Abstract
Malignant gliomas are diffusively infiltrative and remain among the deadliest of all cancers. NF-κB is a transcription factor that mediates cell growth, migration and invasion, angiogenesis and resistance to apoptosis. Normally, the activity of NF-κB is tightly regulated by numerous mechanisms. However, in many cancers, NF-κB is constitutively activated and may function as a tumor promoter. Herein, we show that in gliomas, NF-κB is constitutively activated and the levels of cIAP2, Bcl-2, Bcl-xL and Survivin are elevated. These genes are regulated by NF-κB and can inhibit apoptosis. To understand the potential role of NF-κB p65 in suppressing apoptosis, we generated human glioma cell lines that inducibly express shRNA molecules specific for p65. We demonstrate that in the absence of p65, TNF-α induced cIAP2 expression is significantly reduced while the levels of Bcl-2, Bcl-xL and Survivin are not affected. These data suggest that of these genes, only cIAP2 is a direct target of p65. Using RT-PCR and chromatin immunoprecipitation assays, we have confirmed that cIAP2 is a transcriptional target of NF-κB p65. As a consequence of reduced p65 and cIAP2 levels, we demonstrate that the levels of RIP poly-ubiquitination are reduced, and that p65-deficient glioma cells are more sensitive to the cytotoxic effects of TNF-α than glioma cells expressing p65. Specifically, in the presence of TNF-α, glioma cells lacking p65 showed cellular proliferation defects and underwent apoptosis. Moreover, glioma cells were similarly sensitized to the effects of TNF-α if the levels of cIAP2 were reduced through lentivirus shRNA expression. These data suggest that NF-κB and/or cIAP2 may be therapeutically relevant targets for the treatment of malignant gliomas.
Keywords: NF-κB, cIAP2, TNF-α, Gliomas, Apoptosis
INTRODUCTION
The NF-κB proteins are a family of five transcription factors; RelA/p65, RelB, c-Rel, p105/p50 and p100/p52 [1]. Each member of this family contains a Rel homology domain, which allows them to homo- and heterodimerize in nearly every permutation possible. Additionally, the C-terminus of p65, RelB and c-Rel contains a transactivation domain (TAD), which allows NF-κB dimers that contain p65, RelB or c-Rel to positively regulate transcription. In the central nervous system (CNS) and other cell types, the most common NF-κB dimer consists of p65 and p50, and this form is specifically referred to as NF-κB [2–4]. Normally, NF-κB is inactive and maintained in the cytosol through interactions with Inhibitor of NF-κB alpha (IκBα), which binds to NF-κB and masks its DNA binding domain and nuclear localization signal. NF-κB is activated in response to numerous stimuli, which activate the IκB kinase (IKK) complex. IKK phosphorylates IκBα, which targets it for proteasomal mediated degradation. Once released, the NF-κB dimer translocates to the nucleus, where it binds to specific DNA sequences, known as κB elements, to induce the expression of numerous genes. To date, hundreds of NF-κB target genes have been identified, which mediate various processes including cell growth, angiogenesis, cell migration and invasion, and resistance to apoptosis [5].
Malignant gliomas are aggressive, neurologically destructive and deadly tumors of the CNS [6, 7]. When possible, gliomas are debulked through surgical resection [8, 9]. However, because of their diffusive nature and localization, this treatment is limited and the tumor often recurs at the original or distant sites [6–8]. Attempts to improve patient survival have utilized radiotherapy and/or chemotherapy [9, 10]. However, gliomas remain notoriously resistant to chemo- and immunotherapies, and the current goal of adjuvant therapy is to prolong the patient’s survival in a clinically good condition, since eradication of the disease remains highly unlikely.
In gliomas, NF-κB is constitutively activated [11–13] and is associated with apoptotic resistance and poor disease prognosis [14–16]. Previous studies with glioma cells have shown that the use of either a dominant negative form of NF-κB or a non-degradable form of IκBα increased apoptosis in response to TNF-α and chemotherapeutic agents [17–19]. Additionally, non-specific inhibitors of NF-κB activation such as sulfasalazine and curcumin have also been shown to inhibit glioma cell growth and increase sensitivity to apoptosis [20, 21]. The ability of NF-κB to mediate resistance to apoptosis may depend on its ability to induce one or more target genes. In fibrosarcoma cells, NF-κB induced expression of TNF-α receptor-associated factor 1 (TRAF1), TRAF2, cellular Inhibitor of Apoptosis 1 (cIAP1) and cIAP2 were requisite to mediate resistance to TNF-α induced apoptosis [22]. In T cells, TNF-α was shown to induce cIAP2 expression, and this was sufficient to inhibit TNF-α induced cell death [23]. However, in gliomas, the specific role of one NF-κB family member, p65, and its role in mediating apoptotic resistance, remains unclear.
