Abstract
Background / Objectives
Diet quality indices are increasingly used in nutrition epidemiology as dietary exposures in relation to health outcomes. However, literature on long-term stability of these indices is limited. We aimed to assess the stability of the validated Framingham Nutritional Risk Score (FNRS) and its component nutrients over 8 years as well as the validity of the follow-up FNRS.
Subjects / Methods
Framingham Offspring/Spouse Study women and men (n=1 734) aged 22-76 years wwver 8 years. Individuals' nutrient intake and nutritional risk scores were assessed using 3-day dietary records administered at baseline (1984-1988) and at follow-up (1992-1996). Agreement between baseline and follow-up FNRS and nutrient intakes was evaluated using Bland-Altman method; stability was assessed using intra-class correlation (ICC) and weighted Kappa statistics. The effect of diet quality (as assessed by the FNRS) on cardiometabolic risk factors was evaluated using ANCOVA.
Results
Modest changes from baseline (≤15%) were observed in nutrient intake. Stability coefficients for the FNRS (ICC: women=0.49; men=0.46; P<0.0001) and many nutrients (ICC ≥0.3) were moderate. Over half of women and men (58%) remained in the same or contiguous baseline and follow-up quartile of the FNRS and few (3-4%) shifted >1 quartile. The FNRS was directly associated with BMI in women (P<0.01) and HDL-cholesterol among both women (P<0.001) and men (P<0.01).
Conclusion
The FNRS and its constituent nutrients remained relatively stable over 8 years of follow-up. The stability of diet quality has implications for prospective epidemiological investigations.
Keywords: long-term stability, dietary quality indices, nutrients
Introduction
Diet is a key determinant of health outcomes (Willett, 1998). Historically, studies have focused on single nutrient or food exposures. However, such approaches are inherently confounded by food and nutrient collinearities and interactions (Moeller et al., 2007; Willett, 1998); subsequently, researchers are focusing on broader measures of dietary intake to address the confounding.
Dietary quality indices (theoretical dietary patterns) are typically defined relative to expert dietary guidelines or composite nutrient scores; conversely, empirically-derived patterns define habitual food intake and are derived by cluster and factor analysis or reduced rank regression (Moeller et al., 2007). However, a major concern has been that patterns might not be stable over time due to changes in dietary behaviors and/or dietary assessment methods resulting in failure to detect important diet-disease relationships or attenuation of observed effects (Moeller et al., 2007; Willett, 1998). Thus evaluating stability of dietary patterns is crucial. While there is increasing evidence of reproducibility and stability of empirically-derived dietary patterns (Borland et al., 2008; Mishra et al., 2006; Newby & Tucker, 2004), data on the stability of dietary quality indices are limited and few studies have examined long-term stability of individual or composite nutrient intake despite considerable research interest on the importance of this topic (Moeller et al., 2007).
In this report we evaluated the stability of the validated Framingham Nutritional Risk Score (FNRS) and its 19 component nutrients over 8 years in the Framingham Heart Study (FHS) Offspring/Spouse cohort; we also examined the validity of the FNRS derived at follow-up. This study will help ascertain the utility of theoretical patterns in longitudinal nutrition studies.
Subjects and Methods
Study Population and Sample
The FHS was initiated in 1948 as a longitudinal study of cardiovascular disease (CVD) and other chronic diseases (Dawber, 1980). In 1971, a second generation cohort of 5 124 FHS offspring and their spouses was enrolled, composing the Framingham Offspring/Spouse study (FOS). Members of the FOS cohort participate in standardized medical assessments about every 4 years (Kannel et al., 1979). At FOS exam 3 (1984-1988), the cohort's dietary intake was comprehensively examined and characterized as the Framingham Nutrition Study (FNS). These participants, 3 729 women and men aged 18-77 years (73% of the original offspring), completed a single 24-h recall and the Framingham food frequency questionnaire (FFQ); 67% also completed 3-day dietary records (Millen et al., 2001; Millen et al., 2005). Our study sample comprised 949 women and 785 men who participated in exams 3 and 5 (1992-1996) and provided 3-day dietary record data. A subsample (1 398; 81%) who were free of CVD, diabetes mellitus, and cancer at exam 5 were used for the validation of the follow-up FNRS. We used the unrestricted sample for the primary analyses in order to maximize the statistical power.
Compared to non-participants, FNS participants were older and less likely to smoke (P<0.0001). Additionally, FNS women had lower body mass index (BMI), a smaller waist circumference (WC), higher HDL-cholesterol concentration, and were more likely to be post-menopause while FNS men were less physically active (all P<0.05, data not shown).
The Boston University Medical Center's Human Subjects Institutional Review Board approved the study protocol and all participants provided informed consent.
Nutrient Intake and the Framingham Nutritional Risk Score
Nutrient intake was estimated from 3-day dietary records using the Minnesota Nutrition Data System (NDS) software (version 2.6; Food Database 6A; Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN (Schakel et al., 1988) as described elsewhere (Posner et al., 1992a). A registered dietitian instructed participants to record their intake over 2 weekdays and 1 weekend day, while adhering to their usual eating practices. Participants estimated food portion sizes using a validated 2-dimensional food portion model (Posner et al., 1992b). Trained coders processed dietary records using standardized protocols.
