Abstract
Objective
Despite widespread highly active antiretroviral therapy use, HIV disease remains associated with increased risk of kidney disease. Whether tenofovir use is associated with higher risk of kidney disease is controversial.
Design
We evaluated the association of cumulative and ever exposure to tenofovir on kidney outcomes in 10,841 HIV-infected patients from the Veterans Health Administration who initiated antiretroviral therapy from 1997-2007.
Methods
Cox proportional hazards and marginal structural models evaluated associations between tenofovir and time to first occurrence of 1) proteinuria (two consecutive urine dipstick measurements ≥30mg/dL), 2) rapid decline in kidney function (≥3ml/min/1.73m2 annual decline), and 3) CKD (estimated glomerular filtration rate <60ml/min/1.73m2).
Results
Median follow-up ranged from 3.9 years (proteinuria) to 5.5 years (CKD), during which 3400 proteinuria, 3078 rapid decline, and 533 CKD events occurred. After multivariable adjustment, each year of exposure to tenofovir was associated with 34% increased risk of proteinuria (95%CI 25-45%, p<0.0001), 11% increased risk of rapid decline (3-18%, p=0.0033), and 33% increased risk of CKD (18-51%; p<0.0001). Pre-existing renal risk factors did not appear to worsen the effects of tenofovir. Other ARVs showed weaker or inconsistent associations with kidney disease events. Among those who discontinued tenofovir use, risk of kidney disease events did not appear to decrease during follow-up.
Conclusions
Tenofovir exposure was independently associated with increased risk for three types of kidney disease events, and did not appear to be reversible. Because subtle kidney function decline affects long-term morbidity and mortality, the balance between efficacy and probable adverse effects requires further study.
Keywords: HIV, antiretroviral therapy, kidney disease, tenofovir
Introduction
Despite the widespread use of highly active antiretroviral therapy (HAART), HIV disease remains associated with increased kidney disease risk.[1] Causes and risk factors for kidney disease in the setting of HIV infection include hypertension, diabetes, hepatitis C, and certain antiretroviral drugs (ARV).[2] Consequences of kidney disease in HIV-infected persons include increased risk of atherosclerosis and mortality.[3-4]
Tenofovir (TDF) is a first-line treatment of HIV infection that is currently used in approximately half of all antiretroviral regimens and as part of post-exposure prophylaxis (PEP). Whether tenofovir use is associated with higher risk of kidney disease is controversial.[5] Prior to FDA approval, early tenofovir studies found no or only limited nephrotoxicity; these studies, however, excluded those with pre-existing renal impairment and generally enrolled populations without other risk factors for kidney disease.[6-7] A higher risk of tenofovir-induced toxicity has been associated with older age,[8] lower CD4 count,[9] and other comorbidities.[10] A retrospective study of 1,647 ARV-naïve patients[11] found a steeper decline in estimated glomerular filtration rate (eGFR) in patients on tenofovir-containing versus tenofovir-sparing regimens. Another study of 324 ARV-naïve patients found a greater incidence of proximal tubular dysfunction and greater decline in eGFR over 24 months in tenofovir-treated patients.[12] By contrast, a randomized study of ABC/3TC versus TDF/FTC in 333 persons found no statistically significant differences in eGFR over 48 weeks.[13] Furthermore, tenofovir did not appear to be associated with worsening kidney function in the multicenter, observational FRAM study, despite widespread use at the follow-up visit.[14] A 1-year prospective study of 424 HIV-infected persons also reported no association between tenofovir use and tubular damage.[15] Possible reasons for these disparate findings include variable patient populations, limited sample sizes and lack of access to appropriate laboratory data.
Our objective was to evaluate the association of tenofovir use with kidney disease events in a national sample of 10,841 HIV-infected persons who initiated antiretroviral therapy between 1997 and 2007 within the Veterans Health Administration. We utilized an advanced statistical method, marginal structural models, to account for the possibility that time-dependent covariates may both confound and mediate the effects of antiretroviral treatment, a complexity that conventional methods of analysis cannot address.
Methods
We analyzed kidney disease outcomes in a national sample of HIV-infected US veterans. Data sources used to assemble the analytic cohort have been described in detail.[4] In brief, the Department of Veterans Affairs (VA) HIV Clinical Case Registry (CCR) actively monitors all HIV-infected persons receiving care in the VA nationally and automatically extracts demographic, clinical, laboratory, pharmacy, utilization, and death information from the VA electronic medical record to a centralized database.[16]
Patients
The target population for this analysis was treatment-naïve HIV-infected veterans (i.e., no prior exposure to any ARV) at the time they entered clinical care in the Veterans Health Administration (VHA) system, who subsequently received mono or combined ARV with regular care and laboratory monitoring. Among 59,479 HIV-infected persons treated in the VHA between 1985 and 2007, 19,715 patients initiated ARV in the modern era of combination antiretroviral therapy (after 1997). Baseline was defined as the date of starting antiretroviral therapy. We excluded patients with prevalent kidney failure (receipt of chronic dialysis treatment or kidney transplant), and those who did not have at least one HIV-1 viral load, CD4 count, outpatient visit, and assessment of kidney function, leaving 10,841 patients in the analytic cohort. Participants excluded from the analysis were similar in terms of age, race, and gender, but appeared to have a lower prevalence of some comorbid conditions, as well as somewhat higher CD4 and lower HIVRNA levels (Table, Supplemental Digital Content 1).