Members of the cIAP family, which include cIAP1, cIAP2, Survivin, X-linked Inhibitor of Apoptosis (XIAP) and BIR-containing ubiquitin-conjugating enzyme (BRUCE), are structurally similar proteins that minimally contain at least one Baculoviral IAP Repeat (BIR) domain [24]. Most cIAP proteins also contain a C-terminal RING domain that enables them to function as E3 ubiquitin ligases [24, 25], while cIAP1 and 2 also contain a caspase recruitment domain (CARD), whose function is not yet clear [24]. The cIAP proteins are potent inhibitors of apoptosis, although the mechanism(s) remain poorly understood. Interestingly, cIAP1, cIAP2 and XIAP have all been shown to bind to caspases, yet only XIAP is capable of directly inhibiting caspase activity [26–28]. Instead, it is postulated that cIAP proteins may inhibit apoptosis largely through their intrinsic ubiquitin ligase activity [24]. Thus far, cIAP1 can auto-ubiquitinate itself and trans-ubiquitinate both XIAP and cIAP2 in order to regulate the overall levels of cIAP proteins. Additionally, both cIAP1 and cIAP2 can trans-ubiquitinate receptor interacting protein 1 (RIP1) [29–31]. RIP1 is a serine/threonine kinase that interacts with death receptors such as TNF receptor 1 (TNFR1) [30]. Upon binding to TNF-α, the TNFR1 recruits several proteins including TRADD, TRAF2, cIAP1, cIAP2 and RIP1, which culminates in RIP1 becoming lysine 63-linked (K63-linked) poly-ubiquitinated [31]. When poly-ubiquitinated, RIP1 recruits two additional kinases, IKK and TAK1, which mediate the activation of NF-κB and promote cell survival [31]. In the absence of cIAP1 and cIAP2, RIP1 is still recruited to the TNFR1 but fails to become poly-ubiquitinated. As a consequence, the non-ubiquitinated RIP1 protein binds to and activates caspase 8, thus re-routing TNF-α signaling towards a pro-apoptotic pathway [29].
In gliomas, it remains unclear whether constitutively activated NF-κB induces the expression of genes that mediate resistance to TNF-α. In the present study, we demonstrate that in gliomas, NF-κB is constitutively activated and the levels of cIAP2, Bcl-2, Bcl-xL and Survivin are elevated. Using human glioma cells that were engineered to inducibly express shRNA molecules specific for p65, we addressed the role of NF-κB in regulating the expression of these proteins, and their potential role(s) in mediating resistance to TNF-α induced apoptosis. In the presence of NF-κB p65, TNF-α induced cIAP2 expression, which was abrogated upon the loss of p65 expression. In contrast, the loss of endogenous p65 protein levels did not affect the levels of Bcl-2, Bcl-xL or Survivin, suggesting these genes are not totally dependent on NF-κB p65 for expression. Additionally, we show that glioma cells with reduced p65 and cIAP2 levels express lower levels of poly-ubiquitinated RIP1, and were re-sensitized to the cytotoxic effects of TNF-α. As such, these data suggest that NF-κB p65 and cIAP2 may be key targets in developing novel therapeutic strategies to overcome apoptotic resistance in gliomas.
MATERIALS AND METHODS
Reagents
Recombinant human TNF-α was purchased from R & D Systems (Minneapolis, MN). Secondary peroxidase-conjugated antibodies were purchased from Amersham (Arlington Heights, IL). ECL reagents were purchased from Pierce (Rockford, IL). Antibodies specific for p65, c-Rel, RelB, p105/p50, p100/p52, Survivin, IκBα, ubiquitin, TRAF2, TRADD, RIP1 and normal rabbit serum were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for caspase 8, cIAP2 and phosphorylated-p65 (S536) were purchased from Cell Signaling Technology (Danvers, MA). Anti-p65 antibody for chromatin immunoprecipitation (ChIP), and antibodies specific for GAPDH and caspase 3 were obtained from Abcam (Cambridge, MA). Monoclonal antibodies specific for Bcl-2 and Bcl-xL were a generous gift of Dr. Tong Zhou (University of Alabama at Birmingham).
Plasmids
A plasmid encoding shRNA specific for p65 was generated by annealing double stranded oligonucleotides specific for a 19 bp region of the p65 ORF (CTG TTC CCC CTC ATC TTC C) into the plasmid, pBABE-HI-TetO, which is under the dual control of the tet operator and the HI Pol III promoter. The plasmid, pBABE-HI-TetO, was a generous gift of Dr. Xinbin Chen (University of California, Davis). The U251-MG glioma cell line was obtained from ATCC, and maintained as previously described [32]. U251-MG cells that stably express the Tet repressor (TetR) protein (U251-TR) and U251-TR cells that inducibly express shRNA specific for p65 (sh-p65) (U251-TR/sh-p65) were generated as previously described [12]. Plasmids encoding shRNA specific for cIAP2 were generated by annealing double stranded oligonucleotides specific for cIAP2 into pLVX-shRNA2 (Clontech, Palo Alto, CA). Lentiviral molecules expressing shRNA molecules specific for cIAP2 were generated using the Lenti-X-HT kit and used according to manufacturer’s instructions (Clontech, Palo Alto, CA).
Human Control and Glioma Tissues
Resected nonmalignant (control) and malignant (glioma) brain tissue was obtained from the UAB Brain Tumor Tissue Core facility (IRB X050415007), snap-frozen in liquid nitrogen, and stored at −80°C until processed. Tissues were ground with liquid nitrogen in a pre-chilled mortar and pestle at 4°C as previously described [12]. The ground tissue was collected and processed using the PARIS kit according to the manufacturer’s instructions (Ambion, Austin, TX). Protein was analyzed by immunoblotting, and RNA was analyzed by RT-PCR as previously described [12].