The FNRS, which has been published (Kimokoti et al., 2010; Millen et al., 2006; Wolongevicz et al., 2009), was computed from the sum of the mean scores of 19 rank-ordered nutrients among FOS women (n=1 265) and men (n=1 200) using a validated methodology (Millen et al., 2001; Quatromoni et al., 2002). In brief, FNRS nutrients were CVD risk-related according to 3 categories: selected macronutrients (energy, protein, monounsaturated fat (MUFA), polyunsaturated fat (PUFA)); risk nutrients (total fat, saturated fat (SFA), alcohol, cholesterol, sodium); and protective nutrients (carbohydrate, dietary fiber, calcium, selenium, vitamin C, vitamin B-6, vitamin B-12, folate, vitamin E, β-carotene). A desirable intake level (e.g. lower fat or higher vitamin intake) was assigned a lower rank and a less desirable intake level (e.g. higher fat or lower vitamin intake) a higher rank. A higher MUFA intake received a higher rating since it was derived mainly from animal sources (e.g. beef fat) rather than vegetable sources (e.g. olive oil) among FNS participants. Individuals' nutrient intake and nutritional risk score were estimated at baseline (1984-1988) and follow-up (1992-1996). Recommended intake levels were specified according to expert nutrition Panels and are consistent with Dietary Guidelines for Americans guidelines (AHA, 2006; FNB, 2005; NCEP, 2001; US DHHS, 2005) (Appendix). The FNRS was developed specifically to assess CVD, which is a focus of the FHS (Dawber, 1980) in contrast to the Healthy Eating Index-2005, a mainly food-based index, which measures compliance to Dietary Guidelines for Americans that focus on chronic disease prevention (Guenther et al., 2007). However, the FNRS comprises many of the nutrients that are related to major chronic diseases.
Covariates and cardiometabolic risk factors
CVD risk factors are routinely assessed at Framingham exams (Cupples & D'Agostino, 1987). Age, menopausal status, smoking status, physical activity, hypertension and lipid treatment, and hormone replacement therapy (HRT) were self-reported (Cupples & D'Agostino, 1987). Physical activity was assessed by a standardized questionnaire (Kannel & Sorlie, 1979). To calculate BMI (weight (kg)/height (m2)), participants were weighed using a calibrated scale and height was measured using a stadiometer (Abraham et al., 1979). WC was measured with an anthropometric tape (Stoudt et al., 1970). Blood pressure was determined using a mercury sphygmomanometer (Thomas et al., 1981). Plasma glucose was measured with hexokinase reagent kit (Meigs et al., 1997). HDL-cholesterol and triglyceride were measured enzymatically (McNamara & Schaefer, 1987; Warnick et al., 1982). LDL-cholesterol was estimated by the method of Friedewald et al. (Friedewald et al., 1972). All covariates and cardiometabolic risk factors (CMRFs) were derived from exam 5.
Statistical Analysis
We conducted sex-specific analyses a priori given the gender differences in dietary exposures (Kimokoti et al., 2010; Millen et al., 2001; Millen et al., 2005).
ANCOVA was used to calculate age-adjusted least-squares means ± SE for nutrient intake and where indicated to identify post-hoc pair-wise mean differences between FNRS tertiles using Tukey's HSD test at baseline and follow-up (Lomax, 2007).
To assess stability, raw means and mean differences ± SD for the FNRS and constituent nutrients at baseline and follow-up were calculated. Agreement between baseline and follow-up FNRS and nutrient intakes was assessed using Bland-Altman method. Limits of agreement were calculated as mean difference ±2 SD (Bland & Altman, 1986). Stability of the FNRS and nutrient intakes was evaluated using intra-class correlation (ICC) (Koch, 1982). Intra-individual changes of the FNRS and nutrient intakes were assessed using shift tables of FNRS quartiles at baseline and follow-up; analyses utilized the weighted Kappa statistic for symmetry (Fleiss & Cohen, 1973). In secondary analyses, we examined stability of the FNRS using shift tables of FNRS quintiles.
The follow-up FNRS was validated by relating it to CMRFs (BMI, WC, systolic blood pressure, diastolic blood pressure, glucose, triglycerides, HDL-cholesterol, LDL-cholesterol). ANCOVA was used to calculate multivariable-adjusted least-squares means ± SE of the factors and to calculate pair-wise mean differences between FNRS tertiles using Tukey's HSD test (Lomax, 2007). Models were adjusted for age, BMI, WC, physical activity index, smoking status, hypertension treatment, lipid treatment, HRT (women), and menopausal status (women). The association between the FNRS and CMRFs was evaluated using Pearson correlation coefficient. In secondary analyses, models were adjusted for covariates in the primary model and energy intake. We also conducted analyses in FNRS quartiles and quintiles. The effect of diet quality on CMRFs was assessed using ANCOVA (Lomax, 2007); results were summarized as linear regression coefficients ± SE. In secondary analyses, the FNRS was modeled as a repeated measure using the cumulative average diet approach (Hu et al., 1999).
All analyses were performed using the Statistical Analysis Software (version 9.2, 2008, SAS Institute Inc. Cary, NC) (SAS, 2008). All statistical tests were two-sided (P<0.05).
Results
At both baseline and follow-up, participants with lower diet quality (tertile 3) had higher mean consumption of dietary lipids and alcohol and lower mean intakes of carbohydrate, fiber, micronutrients, sodium, and energy than those with higher diet quality (all P<0.05, data not shown).
Secular improvement was evident in nutrient intake overall apart from energy, protein, and sodium, whose intakes increased over the 8-year period. Most changes in the consumption of the 19 nutrients were modest (≤15%) except for SFA, alcohol, vitamin C, vitamin B-6, folate, and beta-carotene in women as well as alcohol and vitamin B-12 in men. Stability coefficients for many nutrients were moderate (ICC ≥0.3) as was agreement; energy, sodium, calcium, and β-carotene in both sexes had lowest agreement levels (Tables 1 and 2).
