue-food associations to guide choice behavior. That is, they showed identical effects of reinforcer devaluation on both tasks, returning to the devalued-food box earlier in their foraging search than did controls.
Long-Lasting Effects of Neonatal Hippocampal Damage
Given the multitude of findings in rodents, monkeys, and humans indicating that neonatal hippocampal lesions permanently alter spatial memory abilities (Burgess, Maguire, & O’Keefe, 2002; Chambers, Moore, McEvoy, & Levin, 1996; Dyck, Sutherland, & Bunday, 1985; Kazama, Lay, & Bachevalier, 2003; Killiany, Rehbein, & Mahut, 2005; Lipska, Aultman, Verma, Weinberger, & Moghaddam, 2002; Rehbein, Killiany, & Mahut, 2005; Spiers, Burgess, Hartley, Vargha-Khadem, & O’Keefe, 2001; van Praag, Qu, Elliod, Dreyfus, & Black, 1998; Vargha-Khadem et al., 1997; but see Lavenex et al., 2007, for negative findings), we predicted that neonatal hippocampal damage would impair performance in the spatial version of this task. In fact, the impairment in food/place association learning in the monkeys of the present study is reminiscent to the impairment the same monkeys showed in object-place associations as measured with an incidental recognition task (Kazama et al., 2003; Blue et al., 2009), as well as the impairment in monitoring information in nonspatial working memory described earlier (Heuer & Bachevalier, 2011).
Control subjects seemed to have formed stronger associations with the unique identifiers of each box in the cued task than to the unique location of the box in the spatial task. However, the pattern of behavior displayed by Group Neo-Hibo also suggests that they may have used different strategies to successfully forage for preferred foods. For example, they could group the locations of their preferred foods and nonpreferred foods, essentially categorizing “preferred” and “nonpreferred” locations, without specific knowledge of the contents of each box. By only remembering the locations of the preferred foods, the amount of information needed to successfully forage was cut in half. There would be only four locations to remember rather than eight. Furthermore, there was no requirement to remember that a specific preferred food is in a particular location because all four food items were preferred. Thus, monkeys in Group Neo-Hibo may have learned only the location of their most preferred food (i.e., the usual starting point), perhaps using a specific view or local cue allowing them to shift their start position. Such pattern of behavior may in fact indicate an inability to form specific food/place associations, and perhaps a deficit in spatial reference memory, that is, a form of trial independent information that remains relevant throughout the entire experiment (Olton et al., 1979). Further support for this idea comes from Angeli, Murray, and Mishkin (1993) who found that monkeys with hippocampal damage can remember one place after a delay, but not two.
Conclusions
The present study demonstrated impaired spatial relational memory following neonatal neurotoxic lesions to the hippocampus using a novel paradigm to test spatial memory in freely moving macaques. We also demonstrated that normal adult monkeys can form food/place associations, although these results might have been stronger had we used only preferred foods, thus encouraging foraging in all eight locations in addition to increasing the memory load. Although our findings need to be confirmed in monkeys with adult-onset hippocampal damage, these results further strengthen our earlier findings in these monkeys that neonatal hippocampal damage permanently impairs spatial relational memory when tested either in a maze environment or in an incidental spatial recognition task (Blue et al., 2009; Kazama et al., 2003). They also extend previously reported findings of specific working memory impairments (Heuer & Bachevalier, 2011).
Acknowledgments
This work was supported by the National Institute of Mental Health (MH-58846), National Center for Research Resources P51RR165, and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132, and by Center for Behavioral Neuroscience grant NSF IBN-9876754. We thank the veterinary and animal husbandry staff at the University of Texas Health Science Center at Houston and the Yerkes National Primate Research Center for expert animal care, Roger E. Price, Belinda Rivera for the care and handling of animals during the MR imaging procedures, Edward F. Jackson for assistance in neuroimaging techniques, and Andrew Kazama and Zachary Torrey for assistance in design of the foraging boxes.
Footnotes
Data in this article contributed to the Master’s Thesis of Courtney Glavis-Bloom, and a report of preliminary results was presented at the 2006 Society for Neuroscience meeting in Atlanta, GA.