Immunoblotting
Where indicated, U251-TR/sh-p65 cells were grown in the absence (−) or presence (+) of Tet (4 µg/ml) for 24 h. Cells were then incubated in serum free media in the absence or presence of Tet for 24 h and/or TNF-α (10 ng/ml) for the times indicated. Protein concentrations were measured using BioRad Assay and equal amounts of total protein were analyzed by SDS-PAGE using the antibodies specified, as previously described [12].
Immunoprecipitation
U251-TR/sh-p65 cells were grown in the absence (−) or presence (+) of Tet for 24 h. Cells were then incubated in serum free media in the absence or presence of Tet for 24 h. Next, cells were left untreated (−) or treated (+) with TNF-α (10 ng/ml) for the times indicated. Cells were washed twice in cold PBS and collected in ice cold RIPA. Protein concentrations were measured using BioRad Assay and equal amounts of total protein were pre-cleared using protein A/G beads, and then subjected to immunoprecipitation using anti-RIP1 antibodies overnight at 4°C. The next day, protein A/G beads were added for 2 h at 4°C. Immunoprecipitated complexes were washed three times with RIPA buffer and analyzed by immunoblot assays using antibodies specific for ubiquitin.
Total RNA Isolation and Reverse Transcriptase-PCR (RT-PCR)
Where indicated, U251-TR/sh-p65 cells were grown in the absence (−) or presence (+) of Tet for 24 h. Cells were then incubated in serum free media in the absence or presence of Tet for 24 h and/or TNF-α (10 ng/ml) for various times. Total RNA was isolated as previously described [33]. Two µg of total RNA was reverse transcribed and analyzed by PCR using primers specified. To detect p65, the following primers were used: p65-F 5’ GCG AAT GGC TCG TCT GTA GT 3’ and p65-R 5’ CCT GGT CCT GTG TAG CCA TT 3’. To detect cIAP2, the following primers were used: cIAP2-F 5’ AGT CTT GCT CGT GCT GGT TT 3’ and cIAP2-R 5’ TGC TTT TGC CAG ATC TGT TG 3’.
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed as previously described [12, 33, 34]. Five µg of appropriate antibody was used to immunoprecipitate proteins of interest. Immune complexes were precipitated with protein A beads (Millipore, Billirica, MA) blocked with BSA and salmon sperm DNA. Immunoprecipitated DNA was subjected to semi-quantitative PCR using primers specific for the cIAP2 promoter. PCR products were analyzed by gel electrophoresis. To detect the cIAP2 promoter, the following primers were used: cIAP-Pro-F 5’ AAA GTG TAT GGC GGA TGG AG 3’ and cIAP-Pro-R 5’ GTG CAC TGG TGC TTT CCT TT 3’.
Growth Rate Analyses
To determine the rate of cell growth, cells were seeded at approximately 6 × 104 cells/60-mm plate in the absence or presence of Tet for 48 h. Next, the medium was replaced and cells were grown in the absence or presence of Tet and/or TNF-α (10 ng/ml). The medium was replaced every 72 h. At times indicated, three plates per condition were rinsed twice with PBS to remove dead cells and debris. Live cells on the plates were trypsinized and collected separately. Cells from each plate were counted three times using the Coulter cell counter. The average number of cells from three plates was used for growth rate determination [35, 36].
DNA Histogram Analyses
Cells were seeded at 2 × 105 per 90-mm plate in the absence or presence of Tet for 48 h. Next, the medium was changed and cells were grown in the absence or presence of Tet and/or TNF-α. At the times indicated, both floating and dead cells in the medium and live cells on the plate were collected and fixed with 5 ml of 100% ethanol for 4 h and then centrifuged, washed twice with PBS and resuspended in 1 ml of PBS solution containing 50 µg/ml each of RNase A (Sigma) and propidium iodide (Sigma). The stained cells were analyzed in a fluorescence-activated cell sorter (FACS) (FACSCaliber, Becton Dickinson) within 4 h. The percentage of cells in sub-G1, G1, S, and G2-M phases was determined using the Cell Quest program and ModFit program. The percentage of cells in sub-G1 phase was used as an index for the degree of apoptosis [35, 36].
Statistical Analysis
Levels of significance for comparison between samples were determined by the Student’s t-test distribution. p values of less than or equal to 0.05 were considered to be statistically significant.