Table 1. Raw means, mean changes, Bland-Altman limits of agreement, and intra-class correlations (ICC) for the Framingham Nutritional Risk Score and nutrient intakes for Framingham Offspring-Spouse Nutrition Study women at baseline (1984–1988) and follow-up (1992–1996) (n = 949)1.
| Framingham Nutritional Risk Score and its Nutrient components | Baseline (1984–1988) | Follow-up (1992–1996) | |||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD of difference | Limits of agreement | ICC | ||
| Framingham Nutritional Risk Score | 661.5 ± 140.3 | 678.1 ± 145.3 | 16.6 ± 143.92 | −271.2, 304.5 | 0.493 |
| Energy (kJ)4 | 6 588.3 ± 1 986.3 | 6 949.2 ± 2,029.8 | 360.8 ± 2 031.53 | −884.5, 1 056.9 | 0.483 |
| Protein (% Energy) | 17.4 ± 4.2 | 17.5 ± 3.9 | 0.2 ± 4.6 | −9.1, 9.4 | 0.343 |
| Total fat (% Energy) | 36.5 ± 6.9 | 31.6 ± 7.2 | −4.9 ± 8.23 | −21.2, 11.5 | 0.193 |
| Monounsaturated fat (% Energy) | 13.2 ± 2.9 | 11.8 ± 3.0 | −1.4 ± 3.53 | −8.4, 5.5 | 0.233 |
| Polyunsaturated fat (% Energy) | 7.7 ± 2.7 | 6.9 ± 2.4 | −0.8 ± 3.23 | −7.3, 5.6 | 0.183 |
| Saturated fat (% Energy) | 12.7 ± 3.2 | 10.4 ± 3.2 | −2.3 ± 3.73 | −9.6, 5.0 | 0.213 |
| Carbohydrate (% Energy) | 44.8 ± 8.4 | 50.3 ± 8.7 | 5.5 ± 9.33 | −13.1, 24.0 | 0.292 |
| Dietary fiber (g/4 186 kJ) | 13.1 ± 5.4 | 16.0 ± 6.7 | 2.9 ± 6.43 | −9.8, 15.6 | 0.383 |
| Alcohol (% Energy) Median IQR | 0.05 (0, 4.3) | 0.02 (0, 3.0) | −0.6 ± 4.03 | −8.6, 7.4 | 0.623 |
| Cholesterol (mg/4 186 kJ) | 229.4 ± 110.5 | 202.1 ± 103.6 | −27.3 ± 128.03 | −283.3, 228.7 | 0.273 |
| Sodium (mg/4 186 kJ) | 2 503.6 ± 899.2 | 2 678.6 ± 997.5 | 175.0 ± 1 068.33 | −1 961.6, 2 311.7 | 0.363 |
| Calcium (mg/4 186 kJ) | 628.1 ± 275.7 | 676.0 ± 310.2 | 47.9 ± 302.23 | −556.4, 652.2 | 0.463 |
| Selenium (μg/4 186 kJ) | 98.6 ± 34.2 | 105.5 ± 33.9 | 6.9 ± 41.13 | −75.3, 89.2 | 0.263 |
| Vitamin C (mg/4 186 kJ) | 93.9 ± 59.0 | 113.4 ± 69.3 | 19.5 ± 70.13 | −120.7, 159.7 | 0.383 |
| Vitamin B-6 (mg/4 186 kJ) | 1.4 ± 0.6 | 1.7 ± 0.7 | 0.3 ± 0.73 | −1.2, 1.8 | 0.313 |
| Vitamin B-12 (μg/4 186 kJ) Median IQR | 3.7 (2.6, 5.3) | 3.6 (2.4, 5.5) | −0.1 ± 14.5 | −29.1, 28.8 | 0.02 |
| Folate (μg/4 186 kJ) | 225.9 ± 114.2 | 271.5 ± 136.0 | 45.7 ± 139.43 | −233.1, 324.4 | 0.343 |
| Vitamin E (mg/4 186 kJ) | 7.9 ± 4.2 | 8.9 ± 5.1 | 1.0 ± 5.83 | −10.5, 12.6 | 0.223 |
| β-carotene (μg/4 186 kJ) Median IQR | 2 108.9 (1 003.9, 4 319.7) | 2 859.9 (1 425.4, 5 605.8) | 994.7 ± 5 024.83 | −9 054.9, 11 044.4 | 0.153 |
Bland-Altman limits of agreement between baseline and follow-up Framingham Nutritional Risk Score (FNRS) and nutrient intakes were calculated as mean difference ± 2 SD of the difference. Stability of the FNRS and nutrient intakes was evaluated using intra-class correlation.
P<0.001
P<0.0001
To convert kJ to kcal divide by 4.186.
Table 2. Raw means, mean changes, Bland-Altman limits of agreement, and intra-class correlations (ICC) for the Framingham Nutritional Risk Score and nutrient intakes for Framingham Offspring-Spouse Nutrition Study men at baseline (1984–1988) and follow-up (1992–1996) (n=785)1.