Outcomes
Primary study outcomes were time to first occurrence of: (1) proteinuria, (2) rapid decline in kidney function, and (3) eGFR <60 ml/min/1.73m2. Proteinuria was defined as two consecutive urine dipstick measurements ≥30mg/dL. Estimated GFR was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) formula based on age, sex, race, and serum creatinine, as this equation is used in VA clinical practice.[17] Estimated GFR levels above 120 ml/min/1.73m2 were capped at this level, as higher estimates are unlikely to be accurate or precise.[14] Rapid decline in kidney function was defined as an annual decline of 3 ml/min/1.73m2 or more for two consecutive years, a level shown to correspond to elevated mortality risk.[18] Chronic kidney disease (CKD) was defined by two consecutive measures of eGFR <60ml/min/1.73m2, where consecutive measures were required to be at least 3 months apart, and not obtained during inpatient periods. For analyses of proteinuria and CKD, we excluded those with proteinuria or CKD present at baseline (respectively).
Additional sensitivity analyses were performed for time to first occurrence of: (1) doubling of serum creatinine, (2) presence of CKD and proteinuria, (3) annual eGFR decline of >3%, (4) annual eGFR decline of >5%, (5) presence of eGFR <45, and (6) presence of eGFR <30.
Independent Variables
We ascertained drug utilization in CCR medication files based on pharmacy-fill information. Medication exposure was used to define ARV predictor variables, and identify individuals with chronic diseases based on validated algorithms. Previous work demonstrated that VA pharmacy data are comprehensive and reliable for assessing medication use.[19-23] HAART use was defined as in previous reports.[19, 24]
Demographic information (age, sex, and race) from CCR was supplemented with Medicare database information. We defined comorbid conditions as described previously.[24] Blood pressure, body mass index, CD4 T-cell counts, HIV RNA level, LDL, HDL, total cholesterol, and serum glucose were included in statistical models or used to define clinical characteristics. At any given time, the most recent previous measurement was used to define time-dependent covariates.
Statistical Analysis
Our primary objective was to estimate the effects of cumulative and ever use of tenofovir on kidney disease outcomes. We estimated tenofovir treatment effects using two Cox proportional hazards regression models: (1) adjusted for demographic characteristics only; and (2) adjusted for demographics and time-dependent prognostic covariates. Time-dependent multivariable adjusted models included exposure to tenofovir and all other antiretroviral drugs, age, sex, race, baseline comorbid conditions (diabetes, hypertension, dyslipidemia, prevalent cardiovascular disease, smoking, drug abuse, hepatitis B and C virus infection), baseline measurements (CKD or proteinuria, BMI category), and current measurements (CD4 count (log2), viral load (log10), CKD or proteinuria, lipids, diabetes, and hypertension).
In a final step, we used marginal structural models (MSM) to re-estimate effects of cumulative and ever use of tenofovir, while accounting for the fact that the decision to prescribe a particular antiretroviral drug may be modified over time as a result of changing values for a patient’s covariates.[25-27] MSMs are a useful method to minimize selection bias of treatment allocation. To reduce bias due to informative censoring, we also generated stabilized censoring weights, and calculated final weights as the product of the stabilized treatment and censoring weights.
Tests for interaction were performed using cross-product terms between tenofovir and characteristics of interest defined at baseline. Patients were censored at time of death or last day of follow-up, December 31, 2007, due to lack of availability of additional data. Cox regression model assumptions were checked by comparing plots of log (−log(survival)) versus log of survival time and the Schoenfeld test.
We tested linearity of the relationship of tenofovir exposure with kidney disease outcomes by adding quadratic terms to the models and by constructing linear splines. Because tenofovir did not receive FDA approval until 2001, we examined the interaction between “era” of use (early = before 2003, mid = 2003-04, late = 2005-07) and tenofovir use on kidney disease. Of the 4,303 participants with exposure to tenofovir by the end of the study, 17% had used tenofovir before 2003, 59% during 2003-04, and 84% in 2005-2007.
Analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC). This study was approved by the Committee on Human Research at the San Francisco VA Medical Center and the VA Public Health Strategic Health Care Group.
Results
Baseline demographic and clinical characteristics of the 10,841 HIV-infected persons included in this analysis are shown in Table 1, stratified by end-of-study tenofovir exposure status. The overall mean age was 46 years, and females comprised 2.3% of the study sample. Individuals exposed to tenofovir were more often white (46% vs. 39%, p<0.0001) compared to those without tenofovir exposure, but were similar in terms of comorbid conditions such as hypertension, diabetes, dyslipidemia, smoking and hepatitis C, and by CD4 count, viral load, blood pressure, and proteinuria. Proportion with baseline eGFR<60 was slightly higher among those unexposed to tenofovir.
Table 1. Baseline clinical characteristics of 10,841 HIV-infected persons, stratified by tenofovir exposure status at end of study*.
| No Tenofovir Exposure (n=6,538) |
Tenofovir Exposure (n=4,303) |
|
|---|---|---|
| Age, years | 47 (40, 52) | 45 (39, 52) |
| Race, % | ||
| White | 2526 (39%) | 1962 (46%) |
| Black | 3320 (51%) | 2019 (47%) |
| Other | 692 (11%) | 322 (7%) |
| Female, % | 145 (2.2%) | 106 (2.5%) |
| Comorbid Conditions, % | ||
| Hypertension | 2557 (39%) | 1629 (38%) |
| Diabetes | 516 (7.9%) | 292 (6.8%) |
| Dyslipidemia | 960 (15%) | 649 (15%) |
| Smoking | 1266 (19%) | 762 (18%) |
| Hepatitis C Virus | 1087 (17%) | 594 (14%) |
| Measurements | ||
| CD4+ Count, cells/mm3 | 226 (50, 419) | 207 (52, 394) |
| HIV Viral Load (1000 copies/mL) | 60 (15, 258) | 70 (18, 228) |
| Systolic blood pressure, mmHg | 127 (114, 140) | 126 (115, 138) |
| Diastolic blood pressure, mmHg | 77 (70, 85) | 76 (69, 84) |
| Body mass index, kg/m2 | 24 (22, 27) | 24 (22, 27) |
| Total cholesterol, mg/dL | 166 (139, 198) | 168 (142, 196) |
| Triglycerides, mg/dL | 138 (95, 210) | 138 (95, 215) |
| Low density lipoprotein, mg/dL | 99 (76, 124) | 98 (75, 122) |
| High density lipoprotein, mg/dL | 38 (30, 48) | 36 (29, 45) |
| Glucose, mg/dL | 95 (86, 107) | 94 (86, 105) |
| Albumin, g/dL | 3.9 (3.4, 4.2) | 3.9 (3.5, 4.3) |
| eGFR, mL/min/1.73m2 | 96 (82, 114) | 97 (82, 113) |
| eGFR <60mL/min/1.73m2 | 476 (7.3%) | 203 (4.7%) |
| Proteinuria | 1392 (21%) | 814 (19%) |
Continuous variables reported as median (IQR). Proteinuria defined by urinalysis protein 30 mg/dL or greater.