RESULTS
NF-κB is Elevated and Constitutively Activated in Gliomas
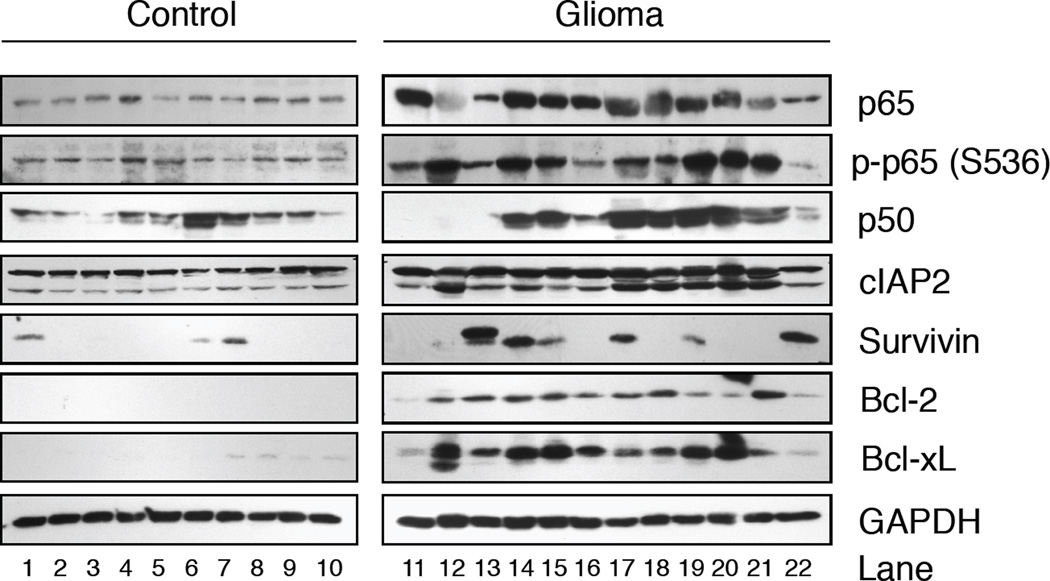
Previously, we have shown that NF-κB is constitutively activated in gliomas [12]. To extend this finding, we evaluated the status of NF-κproteins in 10 non-malignant (Fig. 1, control; lanes 1–10) and 12 malignant glioma (glioma; lanes 11–22) samples. All control tissues displayed moderate levels of total p65, while p65 levels were elevated in 9 of 12 glioma tissues. To assess NF-κB activation, we performed immunoblot assays using antibodies specific for p65 phosphorylated on serine residue 536 (p-p65 (S536)), which is correlated with transcriptional activation [37]. Although basal levels of p-p65 (S536) were detected in the control tissues, these levels were elevated in 11 of 12 glioma samples evaluated (Fig. 1). In control tissues, the levels of p50 varied amongst samples and appeared elevated in several glioma tissues. These results demonstrate that NF-κB is elevated and constitutively activated in gliomas.
Figure 1. NF-κB is Constitutively Activated in Human Gliomas and Correlates with Elevated Levels of Anti-Apoptotic Genes.
Thirty micrograms of total protein from fresh snap-frozen brain tissue resected from nonmalignant (Control) or patients diagnosed with gliomas (Glioma) were evaluated by immunoblotting using antibodies specific for total p65, phospho-p65 (p-p65(S536)), p50, cIAP2, Survivin, Bcl-2, Bcl-XL and GAPDH. Representative of three experiments.
Anti-Apoptotic Proteins Regulated by NF-κB are Overexpressed in Gliomas
NF-κB regulates the expression of several anti-apoptotic genes, including cIAP2, Survivin, Bcl-2 and Bcl-xL [38–41]. To address whether these proteins were elevated in gliomas, we performed immunoblot analyses. As shown in Figure 1, in control tissues, the levels of Survivin, Bcl-2 and Bcl-xL expression were low or absent while cIAP2 was moderately expressed in all, and the levels of these proteins were elevated in gliomas. To confirm these data in a larger sample population, we analyzed the expression of cIAP2, Survivin, Bcl-2 and Bcl-xL mRNA levels using Oncomine, a cancer microarray database [42]. In a comparison of 981 samples, we determined that the levels of cIAP2, Bcl-xL and Survivin mRNA were elevated in glioblastoma compared to control brain (p < 2.35 × 10−4, p < 4.43 × 10−6 and p < 7.11 × 10−14, respectively) [43]. The levels of Bcl-2 mRNA varied between and within glioblastoma and control brain tissues (p-values ranged from 0.005 to 0.967). These data suggest that in gliomas, NF-κB is activated and the levels of cIAP2, Bcl-xL and Survivin are elevated.
Generation of Glioma Cells that Inducibly Regulate Endogenous p65 Levels
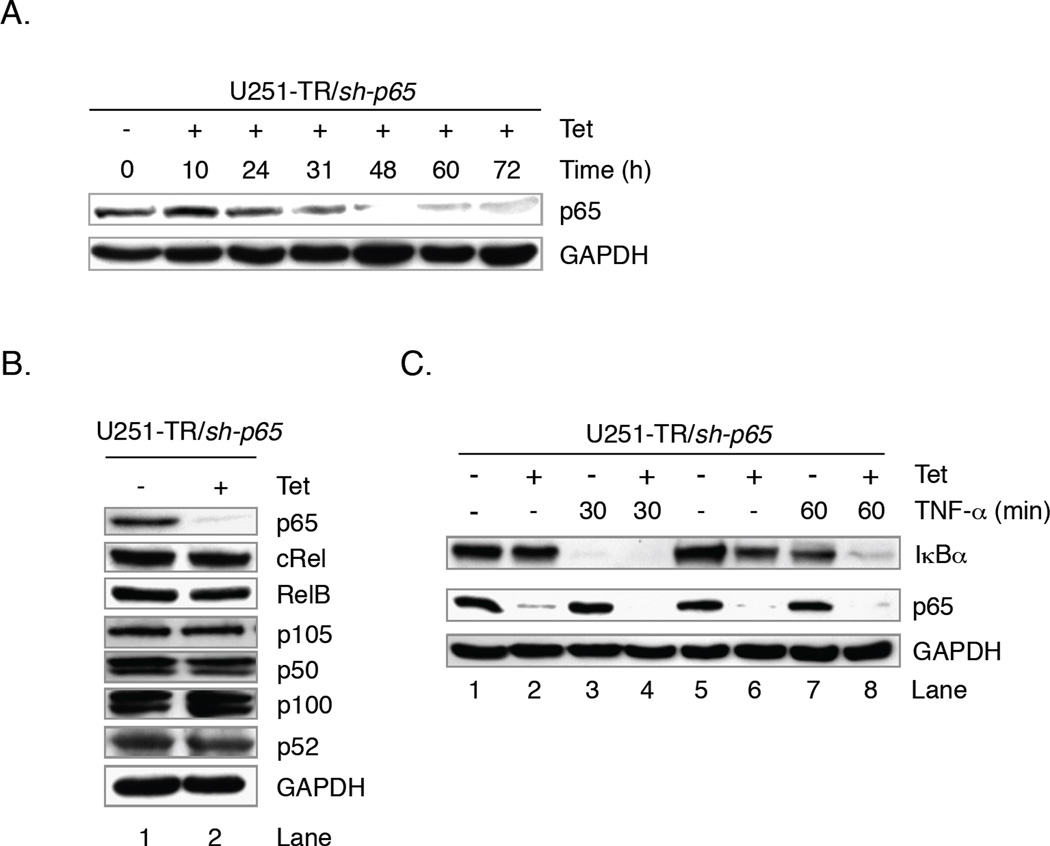