| Framingham Nutritional Risk Score and its Nutrient components | Baseline (1984–1988) | Follow-up (1992–1996) | |||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD of difference | Limits of agreement | ICC | ||
| Framingham Nutritional Risk Score2 | 578.0 ± 119.3 | 560.8 ± 119.0 | −17.2 ± 122.84 | −262.9, 228.4 | 0.464 |
| Energy (kJ)5 | 9 025.4 ± 2 590.7 | 9 301.3 ± 2 740.2 | 275.9 ± 2 849.43 | −1 295.5, 1 427.3 | 0.434 |
| Protein (% Energy) | 16.6 ± 3.5 | 16.9 ± 3.8 | 0.3 ± 4.22 | −8.1, 8.8 | 0.324 |
| Total fat (% Energy) | 36.0 ± 7.0 | 32.7 ± 7.3 | −3.3 ± 8.54 | −20.3, 13.8 | 0.234 |
| Monounsaturated fat (% Energy) | 13.5 ± 3.0 | 12.4 ± 3.1 | −1.1 ± 3.84 | −8.7, 6.5 | 0.204 |
| Polyunsaturated fat (% Energy) | 7.2 ± 2.7 | 6.7 ± 2.2 | −0.5 ± 3.24 | −7.0, 5.9 | 0.134 |
| Saturated fat (% Energy) | 12.5 ± 3.2 | 11.0 ± 3.3 | −1.5 ± 3.64 | −8.7, 5.8 | 0.314 |
| Carbohydrate (% Energy) | 43.5 ± 8.9 | 48.2 ± 8.9 | 4.7 ± 9.54 | −14.3, 23.7 | 0.344 |
| Dietary fiber (g/4 186 kJ) | 16.7 ± 6.8 | 19.3 ± 8.6 | 2.6 ± 8.14 | −13.7, 18.8 | 0.414 |
| Alcohol (% Energy) Median IQR | 2.7 (0, 8.1) | 1.4 (0, 5.5) | −1.6 ± 5.94 | −13.4, 10.2 | 0.504 |
| Cholesterol (mg/4 186 kJ) | 305.2 ± 143.1 | 282.4 ± 135.4 | −22.8 ± 161.94 | −346.6, 301.0 | 0.324 |
| Sodium (mg/4 186 kJ) | 3 379.4 ± 1 216.8 | 3 544.7 ± 1 267.5 | 165.3 ± 1 470.03 | −2 774.7, 3 105.2 | 0.294 |
| Calcium (mg/4 186 kJ) | 769.3 ± 353.4 | 829.3 ± 370.5 | 60.0 ± 384.14 | −708.3, 828.2 | 0.434 |
| Selenium (μg/4 186 kJ) | 132.3 ± 41.9 | 138.5 ± 45.7 | 6.1 ± 53.43 | −100.7, 113.0 | 0.254 |
| Vitamin C (mg/4 186 kJ) | 108.8 ± 74.6 | 120.3 ± 78.7 | 11.5 ± 83.34 | −155.1, 178.0 | 0.404 |
| Vitamin B-6 (mg/4 186 kJ) | 2.0 ± 0.8 | 2.2 ± 1.0 | 0.3 ± 1.04 | −1.7, 2.3 | 0.364 |
| Vitamin B-12 (μg/4 186 kJ) Median IQR | 5.2 (3.6, 7.8) | 4.8 (3.2, 7.2) | −1.6 ± 16.83 | −35.2, 32.0 | 0.02 |
| Folate (μg/4 186 kJ) | 287.3 ± 147.2 | 329.6 ± 172.8 | 42.4 ± 176.64 | −310.9, 395.6 | 0.374 |
| Vitamin E (mg/4 186 kJ) | 10.2 ± 5.9 | 10.9 ± 6.6 | 0.7 ± 7.93 | -15.0, 16.5 | 0.214 |
| β-carotene (μg/4 186 kJ) Median IQR | 2 147.8 (1 178.7, 4 504.4) | 2 618.7 (1 264.9, 5 178.0) | 485.3 ± 4 6663 | −8 846.7, 9 817.3 | 0.224 |
Bland-Altman limits of agreement between baseline and follow-up Framingham Nutritional Risk Score (FNRS) and nutrient intakes were calculated as mean difference ± 2 SD. Stability of the FNRS and nutrient intakes was evaluated using intra-class correlation.
P<0.05
P<0.01
P<0.0001
To convert kJ to kcal divide by 4.186.
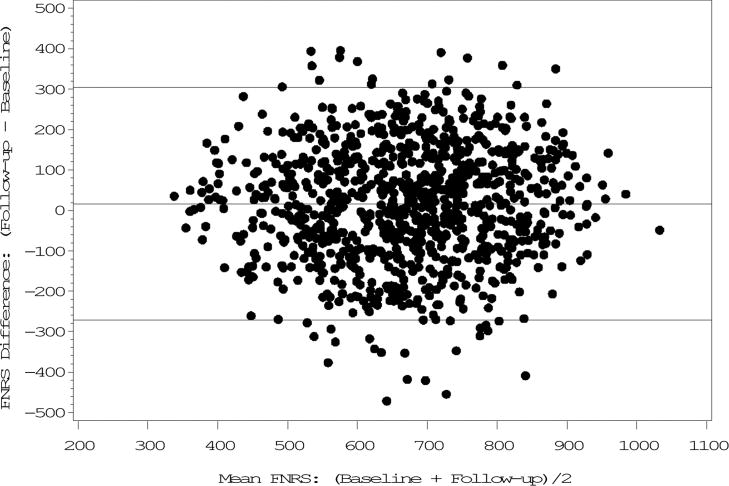
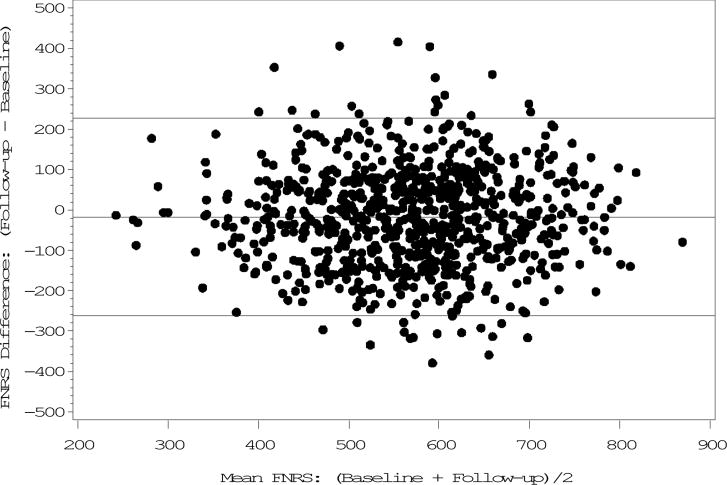
The FNRS changed the least (3%) among both sexes and, excepting alcohol, correlations were higher than those of individual nutrients (women: ICC=0.49; men: ICC=0.46, P<0.0001). Both correlation and agreement were moderate (Tables 1 and 2), (Figures 1 and 2).