Abbreviations: Estimated glomerular filtration rate (eGFR);
There were 3,400 proteinuria events in 38,132 person-years (PY) of follow-up, 3,078 rapid decline events (51,589 PY), and 533 CKD events (56,416 PY) (Table, Supplemental Digital Content 2). The median period of observation (before event or censoring) per individual ranged from 3.9 years for proteinuria to 5.5 years for CKD (maximum 11.0 years). At the end of the study, the 4,303 participants with tenofovir exposure had a mean (±SD) duration of 1.3±1.1 years of use (median 1 year, IQR 0.5-1.9, maximum 6.3 years).
Association of Tenofovir Exposure with Risk of Kidney Disease Outcomes
In Cox proportional hazards models that adjusted for age, gender, and race, each year of cumulative exposure to tenofovir was associated with a 30% increase in the risk of proteinuria (p<0.0001, Table 2). After further adjustment for baseline comorbid conditions, other antiretroviral drugs, and current measurements of HIV-related and other factors, there was little change in the association of tenofovir with proteinuria (34% increase per year, p<0.0001). Tenofovir use was also associated with an 11% increased risk of rapid decline per year of exposure (p=0.0033), and a 33% increased risk of CKD per year of exposure (p<0.0001) in fully adjusted time-dependent Cox models. Results were similar in marginal structural models designed to correct for drug channeling bias. Ever exposure to tenofovir was also strongly associated with ~50% higher risk of proteinuria, rapid decline, and CKD during follow-up (all p<0.0001). When we modified the analysis to begin follow-up when participants initiated combination ART, results were nearly unchanged. We found similar results for the association of tenofovir with CKD after adjustment for baseline eGFR (HR=1.26 per year, 95%CI 1.10-1.44).
Table 2. Association of tenofovir exposure with risk* of kidney disease outcomes.
| Demographic-Adjusted Model† |
Time-Dependent Cox Model‡ |
Marginal Structural Model§ |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P Value | Hazard Ratio (95% CI) |
P Value | Hazard Ratio (95% CI) |
P Value | |
| Cumulative Exposure to Tenofovir (per year) | ||||||
| Proteinuria (n=3400 events) | 1.30 (1.22-1.37) | <0.0001 | 1.34 (1.25-1.45) | <0.0001 | 1.24 (1.17-1.32) | <0.0001 |
| Rapid decline** (n = 3078 events) | 1.17 (1.11-1.24) | <0.0001 | 1.11 (1.03-1.18) | 0.0033 | 1.16 (1.09-1.23) | <0.0001 |
| CKD (n=553 events) | 1.44 (1.30-1.60) | <0.0001 | 1.33 (1.18-1.51) | <0.0001 | 1.36 (1.22-1.51) | <0.0001 |
| Ever Exposure to Tenofovir | ||||||
| Proteinuria (n=3400 events) | 1.70 (1.57-1.85) | <0.0001 | 1.68 (1.52-1.85) | <0.0001 | 1.51 (1.36-1.66) | <0.0001 |
| Rapid decline (n = 3078 events) | 1.51 (1.39-1.64) | <0.0001 | 1.36 (1.23-1.50) | <0.0001 | 1.50 (1.36-1.67) | <0.0001 |
| CKD (n=553 events) | 2.11 (1.76-2.54) | <0.0001 | 1.71 (1.38-2.12) | <0.0001 | 1.88 (1.50-2.36) | <0.0001 |
Each analysis excludes patients who had the condition at baseline. Abbreviations: Confidence Interval (CI); Chronic kidney disease (CKD).
Demographic adjusted Cox model includes drug exposure, age, sex, race, and time.
Time-dependent multivariable adjusted model includes exposure to tenofovir and all other antiretroviral drugs, age, sex, race, baseline comorbid conditions (diabetes, hypertension, dyslipidemia, prevalent cardiovascular disease, smoking, drug abuse, hepatitis B and C virus infection), baseline measurements (CKD or proteinuria, BMI category), and current measurements (CD4 count, viral load, CKD or proteinuria, lipids, diabetes, and hypertension). Rapid decline model controls for both baseline CKD and proteinuria. Proteinuria model controls for baseline CKD, and CKD model controls for baseline proteinuria.
Marginal structural model includes all baseline variables in multivariable model.