In the CNS, the most common NF-κB dimer consists of p65 and p50, and of these two subunits, only p65 contains a transactivation domain [2–4]. Therefore, to directly address the role of NF-κB in mediating apoptotic resistance in gliomas, we generated human glioma cell lines that inducibly expressed shRNA molecules specific for p65 (U251-TR/sh-p65) [12]. To evaluate the efficacy of p65 shRNA expression and determine the time needed to maximally reduce endogenous p65 protein levels, U251-TR/sh-p65 cells were grown in the absence (−) or presence of Tet (+) for various times and protein levels analyzed by immunoblot assays. The levels of endogenous p65 protein are noticeably diminished at 31 h post-Tet, are nearly absent at 48 h and this effect persists until at least 72 h (Fig. 2A). To address whether the expression of p65 shRNA affected the levels of other NF-κB family members, U251-TR/sh-p65 cells were grown in the absence (−) or presence (+) of Tet for 48 h and protein expression analyzed by immunoblot analyses. We determined that neither the expression of p65 specific shRNA molecules, nor the absence of p65 affected the expression of additional NF-κB family members (Fig. 2B). To determine whether reductions in endogenous p65 protein levels were sufficient to inhibit the expression of an NF-κB target gene, we analyzed the expression of IκBα. In the absence of Tet or TNF-α, both p65 and IκBα proteins were detected (Fig. 2C, lanes 1 and 5). In the presence of Tet, the levels of p65 are reduced and the levels of IκBα were unaffected (lanes 2 and 6). Upon stimulation with TNF-α, the levels of IκBα are reduced by degradation within 30 min (lane 3), and this was not altered by the absence of endogenous p65 (lane 4). At 60 min post-TNF-α, IκBα protein levels were nearly restored in cells that express p65 (lane 7), and this was blocked in cells that lacked p65 protein (lane 8). These data demonstrate that the loss of p65 is sufficient to prevent TNF-α-induced IκBα expression.
Figure 2. Reductions in NF-κB p65 Impair Transcriptional Activity of NF-κB in Glioma Cells.
A. U251-TR/sh-p65 cells inducibly express shRNA molecules specific for p65. In the absence of Tet, endogenous p65 is detected. The levels of p65 are diminished at 31 h post-Tet administration, and remain reduced for at least 72 h. B. Reductions in p65 levels do not affect the levels of other NF-κB proteins. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h and total protein levels were analyzed by immunoblot analyses using the antibodies specified. C. Reductions in p65 do not prevent IκBα degradation but do impair the resynthesis of IκBα. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 30 or 60 min. Total protein levels were analyzed by immunoblotting using the antibodies specified. Representative of three experiments.