Figure 1. Bland-Altman plot of the Framingham Nutritional Risk Score at baseline (1984–1988) and follow-up (1992–1996) in Framingham Offspring-Spouse Nutrition Study women (n = 949)1.
1Limits of agreement: Mean difference +/−2 SD of the difference.
Figure 2. Bland-Altman plot of the Framingham Nutritional Risk Score at baseline (1984–1988) and follow-up (1992–1996) in Framingham Offspring-Spouse Nutrition Study men (n = 785)1.
1Limits of agreement: Mean difference +/−2 SD of the difference.
From baseline to follow-up, over one-third of women (36%) and men (37%) stayed in the same FNRS quartile while 58% remained in the same quartile or changed only one quartile up or down. Changes in extreme quartiles (1 to 4 or 4 to 1) were minimal (women: 3%; men: 4%) (weighted Kappa: women=0.29, P=0.6519; men=0.29, P =0.8980) (Table 3). In secondary analyses, nearly one-third of women (31%) and men (31%) stayed in the same quintile whereas 50% remained in the same or contiguous baseline and follow-up quintile (weighted Kappa: women=0.31, P=0.9076; men=0.29, P=0.7468) (data not shown).
Table 3. Transition of Framingham Offspring-Spouse Nutrition Study women and men among Framingham Nutritional Risk Score quartiles between baseline (1984-1988) and follow-up (1992–1996) (n = 1 734)1,2.
| Women | Men | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exam 5 | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | All | Exam 5 | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | All |
| Exam 3 | Exam 3 | ||||||||||
| Quartile 1 | 109 | 74 | 43 | 11 | 237 | Quartile 1 | 96 | 54 | 32 | 14 | 196 |
| Quartile 2 | 74 | 52 | 63 | 48 | 237 | Quartile 2 | 54 | 48 | 59 | 35 | 196 |
| Quartile 3 | 34 | 64 | 72 | 68 | 238 | Quartile 3 | 31 | 51 | 58 | 57 | 197 |
| Quartile 4 | 20 | 47 | 60 | 110 | 237 | Quartile 4 | 15 | 43 | 48 | 90 | 196 |
| All | 237 | 237 | 238 | 237 | 949 | All | 196 | 196 | 197 | 196 | 785 |
Test of symmetry (S): Women =4.2 (P=0.6519), weighted Kappa =0.29; Men =2.2 (P=0.8980), weighted Kappa =0.29. A total of 343 (36%) women and 292 (37%) men remained in the same Framingham Nutritional Risk Score quartile at both exams.
Framingham Nutritional Risk Score: Overall nutrient risk score based on the consumption of 19 cardiovascular disease risk-related nutrients for each woman or man.
In validation analyses, BMI at follow-up was positively correlated with the follow-up FNRS among women. In multivariable-adjusted analysis, women with lower diet quality (tertile 3) had lower mean triglyceride and LDL-cholesterol concentration and higher mean HDL-cholesterol concentration than those with higher diet quality (P<0.05). In men, follow-up BMI and HDL-cholesterol directly correlated with the FNRS. Mean HDL-cholesterol concentration was higher in men with lower diet quality compared to those with higher diet quality (P<0.05). In secondary analyses among FNRS quartiles and quintiles, comparable findings were observed in both sexes. Adjusting for energy intake did not qualitatively alter the main results (data not shown).
In multivariable mixed model analysis, the FNRS was directly associated with BMI in women and HDL-cholesterol among both sexes (Table 4). Similar results were obtained in cross-sectional (follow-up) linear regression analyses (data not shown).
Table 4. Linear regression beta-coefficients showing the association between the Framingham Nutritional Risk Score (cumulative average from baseline and follow-up) and metabolic risk factors among Framingham Offspring-Spouse Nutrition Study women and men (n = 1 398)1,2.
| Women (n = 795) | Men (n = 603) | |||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| BMI (kg/m2)3 | 0.003 (0.001) | 0.0011 | 0.001 (0.001) | 0.1857 |
| WC (cm)4 | −0.003 (0.002) | 0.1653 | −0.0001 (0.0019) | 0.9551 |
| Systolic BP (mmHg)5 | −0.002 (0.005) | 0.6084 | 0.002 (0.006) | 0.7784 |
| Diastolic BP (mmHg)5 | −0.001 (0.003) | 0.5833 | 0.002 (0.004) | 0.5309 |
| Glucose (mg/dL)5 | 0.004 (0.003) | 0.1125 | 0.004 (0.003) | 0.2454 |
| Triglycerides (mg/dL)5 | −0.001 (0.02) | 0.9470 | −0.07 (0.04) | 0.0791 |
| LDL-cholesterol (mg/dL)5 | −0.01 (0.01) | 0.2994 | 0.012 (0.013) | 0.3558 |
| HDL-cholesterol (mg/dL)5 | 0.013 (0.004) | 0.0022 | 0.014 (0.004) | 0.0018 |
The Framingham Nutritional Risk Score was analyzed as a repeated measurement using cumulative average diet.
Framingham Nutritional Risk Score: Overall nutrient risk score based on the consumption of 19 cardiovascular disease risk-related nutrients for each woman or man.
BMI was adjusted for age, WC, physical activity index, smoking status (non-smokers, smokers), hypertension treatment (yes/no), lipid treatment (yes/no), and among women, HRT (yes/no) and post-menopausal status (yes/no).