Rapid decline in kidney function was defined as an annual decline of 3 ml/min/1.73m2 or more for two consecutive years
A greater percentage of tenofovir users had repeated measures of proteinuria (66% vs. 62%, p<0.0001) and creatinine (96% vs. 94%, p<0.0001) compared with non-users. Controlling for number of assessments did not weaken the association of tenofovir with proteinuria (HR=1.40 per year of exposure, 95%CI: 1.30-1.50), rapid decline (HR=1.11, 95%CI: 1.04-1.19), or CKD (HR=1.36, 95%CI: 1.20-1.55). There was little evidence of interaction of TDF with concomitant PI, NNRTI, and ritonavir use on the three outcomes (all p-values for interaction >0.30).
We also analyzed associations of tenofovir with more stringent measures of kidney disease (Table, Supplemental Digital Content 3). Cumulative exposure to tenofovir was associated with a 10% increased risk of creatinine doubling (432 events) (95%CI: 0.92-1.32, p=0.28), and a 35% (95%CI: 12-62%) increased risk of combined CKD and proteinuria (237 events) (p=0.0014). Findings were similar when rapid kidney decline was defined by >3% or >5% decline in eGFR (Table, Supplemental Digital Content 3). We also considered lower threshholds of risk for CKD (cutpoints of 45 and 30 ml/min). Cumulative tenofovir exposure was associated with a marginally increased risk of eGFR <45 (HR=1.18, 95%CI: 0.97-1.45, p=0.10) but did not appear to be associated with increased risk of eGFR <30 (HR=0.91, 95%CI: 0.62-1.33, p=0.61). However, the incidence was low for both eGFR <45 (237 events) and eGFR <30 (124 events).
Among those who discontinued tenofovir use, the time-period following cessation was not significantly associated with either higher or lower risks of proteinuria (HR=1.05 per year, 95%CI: 0.93-1.18, p=0.41) or rapid decline (HR=1.05 per year, 95%CI: 0.94-1.16, p=0.42), although there was a marginal association of time off tenofovir with CKD (HR=1.22 per year, 95%CI: 0.99-1.50, p=0.055). All hazard ratios remained greater than unity, which suggests that the effects of tenofovir on kidney disease risk were not reversible following discontinuation. When we instead discretized tenofovir use as never, current, or past, we found that past and current use of TDF had increased risk of outcomes, compared to those never exposed (Table, Supplemental Digital Content 4). We also considered the 3,400 participants who experienced a proteinuria event, and looked at subsequent time to resolution of proteinuria. This analysis found that current and past TDF users did not differ statistically from never users in likelihood of resolution of proteinuria (all p>0.2).
We found evidence of non-linear associations of cumulative tenofovir exposure with risk of proteinuria (p=0.0030), rapid decline (p<0.0001), and CKD (p=0.036) (Table 3). Risk of proteinuria appeared strengthened among those with more than 3 years of exposure to tenofovir, while incidence of rapid decline appeared to decrease over time, especially after three years of exposure. Each category of exposure to tenofovir was associated with increased risk of CKD, although associations were not statistically significant for those with <0.5 or more than 3 years of exposure.
Table 3. Association of cumulative tenofovir (in different time ranges) with risk* of kidney disease outcomes.
| Outcome Category of exposure† |
Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Proteinuria | ||
| Tenofovir <0.5 years | 1.72 (1.50-1.96) | <0.0001 |
| Tenofovir 0.5-1 years | 1.59 (1.36-1.86) | <0.0001 |
| Tenofovir 1-3 years | 1.68 (1.44-1.95) | <0.0001 |
| Tenofovir >3 years | 2.17 (1.48-3.20) | 0.0001 |
| Rapid Decline ** | ||
| Tenofovir <0.5 years | 1.35 (1.16-1.56) | 0.0001 |
| Tenofovir 0.5-1 years | 1.59 (1.38-1.84) | <0.0001 |
| Tenofovir 1-3 years | 1.23 (1.07-1.42) | 0.0042 |
| Tenofovir >3 years | 1.04 (0.66-1.63) | 0.88 |
| CKD | ||
| Tenofovir <0.5 years | 1.30 (0.91, 1.86) | 0.15 |
| Tenofovir 0.5-1 years | 1.85 (1.35, 2.53) | 0.0001 |
| Tenofovir 1-3 years | 1.69 (1.26, 2.27) | 0.0005 |
| Tenofovir >3 years | 1.56 (0.73, 3.36) | 0.25 |
All estimates based on multivariable adjusted time-dependent Cox models described in Table 2.
Reference is versus 0 years.
Rapid decline in kidney function was defined as an annual decline of 3 ml/min/1.73m2 or more for two consecutive years
The association of cumulative TDF use with proteinuria appeared to be stronger in the earlier era (HR=2.2, p=0.0051) than in the mid (HR=1.5, p<0.0001) and later eras (HR=1.2, p<0.0001; test for difference: p=0.014) (Table, Supplemental Digital Content 5). Cumulative tenofovir exposure was more strongly associated with rapid decline in the earlier era (HR=1.7, p=0.071) than in the mid era (HR=1.3, p<.0001), with little association in the later era (HR=1.04, p=0.20; test for difference: p=0.0018). Likewise, cumulative tenofovir exposure was more strongly associated with CKD in the earlier era (HR=3.3, p=0.018) compared with mid (HR=1.6, p=0.0002) and later eras (HR=1.4, p<0.0001), although the test for difference did not reach statistical significance (p=0.12). As a sensitivity analysis, we excluded data collected before 2001, but found little change in the association of tenofovir exposure with kidney outcomes. We also controlled for whether participants were ARV-naïve at the time of tenofovir initiation; this analysis found little change in the association of tenofovir exposure with kidney outcomes.