References
- Alvarado MC, Wright AA, Bachevalier J. Object and spatial relational memory in adult rhesus monkeys is impaired by neonatal lesions of the hippocampal formation but not the amygdaloid complex. Hippocampus. 2002;12:421–433. doi: 10.1002/hipo.1115. [DOI] [PubMed] [Google Scholar]
- Angeli SJ, Murray EA, Mishkin M. Hippocampectomized monkeys can remember one place but not two. Neuropsychologia. 1993;31:1021–1030. doi: 10.1016/0028-3932(93)90030-4. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, Bachevalier J. 2009 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2009. The normal development of object-place association memory is altered by neonatal hippocampal lesions in rhesus monkeys [Abstract No. 98.7] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological Psychiatry. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Dyck RH, Sutherland RJ, Bunday MR. The ontogeny of mapping and non-mapping spatial strategies following neonatal hippocampal damage in rats. Society for Neuroscience Abstract. 1985;11:832. [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: Evidence for multiple memory systems in the primate temporal lobe. Experimental Brain Research. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Harrison S. Place memory and scene memory: Effects of fornix transaction in the monkey. Experimental Brain Research. 1989;74:202–212. doi: 10.1007/BF00248293. [DOI] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado MC, Bachevalier J. Neonatal hippocampal damage impairs specific place/food associations in adult macaques. Society for Neuroscience Abstract. 2006;32 doi: 10.1037/a0031498. Retrieved from Abstracts/Annual Meeting Publications, http://www.sfn.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley GE, McGuire A, Berteau-Pavy D, Weiss A, Patel R, Mes-saoudi I, Raber J. Measures of anxiety, amygdala volumes, and hippocampal scopolamine phMRI response in elderly female rhesus macaques. Neuropharmacology. 2012;62:385–390. doi: 10.1016/j.neuropharm.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14:808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Schwartz BL. Episodic memory in nonhumans: What, and where, is when? Current Opinion in Neurobiology. 2004;14:192–197. doi: 10.1016/j.conb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behavioral Neuroscience. 2011;125:137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Working memory for temporal order is impaired after selective neonatal hippocampal lesions in adult rhesus macaques. Behavioral Brain Research. doi: 10.1016/j.bbr.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Bobko P. A weighted index of bilateral brain lesions. Journal of Neuroscience Methods. 1984;12:43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Holscher C. Time, space and hippocampal functions. Reviews in Neuroscience. 2003;14:253–284. doi: 10.1515/revneuro.2003.14.3.253. [DOI] [PubMed] [Google Scholar]
- Hough GE, Bingman VP. Spatial response properties of homing pigeon hippocampal neurons: Correlations with goal locations, movement between goals, and environmental context in a radial-arm arena. Journal of Comparative Physiology. 2004;190:1047–1062. doi: 10.1007/s00359-004-0562-z. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. The Journal of Neuroscience. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Glavis-Bloom C, Bachevalier J. 2008 Abstract Viewer/ Itinerary Planner. Washington, DC: Society for Neuroscience; 2008. Neonatal amygdala and orbital frontal cortex lesions disrupt flexible decision-making in adult macaques. Program No. 791.4. Retrieved from Abstracts/Annual Meeting Publications, http://www.sfn.org. [Google Scholar]
- Kazama AM, Lay DA, Bachevalier J. 2003 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Delayed maturation of spatial memory in infant monkeys as assessed by a visual paired-comparison task. Program No. 324.4. Retrieved from Abstracts/Annual Meeting Publications, http://www.sfn.org. [Google Scholar]
- Kessels RPC, de Haan EHF, Kappelle LJ, Postma A. Varieties of human spatial memory: A meta-analysis on the effects of hippocampal lesions. Brain Research Reviews. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- Killiany R, Rehbein L, Mahut H. Developmental study of the hippocampal formation in rhesus monkeys (Macacca mulatta): II. Early ablations do not spare the capacity to retrieve conditional object-object associations. Behavioral Neuroscience. 2005;119:651–661. doi: 10.1037/0735-7044.119.3.651. [DOI] [PubMed] [Google Scholar]