Absence of p65 Reduces cIAP2 Expression but not Bcl-2, Bcl-xL or Survivin Expression
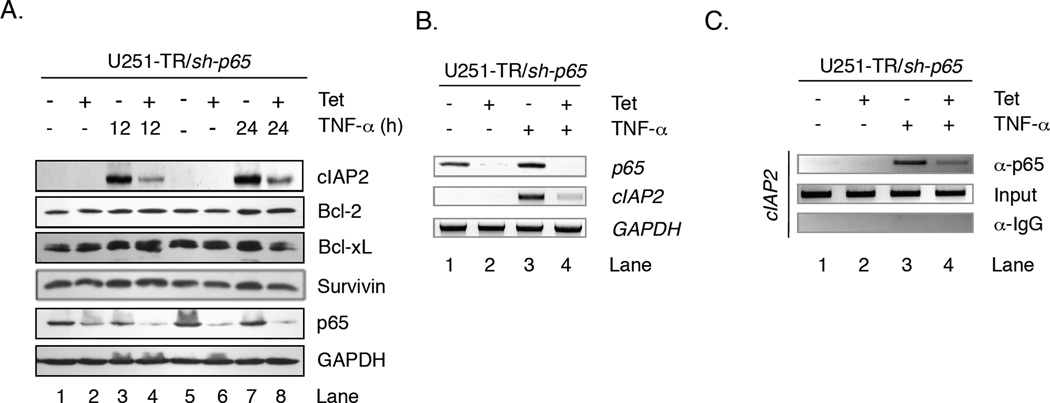
To assess how the loss of p65 protein impacts cIAP2, Bcl-2, Bcl-xL and Survivin expression, U251-TR/sh-p65 cells were grown in the absence (−) or presence (+) of Tet for 48 h to reduce endogenous p65 levels, then in the absence or presence of TNF-α for 12 or 24 h, and protein levels analyzed by immunoblot assays. Because human glioma cells in culture are not subject to the same microenvironment as tumors, TNF-α was added to mimic the environment and ensure NF-κB activation. In the absence of Tet or TNF-α, U251-MG glioma cells constitutively expressed Survivin, Bcl-2 and Bcl-xL but did not express cIAP2 (Fig. 3A, lanes 1 and 5). Interestingly, the levels of Survivin, Bcl-2 and Bcl-xL were relatively unchanged in the absence of p65 (lanes 2 and 6), TNF-α (lanes 3 and 7), or both (lanes 4 and 8). In contrast, TNF-α induced expression of cIAP2 at 12 h (lane 3) and these levels remained elevated at 24 h (lane 7). However, in the absence of p65, TNF-α induced cIAP2 expression was inhibited (lanes 4 and 8). These results indicate that NF-κB p65 is required for the maximal expression of cIAP2 by TNF-α.
Figure 3. TNF-α Induced cIAP2 Expression is Dependent on NF-κB p65.
A. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 12 or 24 h. Total proteins were analyzed by immunoblot analyses using the antibodies specified. B. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 12 h. Total RNA was analyzed by RT-PCR using primers specific for p65, cIAP2 or GAPDH. C. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 1 h. ChIP assays were performed using antibodies specific for p65 or control IgG. Input and IP DNA was analyzed using primers specific for human cIAP2 promoter. Representative of five experiments.
To confirm that these results were due to diminished cIAP2 mRNA expression, U251-TR/sh-p65 cells were grown in the absence or presence of Tet and/or TNF-α, and levels of cIAP2 mRNA evaluated by RT-PCR. In the absence of any stimuli, cIAP2 was not expressed (Fig. 3B, lane 1). cIAP2 mRNA levels were not affected by Tet alone (lane 2), but were substantially increased by TNF-α (lane 3). However, in the presence of both Tet and TNF-α, cIAP2 mRNA levels were reduced (lane 4).
To confirm that cIAP2 is a direct transcriptional target of NF-κB p65, U251-TR/sh-p65 cells were grown in the absence or presence of Tet and/or TNF-α, and chromatin immunoprecipitation (ChIP) assays were performed using antibodies specific for p65. In the absence of any stimulus or in the presence of Tet alone, p65 was not detected at the cIAP2 promoter (Fig. 3C, lanes 1 and 2). After 1 h of TNF-α stimulation, p65 was detected at the cIAP2 promoter (lane 3), and these levels were reduced in the presence of Tet (lane 4). These data confirm cIAP2 is a direct transcriptional target of NF-κB p65, and that NF-κB p65 is required for maximal induction of cIAP2 in response to TNF-α stimulation.