Interactions between Tenofovir and Baseline Comorbidity
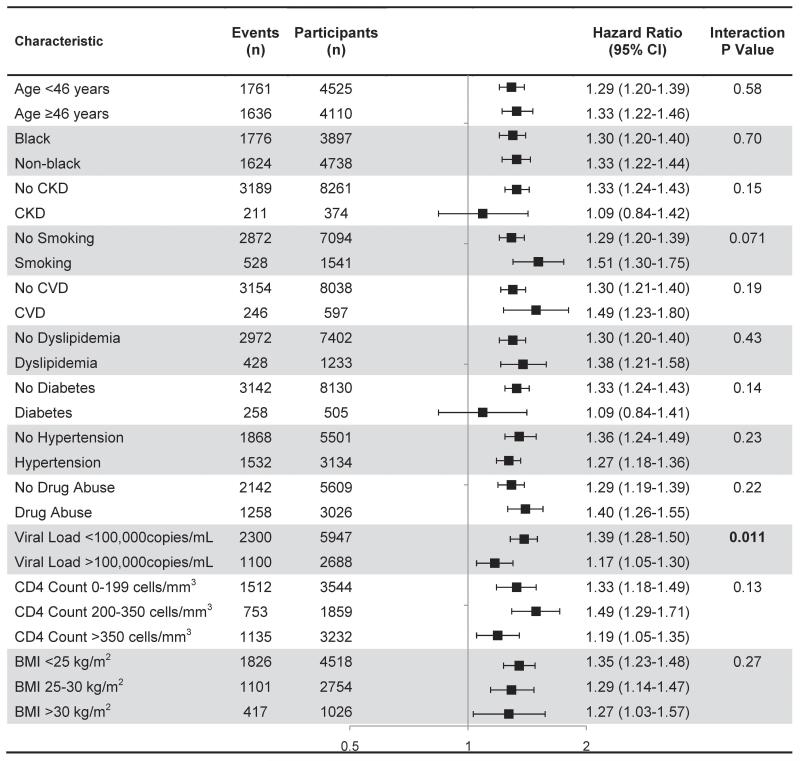
We examined interactions of tenofovir and subgroups (age, race, baseline CKD, smoking, and others) on proteinuria (Figure 1). Tenofovir use was associated with increased risk of proteinuria in all subgroups, but appeared somewhat stronger in persons with lower compared with higher HIV-1 RNA levels (p-value for interaction =0.011). There was no evidence that pre-existing CKD, diabetes or hypertension increased the risk of proteinuria, relative to those without pre-existing comorbidities.
Figure 1. Association between cumulative tenofovir exposure and risk of proteinuria in subgroups defined by baseline characteristics (excluding those with proteinuria at baseline)*.
*CV risk category based on Framingham risk score (<10% = low, 10-20% = moderate, >20% = high). All estimates based on multivariable adjusted Cox models described in Table 2. P value for test of interaction between cumulative tenofovir use and characteristic reported. Abbreviations: CI (confidence interval), eGFR (estimated glomerular filtration rate), CVD (cardiovascular disease), BMI (body mass index).
Tenofovir use was associated with increased risk of CKD in nearly all subgroups (Figure, Supplemental Digital Content 6). The association of tenofovir use with CKD was weaker in older versus younger subjects (p=0.043), in those with vs. without CVD (p=0.016), in diabetics vs. non-diabetics (p=0.041), and in those with hypertension versus those without hypertension (p=0.018).
Associations of Other Antiretroviral Drugs with Kidney Disease
We evaluated associations of all ARVs in use in this study, summarized by descending prevalence of use (Table 4). Tenofovir was the only ARV that showed statistically significant associations with all three outcomes. Ritonavir was associated with increased proteinuria risk in fully adjusted analysis, whereas efavirenz, lopinavir/ritonavir, and saquinavir appeared to be associated with lower proteinuria risk. Atazanavir was associated with increased risk of rapid decline, but was not associated with proteinuria or CKD risk. Indinavir was associated with increased risk of CKD, while efavirenz and zidovudine were associated with decreased risk.
Table 4. Association of cumulative antiretroviral exposure (per year) with risk* of kidney disease outcomes, ordered by prevalence of use.
| Proteinuria | Rapid Decline** | Chronic Kidney Disease | |||||
|---|---|---|---|---|---|---|---|
| Antiretroviral | % of participants with any exposure at end of study |
Hazard Ratio (95% CI) |
P Value | Hazard Ratio (95% CI) |
P Value | Hazard Ratio (95% CI) |
P Value |
| Tenofovir | 39.7 | 1.34 (1.25, 1.45) | <0.0001 | 1.11 (1.03, 1.18) | 0.0033 | 1.33 (1.18, 1.51) | <0.0001 |
| Lamivudine | 89.5 | 0.98 (0.94, 1.03) | 0.50 | 1.02 (0.97, 1.06) | 0.44 | 0.93 (0.85, 1.02) | 0.11 |
| Zidovudine | 68.3 | 0.98 (0.93, 1.03) | 0.42 | 0.98 (0.93, 1.02) | 0.29 | 0.89 (0.81, 0.98) | 0.020 |
| Efavirenz | 49.0 | 0.94 (0.90, 0.99) | 0.026 | 1.01 (0.97, 1.05) | 0.64 | 0.88 (0.79, 0.98) | 0.018 |
| Stavudine | 43.0 | 1.02 (0.97, 1.07) | 0.54 | 1.02 (0.97, 1.06) | 0.43 | 0.98 (0.89, 1.07) | 0.61 |
| Ritonavir† | 35.7 | 1.18 (1.09, 1.27) | <0.0001 | 0.96 (0.89, 1.04) | 0.34 | 0.97 (0.84, 1.14) | 0.74 |
| Nelfinavir | 31.6 | 0.99 (0.95, 1.04) | 0.68 | 1.02 (0.98, 1.06) | 0.39 | 1.01 (0.92, 1.11) | 0.76 |
| Abacavir | 29.6 | 1.01 (0.96, 1.07) | 0.73 | 1.01 (0.96, 1.06) | 0.65 | 1.07 (0.97, 1.18) | 0.20 |
| Indinavir | 24.6 | 1.04 (0.99, 1.09) | 0.15 | 0.99 (0.95, 1.04) | 0.67 | 1.16 (1.06, 1.27) | 0.0019 |
| Didanosine | 23.0 | 0.94 (0.88, 1.00) | 0.051 | 0.98 (0.93, 1.04) | 0.49 | 0.95 (0.84, 1.07) | 0.37 |
| Nevirapine | 22.8 | 1.01 (0.96, 1.06) | 0.69 | 1.02 (0.97, 1.06) | 0.52 | 0.93 (0.84, 1.03) | 0.18 |
| Atazanavir | 17.1 | 0.93 (0.79, 1.08) | 0.34 | 1.22 (1.07, 1.40) | 0.0035 | 0.96 (0.77, 1.18) | 0.69 |
| Lopinavir/r | 15.3 | 0.77 (0.68, 0.86) | <0.0001 | 1.05 (0.94, 1.17) | 0.39 | 1.21 (0.91, 1.60) | 0.18 |
| Saquinavir | 10.7 | 0.91 (0.83, 0.99) | 0.035 | 1.00 (0.92, 1.08) | 0.97 | 0.89 (0.72, 1.09) | 0.24 |