- King JA, Trinkler I, Hartley T, Vargha-Khadem F, Burgess N. The hippocampal role in spatial memory and the familiarity-recollection distinction: A case study. Neuropsychology. 2004;18:405–417. doi: 10.1037/0894-4105.18.3.405. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. The Journal of Neuroscience. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex PB, Lavenex P. Spatial memory and the monkey hippocampus: Not all space is created equal. Hippocampus. 2009;19:8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavanex PB, Amaral DG. Spatial relational learning persists following neonatal hippocampal lesions in macaque monkeys. Nature Neuroscience. 2007;10:234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghad-dam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Lopez JC, Gomez Y, Rodriguez F, Broglio C, Vargas JP, Salas C. Spatial learning in turtles. Animal Cognition. 2001;4:49–59. [Google Scholar]
- Ludvig N, Tang HM, Eichenbaum H, Gohil BC. Spatial memory performance of freely-moving squirrel monkeys. Behavioural Brain Research. 2003;140:175–183. doi: 10.1016/s0166-4328(02)00325-x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. European Journal of Neuroscience. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Burgess N, Donnett JG, O’Keefe J. Knowing where things are: Parahippocampal involvement in encoding object locations in virtual large-scale space. Journal of Cognitive Neuroscience. 1998;10:61–76. doi: 10.1162/089892998563789. [DOI] [PubMed] [Google Scholar]
- Mahut H. Spatial and object reversal learning in monkeys with partial temporal lobe ablations. Neuropsychologia. 1971;9:409–424. doi: 10.1016/0028-3932(71)90005-4. [DOI] [PubMed] [Google Scholar]
- Mahut H, Zola S. A non-modality specific impairment in spatial learning after fornix lesions in monkeys. Neuropsychologia. 1973;11:255–269. doi: 10.1016/0028-3932(73)90037-7. [DOI] [PubMed] [Google Scholar]
- Málková L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. The Journal of Neuroscience. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. The Journal of Neuroscience. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behavioral Neuroscience. 1998;112:1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan EA, Flint RWJ. Anterior rhinal cortex and amygdala: Dissociation of their contributions to memory and food preference in rhesus monkeys. Behavioral Neuroscience. 1996;110:30–42. [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. The Journal of Neuroscience. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: A replication. Journal of Neuroscience Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. New York, NY: Oxford University Press; 1978. [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behavioral Brain Science. 1979;2:313–365. [Google Scholar]
- Parker A, Gaffan D. The effect of anterior thalamic and cingulated cortex lesions on object-in-place memory in monkeys. Neuropsychologia. 1997;35:1093–1102. doi: 10.1016/s0028-3932(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Parkinson JK, Murray EA, Mishkin M. A selective mnemonic role for the hippocampus in monkeys: Memory for the location of objects. The Journal of Neuroscience. 1988;8:4159–4167. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. The Journal of Neuroscience. 1995;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucet B. Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behavioral Neuroscience. 1989;103:1009–1016. doi: 10.1037//0735-7044.103.5.1009. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Rehbein L, Killiany R, Mahut H. Developmental study of the hippocampal formation in rhesus monkeys (Macaca mulatta): I. Early ablations spare discrimination learning but not recognition memory. Behavioral Neuroscience. 2005;119:635–650. doi: 10.1037/0735-7044.119.3.635. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Pearson C, Kershaw TR, Hodges H, Maclean CJ, Hoyle C, Baker HF. Learning impairment induced by lesion of the CA1 field of the primate hippocampus: Attempts to ameliorate the impairment by transplantation of fetal CA1 tissue. Experimental Brain Research. 1997;115:83–94. doi: 10.1007/pl00005688. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Kolachana BS, Bachevalier J, Weinberger DR. Neonatal lesions of the medial temporal lobe disrupt prefrontal cortical regulation of striatal dopamine. Nature. 1998;393(6681):169–171. doi: 10.1038/30245. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- van Praag H, Qu PM, Elliod RC, Dreyfus CF, Black IB. Unilateral hippocampal lesions in newborn and adult rats: Effects on spatial memory and BDNF gene expression. Behavioural Brain Research. 1998;92:21–30. doi: 10.1016/s0166-4328(97)00117-4. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Von Bonin G, Bailey P. The neocortex of Macaca mulatta. Champagne, IL: University of Illinois Press; 1947. [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. The Journal of Neuroscience. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]