WC was adjusted for age, BMI, physical activity index, smoking status (non-smokers, smokers), hypertension treatment (yes/no), lipid treatment, (yes/no), and among women, HRT (yes/no) and post-menopausal status (yes/no).
All other outcomes were adjusted for age, BMI, WC, physical activity index, smoking status (non-smokers, smokers), hypertension treatment (yes/no), lipid treatment, (yes/no), and among women, HRT (yes/no) and post-menopausal status (yes/no).
Discussion
In this study, the FNRS was moderately stable over 8 years (ICC: women=0.49; men=0.46). Among FNRS nutrient components, changes in consumption were modest and individual nutrient intakes showed low to moderate but significant stability. Nearly two-fifths of women (36%) and men (37%) remained in the same FNRS quartile while 58% remained in the same or contiguous baseline and follow-up FNRS quartile and few (3-4%) shifted to extreme quartiles. The FNRS was associated with HDL-cholesterol in both sexes and BMI among women.
Secular trends in nutrient intake paralleled those found in the general US population. National Health and Nutrition Examination Surveys (NHANES) data show that macronutrient intakes have shifted ≤15% during a similar duration (Briefel & Johnson, 2004) and, consistent with our data, on average meet recommendations for population health and chronic disease reduction except for SFA, which exceeds the recommended levels (albeit half the population continues to fail expert-advised intake levels). National studies also indicate that calcium intake in women has risen somewhat (Briefel & Johnson, 2004) but, similar to our study, still falls below the expert guidelines (FNB, 2005; US DHHS, 2005). Likewise, adult folate intakes have increased but are generally low among African and Mexican Americans and younger (15-44 years) women (Briefel & Johnson, 2004).
Our findings also corroborate those of the UK National Survey of Health and Development (NSHD). Changes of 25 nutrients in adults followed for 17 years were modest (≤15%) except for alcohol, vitamin C, retinol, pyridoxine, and copper in both sexes as well as carotene and thiamine in women. Nutrient intakes improved in accordance with dietary guidelines. However, neither stability of the nutrient intakes nor dietary quality of the cohort were evaluated (Prynne et al., 2005).
Most comparable to FNS results were those obtained in the Health Professionals Follow-up Study (HPFS) (Newby et al., 2003). Correlation for the Dietary Quality Index-Revised (DQI-R) scores assessed 1 year apart was r=0.72 while correlations for total fat, SFA, cholesterol, calcium, and iron ranged from r=0.41 to r=0.69. Agreement of the indices and constituent nutrients were, however, not assessed. Short-term stability (1 year) of the glycemic index has been examined in several studies (Du et al., 2009; Murakami et al., 2008); ICC coefficients ranged between 0.40 and 0.78. Agreement of the indices was likewise not examined. Our study, to the best of our knowledge, is the first to evaluate long-term stability of dietary quality using a nutrient risk score.
Two studies investigating the stability of empirically-derived dietary patterns in adults also provide results consistent with those for the FNRS. In the British NSHD mentioned earlier (Prynne et al., 2005), factor score agreement for 4 of 5 dietary patterns was similar to that of the FNRS; Kappa (95% CI): women=0.28 (0.23-0.34) to 0.36 (0.31-0.42); men=0.38 (0.32-0.44) to 0.39 (0.33, 0.45) (Mishra et al., 2006). Of 2 dietary patterns defined among participants of the Southampton Women's Survey, the Prudent pattern was very stable over 2 years (Bland-Altman limits of agreement: -1.00, 1.26) (Borland et al., 2008). In the Cardiovascular Risk in Young Finns Study, 2 patterns identified were stable over 21 years. Among older adolescents (15-18 years), 27-29% remained in quintile 1 and 38-42% in quintile 5 (Mikkila et al., 2005). Estimates were somewhat lower than those observed in the FNS cohort most likely due to the long follow-up.
Several studies of CVD risk factors in relation to diet quality indices have obtained discrepant findings similar to ours. The Healthy Eating Index (HEI) was inversely related to both BMI and HDL-cholesterol but was not associated with triglycerides and blood pressure in NHANES III participants (Kant & Graubard, 2005). In the SUpplementation en VItamines et Minéraux AntioXydants study, the HEI was inversely associated with BMI and blood pressure but was unrelated to plasma lipids (Drewnoski et al., 2009). The Comprehensive Healthy Dietary Pattern was associated with BMI, WC, HDL-cholesterol, and triglycerides but not with LDL-cholesterol and blood pressure in the Multi-Ethnic Study of Atherosclerosis (Nettleton et al., 2008).
Methods of assessment in the FNS cohort remained constant, as did nutrient databases; however, improvements in nutrient intake attributable to nationwide nutrition education campaigns might have affected our findings (Millen & Quatromoni, 2001; Millen et al., 1997). Of note, however, were the significant ICC coefficients. Improvements in CMRFs due to changes in dietary behavior and improved treatment methods (Millen & Quatromoni, 2001; Millen et al., 1997) may similarly explain our inability to detect associations between some of the factors and dietary quality as evaluated by the FNRS.
Reverse causality may account for the lower mean triglyceride and LDL-cholesterol concentration among women with lower diet quality as well as the higher mean HDL-cholesterol concentration in both women and men with lower diet quality at follow-up. However, greater consumption of TF, SFA, MUFA, and PUFA with concomitant low carbohydrate intake among these participants might also explain the observed associations (ADA, 2007; Miller, 2011). In the mixed model analysis, this nutrient intake pattern may similarly account for the direct relationship of HDL-cholesterol and the FNRS in both sexes. Among women, the direct association of BMI and the FNRS might be attributable to reduction of postprandial and hepatic fatty acid oxidation by estrogen (O'Sullivan, 2009).