| Amprenavir | 4.3 | 0.90 (0.78, 1.05) | 0.20 | 1.03 (0.90, 1.18) | 0.67 | 1.17 (0.94, 1.46) | 0.16 |
| Fosamprenavir | 3.3 | 0.91 (0.63, 1.32) | 0.63 | 1.29 (0.90, 1.85) | 0.16 | 1.00 (0.67, 1.47) | 0.98 |
| Zalcitabine | 1.5 | 1.11 (0.92, 1.35) | 0.29 | 0.91 (0.72, 1.14) | 0.41 | 1.24 (0.70, 2.19) | 0.46 |
| Delavirdine | 1.5 | 1.10 (0.90, 1.35) | 0.35 | 0.85 (0.66, 1.10) | 0.21 | 1.24 (0.84, 1.81) | 0.28 |
| Tipranavir | 0.6 | 0.87 (0.29, 2.68) | 0.81 | 0.34 (0.05, 2.34) | 0.27 | 0.06 (0.00, 66.0) | 0.43 |
Time-dependent multivariable adjusted model includes exposure to tenofovir and all other antiretroviral drugs, age, sex, race, baseline comorbid conditions (diabetes, hypertension, dyslipidemia, prevalent cardiovascular disease, smoking, drug abuse, hepatitis B and C virus infection), baseline measurements (CKD or proteinuria, BMI category), and current measurements (CD4 count, viral load, CKD or proteinuria, lipids, diabetes, and hypertension). Rapid decline model controls for both baseline CKD and proteinuria. Proteinuria model controls for baseline CKD, and CKD model controls for baseline proteinuria.
Refers to both boosted and unboosted ritonavir use.
Rapid decline in kidney function was defined as an annual decline of 3 ml/min/1.73m2 or more for two consecutive years
Discussion
In this large, national sample of predominantly male HIV-infected veterans receiving combination antiretroviral therapy, we found that exposure to tenofovir was associated with increased risk for proteinuria, rapid decline (a ≥3 unit annual decrease) in kidney function, and development of CKD. Even after accounting for demographics, HIV-related factors, comorbidities, and other antiretroviral drugs, tenofovir remained independently associated with elevated risk for each kidney disease outcome. These associations were in general similar across subgroups based on baseline comorbidities and characteristics, and few statistically significant interactions were observed. Presence of traditional CKD risk factors at baseline such as pre-existing CKD, diabetes, and hypertension did not appear to worsen the effects of tenofovir. Together, these findings provide strong evidence that tenofovir may cause clinically significant toxicity to the kidney that is not reversible.
It is noteworthy that tenofovir was associated with both proteinuria and CKD in our study. These outcomes are not highly inter-correlated, and each is independently associated with cardiovascular disease and death in the setting of HIV infection.[3-4] The primary mechanism by which tenofovir causes renal toxicity may involve drug accumulation within proximal renal tubules, leading to mitochondrial injury and depletion.[28] Individuals with certain variants of the ABCC2 gene, the multidrug resistance protein which facilitates tenofovir efflux from proximal tubular cells, may be more prone to tenofovir toxicity.[29] Furthermore, inhibition of tenofovir entry into proximal tubular cells via the organic anion transporter by probenecid prevents recurrent tenofovir renal toxicity.[30] Consistent with this proposed mechanism of drug accumulation in the renal proximal tubule, most case reports describe tenofovir renal toxicity presenting as partial or full Fanconi syndrome characterized by sub-nephrotic proteinuria with or without hyperphosphaturia and normoglycemic glycosuria.[31-33] However, tenofovir renal injury may also present as acute tubular necrosis,[34] eventually leading to tubulointerstitial scarring, which may account for the lack of reversibility of tenofovir renal toxicity in some individuals.[5]
Few previous large, nationally representative studies in HIV-infected patients have looked at associations of tenofovir with kidney disease outcomes. Tenofovir was the only antiretroviral drug that showed statistically significant associations with all three kidney disease outcomes in our study. A study of 10,343 patients designed to evaluate the safety of tenofovir over the first four years of use reported that less than 1% of patients experienced a serious renal adverse event;[35] however, all subjects were taking tenofovir, so its effects on renal function could not be compared with other drugs.