RIP1 is Hypo-Ubiquitinated upon TNF-α Stimulation in the Absence of p65
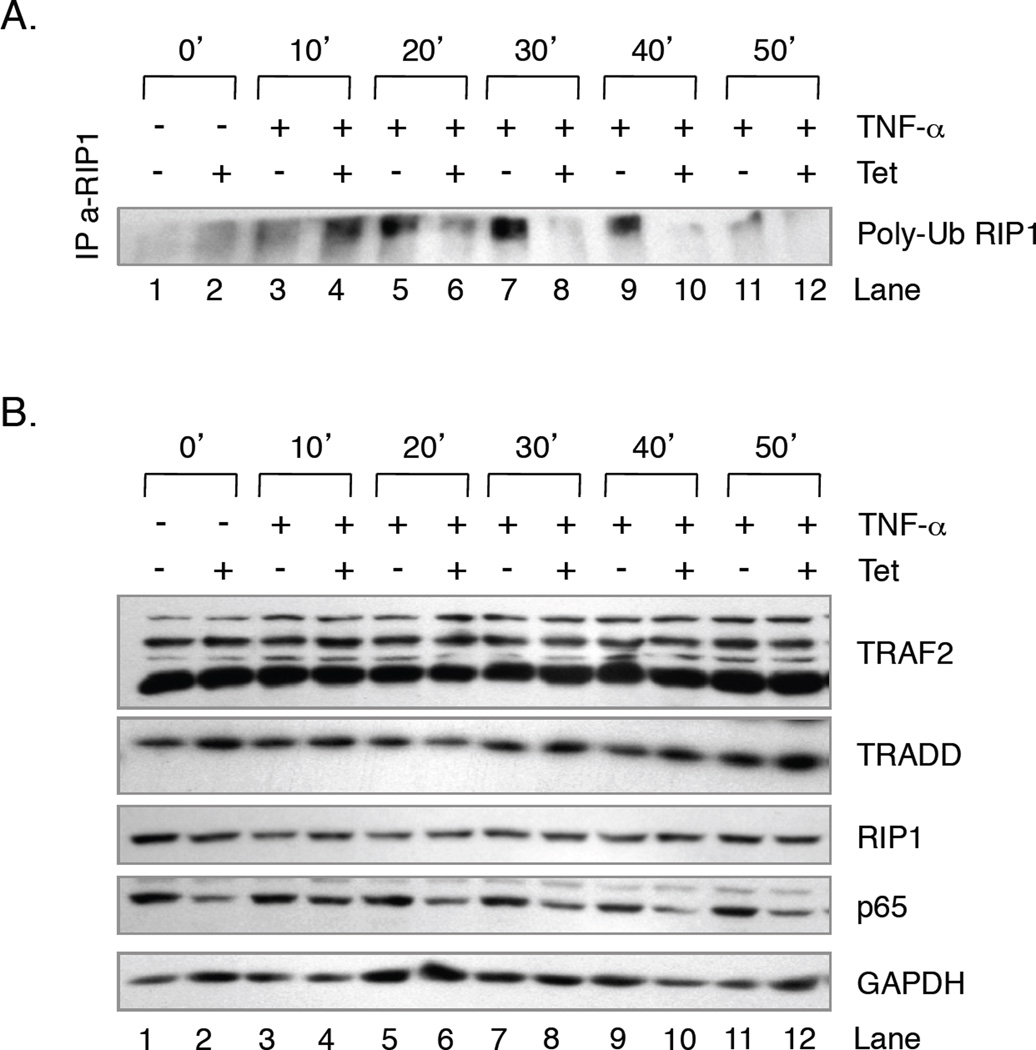
Thus far, our data suggest that in the absence of p65, TNF-α fails to induce cIAP2 expression. Previous studies demonstrated that cIAP1 and cIAP2 mediate the K63-linked ubiquitination of RIP1, which consequently allows RIP1 to interact with the prosurvival kinases TAK1 and IKK [29]. Therefore, we hypothesized that in the absence of p65, U251-MG cells that were challenged with TNF-α may display reduced levels of RIP1 ubiquitination. To address this, U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h and then in the absence or presence of TNF-α for various times. Whole cell extracts were collected and equal amounts of proteins were immunoprecipitated with antibodies specific for RIP1 and then analyzed by immunoblot assays using antibodies specific for ubiquitin. In the absence of any stimuli, these cells display low levels of ubiquitinated RIP1 (Poly-Ub RIP1) (Fig. 4A, lane 1), and these levels were slightly enhanced by the absence of p65 (lane 2) or the presence of TNF-α after 10 min (lane 3). In the presence of TNF-α, the levels of Poly-Ub RIP1 increased in a time dependent manner until 40 min post-TNF-α (lanes 3, 5, 7, and 9), and the levels were reduced at 50 min (lane 11). However, in the absence of p65, the levels of TNF-α-induced Poly-Ub RIP1 were substantially reduced (Fig. 4A, lanes 6, 8 and 10). To determine if these results were due to differences in total RIP1 levels, or the levels of TRAF2 and TRADD, which are also present at the TNF-α receptor, we analyzed their expression using immunoblot assays. As shown in Fig. 4B, the levels of endogenous p65 were reduced by the presence of Tet. However, neither the absence nor presence of Tet and/or TNF-α altered the levels of RIP1, TRAF2 or TRADD, suggesting that the results of Fig. 4A were due to changes in the levels of Poly-Ub RIP1.
Figure 4. RIP1 is Hypo-Ubiquitinated in the Presence of TNF-α and the Absence of NF-κB p65.
U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 10, 20, 30, 40 or 50 min. Whole cell lysates were prepared and 500 µg of total protein was immunoprecipitated using antibodies specific for RIP1. Immunoprecipitated proteins were washed and evaluated by immunoblot assays using antibodies specific for ubiquitin. B. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 10, 20, 30, 40 or 50 min and prepared as described in A. Fifty µg total protein were evaluated by immunoblot analyses using antibodies specific for TRAF2, TRADD, RIP1, p65 and GAPDH.