Use of repeated diet measures, which increases accuracy of dietary data (Hu et al., 1999), is an important strength of our research. Bland-Altman method is considered one of the best procedures for evaluating agreement between two measurements (Bland & Altman, 1986) and ICC is regarded as the best measure of stability for continuous data (Koch, 1982). We believe that our study is the first to show stability of diet quality using an index and its nutrient components. Inclusion of both women and men with a broad age range and the well-characterized study cohort are additional strengths of our study.
The exclusively Caucasian sample of our study may limit generalizability of results obtained as dietary patterns may differ across race/ethnic groups and culture (Moeller et al., 2007) although a comprehensive review (Newby & Tucker, 2004) and the INTERHEART study (Iqbal et al., 2008) provide evidence to show reproducibility of major patterns across these characteristics. Further, diet quality indices are subjective with regard to dietary variables included (e.g. the disease outcome focus of the FNRS), cut-off points and scoring methods (Moeller et al., 2007). Moreover, dietary guidelines across countries may lack scientific consensus (Newby & Tucker, 2004). Comparison across studies is thus not always straightforward.
Only two-thirds of FNS participants had 3-day dietary records and the reduced sample size may have underrated the observed relationships. Dietary records are considered a gold standard for diet assessment and we elected to collect 3-day due to their relatively lower respondent burden compared to multiple-day records. Weighed food records are the most accurate method and are commonly utilized in Europe; we determined food-specific units and volumes, which is the generally used approach in the United States (Willett, 1998). Furthermore, usually random errors that are associated with any dietary self-report may have underestimated our findings (Willett, 1998). Additionally, the ‘healthy cohort’ effect may somewhat limit the generalizability of study findings. Nonetheless, the secular trends found in the FHS population synchronize with those observed in nationally representative samples, and the comparability of our data on nutrient stability provide evidence of our population's representativeness.
In conclusion, the FNRS and nutrient intakes of FNS adults remained relatively stable over 8 years of follow-up. The stability of diet quality has implications for prospective epidemiological investigations. Additional longitudinal studies are needed to further understand the stability and validity of population-specific dietary quality indices and how measures of diet quality assessed over time are associated with health outcomes.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health/National Heart, Lung and Blood Institute grants contracts R01-HL-60700, R01-HL-54776 and N01-HC-25195 Bethesda, MD.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
References
- Abraham S, Johnson CL, Najjar MF. Weight and height of adults 18–74 years of age. United States, 1971–1974. Vital Health Stat 11. 1979;211:1–49. [PubMed] [Google Scholar]
- American Dietetic Association. Position of the American Dietetic Association and Dietitians of Canada: Dietary Fatty Acids. J Am Diet Assoc. 2007;107:1599–1611. [PubMed] [Google Scholar]
- American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Borland SE, Robinson SM, Crozier SR, Inskip HM SWS Study Group. Stability of dietary patterns in young women over a 2-year period. Eur J Clin Nutr. 2008;62:119–126. doi: 10.1038/sj.ejcn.1602684. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- Cupples LA, D'Agostino RB. Some risk factors related to the annual incidence of cardiovascular disease and death by using pooled repeated biennial measurements: Framingham Heart Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Study: an epidemiological investigation of cardiovascular disease. Section 34. Washington, DC: Department of Health and Human Services; 1987. Section 34. [NIH publication 87-2703 (NTIS PB87-177499).] [Google Scholar]
- Dawber T. The Framingham Study: The epidemiology of atherosclerotic disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- Drewnoski A, Fifddler EC, Dauchet L, Galan P, Hercberg S. Diet quality measures and cardiovascular risk factors in France: applying the Healthy Eating Index to the SU.VI.MAX study. J Am Coll Nutr. 2009;28:22–29. doi: 10.1080/07315724.2009.10719757. [DOI] [PubMed] [Google Scholar]
- Du H, van der A DL, van Bakel MM, Verberne LD, Ocké M, Feskens EJ. Reproducibility and relative validity of dietary glycaemic index and glycaemic load assessed by the food-frequency questionnaire used in the Dutch cohorts of the European Prospective Investigation into Cancer and Nutrition. Br J Nutr. 2009;102:601–604. doi: 10.1017/S0007114508207269. [DOI] [PubMed] [Google Scholar]
- Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educational and Psychological Measurement. 1973;33:613–619. [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Washington, DC: National Academy Press; 2005. [accessed 22 October 2010]. Dietary reference intakes: energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) Internet: http://books.nap.edu/openbook.php?isbn=0309085373. [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB, Basiotis PP. U.S. Department of Agriculture. [accessed 10 July 2011];Development and Evaluation of the Healthy Eating Index-2005: Technical Report. 2007 Internet: http://www.cnpp.usda.gov/HealthyEatingIndex.htm.
- Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation. 2008;118:1929–1937. doi: 10.1161/CIRCULATIONAHA.107.738716. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- Kant AK, Graubard BI. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J Am Coll Nutr. 2005;24:294–303. doi: 10.1080/07315724.2005.10719477. [DOI] [PubMed] [Google Scholar]
- Kimokoti RW, Newby PK, Gona P, Jasuja GK, Pencina MJ, McKeon-O'Malley C, et al. Diet quality, physical activity, smoking status, and weight fluctuation are associated with weight change in women and men. J Nutr. 2010;140:1287–1293. doi: 10.3945/jn.109.120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch GG. Intraclass correlation coefficient. In: Kotz S, Johnson NL, editors. Encyclopedia of Statistical Sciences. New York: John Wiley & Sons; 1982. pp. 213–217.pp. 213–217. [Google Scholar]
- Lomax RG. An introduction to statistical concepts. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 2007. [Google Scholar]
- McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chem Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- Meigs JB, D'Agostino RB, Sr, Wilson PWF, Cupples LA, Nathan DM, Singer DE. Risk Variable Clustering in the Insulin Resistance Syndrome: The Framingham Offspring Study. Diabetes. 1997;46:1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- Millen BE, Pencina MJ, Kimokoti RW, Meigs JB, Ordovas JM, D'Agostino RB. Nutritional risk and the metabolic syndrome in women: opportunities for preventive intervention from the Framingham Nutrition Studies. Am J Clin Nutr. 2006;84:434–441. doi: 10.1093/ajcn/84.1.434. [DOI] [PubMed] [Google Scholar]
- Millen BE, Quatromoni PA. Nutritional research within the Framingham Heart Study. J Nutr Health Aging. 2001;5:139–143. [PubMed] [Google Scholar]
- Millen BE, Quatromoni PA, Copenhafer DL, Demissie S, O'Horo CE, D'Agostino RB. Validation of a dietary pattern approach for evaluating nutritional risk: the Framingham Nutrition Studies. J Am Diet Assoc. 2001;101:187–194. doi: 10.1016/s0002-8223(01)00051-7. [DOI] [PubMed] [Google Scholar]
- Millen BE, Quatromoni PA, Franz MM, Epstein BE, Cupples A, Copenhafer DL. Population nutrient intake approaches dietary recommendations: 1991 to 1995 Framingham Nutrition Studies. J Am Diet Assoc. 1997;97:742–749. doi: 10.1016/S0002-8223(97)00184-3. [DOI] [PubMed] [Google Scholar]
- Millen BE, Quatromoni PA, Pencina MJ, Kimokoti R, Nam BH, Cobain S, et al. Unique dietary patterns and chronic disease risk profiles of adult men: The Framingham Nutrition Studies. J Am Diet Assoc. 2005;105:1723–1734. doi: 10.1016/j.jada.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2011;123:1723–1734. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Mishra GD, McNaughton SA, Bramwell GD, Wadsworth ME. Longitudinal changes in dietary patterns during adult life. Br J Nutr. 2006;96:735–744. [PubMed] [Google Scholar]
- Moeller SM, Reedy J, Millen AE, Dixon LB, Newby PK, Tucker KL, et al. Dietary patterns: challenges and opportunities in dietary patterns research. An Experimental Biology workshop, April 1, 2007. J Am Diet Assoc. 2007;107:1233–1239. doi: 10.1016/j.jada.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Murakami K, Sasaki S, Takahashi Y, Okubo H, Hirota N, Notsu A, et al. Reproducibility and relative validity of dietary glycaemic index and load assessed with a self-administered diet-history questionnaire in Japanese adults. Br J Nutr. 2008;99:639–648. doi: 10.1017/S0007114507812086. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR. A priori-defined dietary patterns and markers of cardiovascular disease risk in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88:185–194. doi: 10.1093/ajcn/88.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby PK, Hu FB, Rimm EB, Smith-Warner SA, Feskanich D, Sampson L, et al. Reproducibility and validity of the Diet Quality Index Revised as assessed by use of a food-frequency questionnaire. Am J Clin Nutr. 2003;78:941–949. doi: 10.1093/ajcn/78.5.941. [DOI] [PubMed] [Google Scholar]
- Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ. Does oestrogen allow women to store fa more efficiently? A biological advantage for fertility and gestation. Obes Rev. 2009;10:168–177. doi: 10.1111/j.1467-789X.2008.00539.x. [DOI] [PubMed] [Google Scholar]
- Posner BM, Martin-Munley SS, Smigelski C, Cupples LA, Cobb JL, Schaefer E, et al. Comparison of techniques for estimating nutrient intake: the Framingham Study. Epidemiology. 1992a;3:171–177. doi: 10.1097/00001648-199203000-00016. [DOI] [PubMed] [Google Scholar]
- Posner BM, Smigelski C, Duggal A, Morgan JL, Cobb J, Cupples LA. Validation of two-dimensional models for estimation of portion size in nutrition research. J Am Diet Assoc. 1992b;92:738–741. [PubMed] [Google Scholar]
- Prynne CJ, Paul AA, Mishra GD, Greenberg DC, Wadsworth ME. Changes in intake of key nutrients over 17 years during adult life of a British birth cohort. Br J Nutr. 2005;94:368–376. doi: 10.1079/bjn20041404. [DOI] [PubMed] [Google Scholar]
- Quatromoni PA, Copenhafer DL, Demissie S, D'Agostino RB, O'Horo CE, Nam BH, et al. The internal validity of a dietary pattern analysis. The Framingham Nutrition Studies. J Epidemiol Community Health. 2002;56:381–388. doi: 10.1136/jech.56.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- Statistical Applications Software. SAS Institute, Inc.; Cary, NC: 2008. Version 9.1. [Google Scholar]
- Stoudt H, Damon A, McFarland R. Skinfolds, body girths, biacromial diameter and selected anthropometric indices of adults. United States, 1960-1962. Vital Health Stat 11. 1970;35:1–69. [PubMed] [Google Scholar]
- Thomas JE, Schirger A, Fealey RD, Sheps SG. Orthostatic Hypotension. Mayo Clin Proc. 1981;56:117–125. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans. Washington DC: U.S. Government Printing Office; 2005. [accessed 22 October 2010]. Internet: http://www.health.gov/dietaryguidelines/dga2005/document/default.htm. [Google Scholar]
- Warnick GR, Benderson J, Albers JJ. Dextran sulfate-magnesium precipitation procedure for quantification of high-density lipoprotein cholesterol. Clin Chem. 1982;28:1379–1382. [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- Wolongevicz DM, Zhu L, Pencina MJ, Kimokoti RW, Newby PK, D'Agostino RB, et al. Diet quality and obesity in women: the Framingham Nutrition Studies. Br J Nutr. 2009;24:1–7. doi: 10.1017/S0007114509992893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.