A recent longitudinal study of 6,843 HIV-infected persons found that tenofovir, indinavir, and atazanavir were associated with a higher incidence of CKD, even after controlling for traditional CKD risk factors and other antiretroviral drugs.[36] In our study, atazanavir was associated with increased risk of rapid decline, but not with CKD.
By contrast, efavirenz was associated with a lower risk of both proteinuria and CKD. Similarly, a recent prospective study of 62 HIV-infected patients found lower rates of proteinuria and higher levels of eGFR among those who were treated with tenofovir/lamivudine/efavirenz compared to those treated with tenofovir/lamivudine/nevirapine.[37] Mechanisms accounting for this potential beneficial effect of efavirenz are unknown.
Among those who discontinued tenofovir use in our study, time following cessation was not significantly associated with either higher or lower risks of proteinuria, or rapid decline, and appeared to be weakly associated with increased CKD risk. Past users of tenofovir remained at increased risk of outcomes, compared to those never exposed to tenofovir. Proteinuria appeared to be similarly persistent among users, current and former, as non-users, suggesting that TDF-induced proteinuria is not uniquely transient. A small study of HIV-infected men found little recovery on average in eGFR following tenofovir cessation.[10] Similarly, both current and past tenofovir use were associated with increased risk of proximal renal tubular dysfunction in a cross-sectional study of 399 HIV-infected persons.[38] These findings suggest that kidney damage and loss of function do not quickly reverse after cessation of tenofovir use.
A major strength of our study is the large number of participants, which gave us power to detect relatively small hazard ratios for the risk of renal outcomes per year of tenofovir exposure. Previous studies may have been less powered to detect statistically significant associations between tenofovir use and kidney disease. Assuming 5 years of follow-up and a type I error rate of 5% with equal allocation to treatment arms (tenofovir versus no tenofovir), a study would need to enroll 3,544 participants to achieve 80% power to detect a hazard ratio of 1.3 or greater (the TDF effect observed in our study for CKD and proteinuria).
Study limitations include our inability to measure GFR directly, similar to all large studies of kidney disease. There may have been incomplete or inadequate control for factors that may confound or explain the association between tenofovir and kidney disease. However, we utilized marginal structural models to account for the possibility that the decision to prescribe a particular antiretroviral drug may change over time due to changes in a patient’s covariates. Mean exposure to tenofovir in our study was 1.3 years, and among those who discontinued tenofovir, mean follow-up time was 1.2 years; this limits our ability to extrapolate risk of longer exposure. Antiretroviral drugs other than tenofovir showed inconsistent associations with kidney disease risk; the few results reaching statistical significance may be due to chance despite meeting the conventional cutoff for statistical significance. Prospective studies should be undertaken to validate our findings. Additionally, our results may not generalize to non-veterans, women, or patients not receiving regular clinical care. However, our population includes those who are often excluded from clinical trials and do not qualify or volunteer for cohort studies. Finally, our analyses excluded patients with inadequate data collection, and these persons on average were healthier than patients included in our study; we cannot discern whether this would have a bias on our findings.
In conclusion, this large, national sample of 10,841 HIV-infected persons indicates that tenofovir is associated with increased risk of proteinuria, rapid decline, and CKD. Clinicians treating HIV-infected patients should recognize that while traditional risk factors such as hypertension, older age, and diabetes may increase the risk for kidney disease, tenofovir is associated with elevated risk even in patients without pre-existing kidney risk factors. Despite tenofovir’s association with progressive kidney disease, it is an important component of effective antiretroviral therapy that may be required in many patients to control viral load. The balance between its efficacy and probable adverse effects requires further study.
Supplementary Material
Acknowledgements
The authors would like to thank Eric Vittinghoff, PhD for statistical assistance and Cristin Weekley, BA for assistance with figures and administrative help. RS, MGS, and YL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Funding Sources and Disclosures
This study was supported by the National Institutes of Health (K23DK080645-01A1 (AIC), 1R03AG034871-01 (AIC/MGS), K24AI069994, R01 DK066488-01 (MGS/AIC)), the National Center for Research Resources (KL2 RR024130), the American Heart Association Established Investigator Award (MGS), and the VA Public Health Strategic Health Care Group, which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. These funding sources had no involvement in the design or execution of this study. SGD receives research support from Merck, Bristol-Myers Squibb, Gilead, and Roche Molecular Sciences. CG has received prior research funding and/or honorarium from Merck, Bristol-Myers Squibb, Abbott, Gilead Sciences, EMD Serono and Theratechnologies.
Footnotes
Conflicts of Interest
None declared.
Supplemental Digital Content
- Supplemental Digital Content 1. Table that lists the baseline clinical characteristics of HIV-infected persons, stratified by inclusion/exclusion status. doc
- Supplemental Digital Content 2. Table of the summary of events and person-years by exposure to tenofovir. doc
- Supplemental Digital Content 3. Table that shows the association of tenofovir exposure with risk of alternative kidney disease outcomes. doc
- Supplemental Digital Content 4. Table that shows the association of current and past tenofovir use with risk of kidney disease outcomes. doc
- Supplemental Digital Content 5. Table that shows the association of era of antiretroviral use and cumulative tenofovir with risk of kidney disease outcomes. doc
- Supplemental Digital Content 6. Figure that illustrates the association between cumulative tenofovir exposure and risk of CKD in subgroups defined by baseline characteristics (excluding those with CKD at baseline). doc
References
- 1.Phair J, Palella F. Renal disease in HIV-infected individuals. Curr Opin HIV AIDS. 2011 doi: 10.1097/COH.0b013e3283476bc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci. 2008;335:89–94. doi: 10.1097/MAJ.0b013e31812e6b34. [DOI] [PubMed] [Google Scholar]
- 3.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-Associated Kidney Toxicity in HIV-Infected Patients: A Review of the Evidence. Am J Kidney Dis. 2011;57:773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Squires K, Pozniak AL, Pierone G, Jr., Steinhart CR, Berger D, Bellos NC, et al. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann Intern Med. 2003;139:313–320. doi: 10.7326/0003-4819-139-5_part_1-200309020-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. Jama. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 8.Campbell LJ, Ibrahim F, Fisher M, Holt SG, Hendry BM, Post FA. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40:1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 10.Wever K, van Agtmael MA, Carr A. Incomplete reversibility of tenofovir-related renal toxicity in HIV-infected men. J Acquir Immune Defic Syndr. 2010;55:78–81. doi: 10.1097/QAI.0b013e3181d05579. [DOI] [PubMed] [Google Scholar]
- 11.Horberg M, Tang B, Towner W, Silverberg M, Bersoff-Matcha S, Hurley L, et al. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2010;53:62–69. doi: 10.1097/QAI.0b013e3181be6be2. [DOI] [PubMed] [Google Scholar]
- 12.Calza L, Trapani F, Tedeschi S, Piergentili B, Manfredi R, Colangeli V, et al. Tenofovir-induced renal toxicity in 324 HIV-infected, antiretroviral-naive patients. Scand J Infect Dis. 2011 doi: 10.3109/00365548.2011.572906. [DOI] [PubMed] [Google Scholar]
- 13.Martinez E, Arranz JA, Podzamczer D, Lonca M, Sanz J, Barragan P, et al. A simplification trial switching from nucleoside reverse transcriptase inhibitors to once-daily fixed-dose abacavir/lamivudine or tenofovir/emtricitabine in HIV-1-infected patients with virological suppression. J Acquir Immune Defic Syndr. 2009;51:290–297. doi: 10.1097/QAI.0b013e3181aa12d5. [DOI] [PubMed] [Google Scholar]
- 14.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG. HIV viremia and changes in kidney function. Aids. 2009;23:1089–1096. doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando M, Yanagisawa N, Ajisawa A, Tsuchiya K, Nitta K. Kidney tubular damage in the absence of glomerular defects in HIV-infected patients on highly active antiretroviral therapy. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr020. [DOI] [PubMed] [Google Scholar]
- 16.Backus LI, Gavrilov S, Loomis TP, Halloran JP, Phillips BR, Belperio PS, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] Journal of the American Society of Nephrology. 2000;11:155A. [Google Scholar]
- 18.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi AI, Rodriguez RA, Bacchetti P, Volberding PA, Havlir D, Bertenthal D, et al. Low rates of antiretroviral therapy among HIV-infected patients with chronic kidney disease. Clin Infect Dis. 2007;45:1633–1639. doi: 10.1086/523729. [DOI] [PubMed] [Google Scholar]
- 20.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44:S13–24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Office USGA. GAO Report. Washington, D.C.: 1994. Veterans health care: Use of VA services by Medicare-eligible veterans. [Google Scholar]
- 22.Shen Y, Hendricks A, Zhang S, Kazis LE. VHA enrollees’ health care coverage and use of care. Med Care Res Rev. 2003;60:253–267. doi: 10.1177/1077558703060002007. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JH, Justice AC, Chesney M, Sinclair G, Weissman S, Rodriguez-Barradas M. Patient- and provider-reported adherence: toward a clinically useful approach to measuring antiretroviral adherence. J Clin Epidemiol. 2001;54(Suppl 1):S91–98. doi: 10.1016/s0895-4356(01)00450-4. [DOI] [PubMed] [Google Scholar]
- 24.Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011 Apr 21; doi: 10.1097/QAD.0b013e328347fa16. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Petersen ML, Wang Y, van der Laan MJ, Bangsberg DR. Assessing the effectiveness of antiretroviral adherence interventions. Using marginal structural models to replicate the findings of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S96–S103. doi: 10.1097/01.qai.0000248344.95135.8d. [DOI] [PubMed] [Google Scholar]
- 27.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45:908–915. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89:513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Novoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48:e108–116. doi: 10.1086/598507. [DOI] [PubMed] [Google Scholar]
- 30.Izzedine H, Thibault V, Valantin MA, Peytavin G, Schneider L, Benhamou Y. Tenofovir/probenecid combination in HIV/HBV-coinfected patients: how to escape Fanconi syndrome recurrence? Aids. 2010;24:1078–1079. doi: 10.1097/QAD.0b013e3283313f54. [DOI] [PubMed] [Google Scholar]
- 31.Peyriere H, Reynes J, Rouanet I, Daniel N, de Boever CM, Mauboussin JM, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr. 2004;35:269–273. doi: 10.1097/00126334-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 32.Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36:1070–1073. doi: 10.1086/368314. [DOI] [PubMed] [Google Scholar]
- 33.Irizarry-Alvarado JM, Dwyer JP, Brumble LM, Alvarez S, Mendez JC. Proximal tubular dysfunction associated with tenofovir and didanosine causing Fanconi syndrome and diabetes insipidus: a report of 3 cases. AIDS Read. 2009;19:114–121. [PubMed] [Google Scholar]
- 34.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 35.Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. Aids. 2007;21:1273–1281. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 36.Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. Aids. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 37.Manosuthi W, Mankatitham W, Lueangniyomkul A, Prasithsirikul W, Tantanathip P, Suntisuklappon B, et al. Renal impairment after switching from stavudine/lamivudine to tenofovir/lamivudine in NNRTI-based antiretroviral regimens. AIDS Res Ther. 2010;7:37. doi: 10.1186/1742-6405-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dauchy FA, Lawson-Ayayi S, de La Faille R, Bonnet F, Rigothier C, Mehsen N, et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney Int. 2011 doi: 10.1038/ki.2011.124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.