Reduced NF-κB p65 Levels Do Not Impair Cell Growth but Do Sensitize Glioma Cells to TNF-α
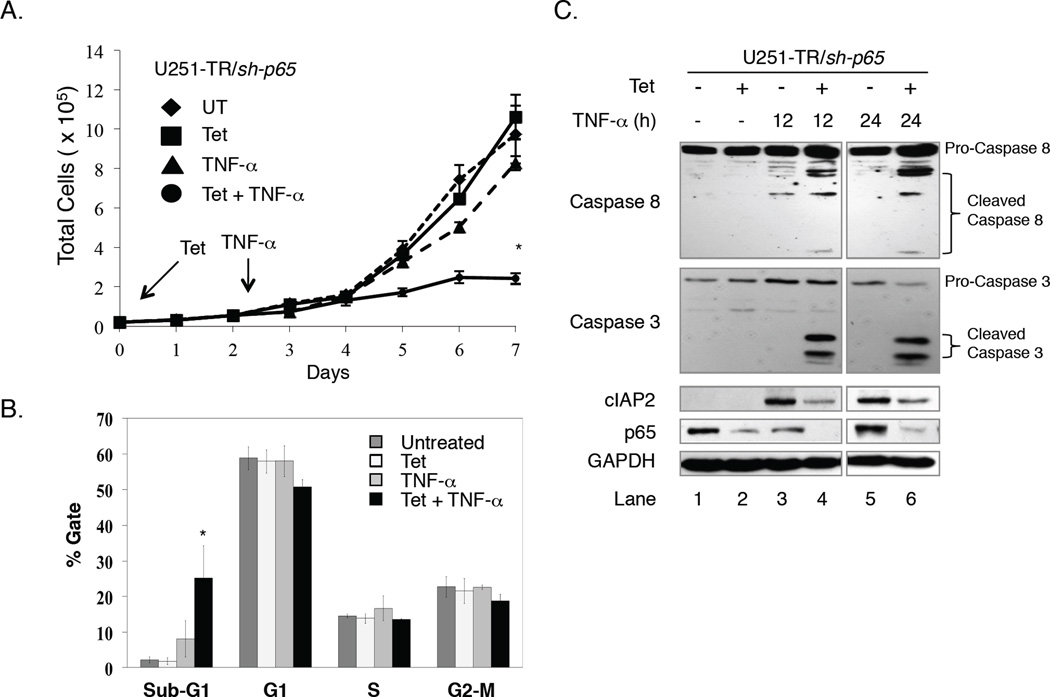
In the absence of p65, U251-MG cells challenged with TNF-α failed to express cIAP2 and harbored reduced levels of poly-Ub RIP1. Therefore, because the ubiquitinated status of RIP1 determines whether TNF-α signaling is pro-survival or pro-apoptotic, we assessed the effects of reduced p65 and cIAP2 expression on cell proliferation in the absence or presence of TNF-α. These cells provided a suitable model since U251-MG cells are resistant to the cytostatic effects of TNF-α. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h to reduce p65 expression, then grown in the absence or presence of Tet and/or TNF-α and growth rate analyses were performed. As shown in Fig. 5A, cells grown in the absence of any stimulus (◆) or in the presence of Tet (■) alone displayed similar growth rates. The addition of TNF-α alone (▲) had only a modest effect on cell growth after 5 days, while those cells grown in the presence of Tet and TNF-α (□) were significantly impaired in their ability to grow. These data indicate that TNF-α impairs glioma growth in the absence of p65 expression.
Figure 5. TNF-α Impairs Cell Growth and Induces Apoptosis in the Absence of NF-κB p65.
A. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for an additional five days. Each day, cells were washed, trypsinized and the number of cells was counted using a Coulter Counter. Medium ± Tet ± TNF-α was changed at 3 and 6 days of the experiment. Data points represents the mean ± SEM of three independent measurements per plate, and three plates per condition were evaluated. *p ≤ 0.001 when compared to TNF-α alone. B. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 24 h. At 72 h after plating, both floating and dead cells in the media and live cells on the plates were collected and were fixed with 1 ml of 100% ethanol overnight and were then centrifuged and resuspended in 300 µl of PBS solution containing 50 µg each of RNase A and propidium iodide per ml. The stained cells were measured by fluorescence-activated cell sorter analysis. The percentage of cells in the sub-G1, G1, S, and G2-M phases was determined by using the Cell Quest program. The percentage of cells in the sub-G1 phase was used as an index for the degree of apoptosis. Columns represent the mean ± SEM of three independent experiments. *p ≤ 0.002 when compared with TNF-α alone. C. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of TNF-α for 12 or 24 h. Whole cell lysates were collected and the levels of pro-caspase 8, cleaved caspase 8, pro-caspase 3, cleaved caspase 3, cIAP2, p65 and GAPDH were analyzed using the antibodies specified.
TNF-α Induces Apoptosis in Glioma Cells that Lack p65 Expression
To assess whether the negative effects of TNF-α on glioma cell growth were due to cell cycle inhibition or apoptosis, DNA histogram analyses were performed. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h to reduce p65 protein levels, and then in the absence or presence of Tet and/or TNF-α for an additional 24 h. In the absence of Tet or TNF-α, approximately 60% of U251-MG cells were in G1, 15% in S, 20% in G2-M and 5% were undergoing apoptosis (sub-G1) (Fig. 5B). The percentage of cells in each phase of the cell cycle was not altered by the presence of Tet or TNF-α. However, cells grown in the presence of Tet and TNF-α showed modest reductions in the percentage of cells in G1, S and G2-M, while the percentage of cells undergoing apoptosis (sub-G1) increased to approximately 25% (Fig. 5B).
To confirm the incidence of apoptosis, we evaluated cIAP2 levels and caspase activation by immunoblot assays. U251-TR/sh-p65 cells were grown in the absence or presence of Tet for 48 h, and then in the absence or presence of Tet and/or TNF-α for 12 or 24 h. We did not detect cIAP2 expression, or activation of caspase-8 or caspase-3 in cells grown in the absence of any stimulus (Fig. 5C, lane 1) or in the presence of Tet alone (lane 2). Although TNF-α alone increased the levels of cIAP2 and caused modest activation of caspase-8 at 12 and 24 h, we failed to detect concomitant activation of caspase-3 (lanes 3 and 5). However, cells grown in both Tet and TNF-α displayed reduced levels of cIAP2 concommittant with caspase-8 and caspase-3 activation (lanes 4 and 6). These data indicate that TNF-α induced apoptosis in glioma cells that lack p65 expression.
Loss of cIAP2 Sensitizes Gliomas to Apoptosis Induced by TNF-α
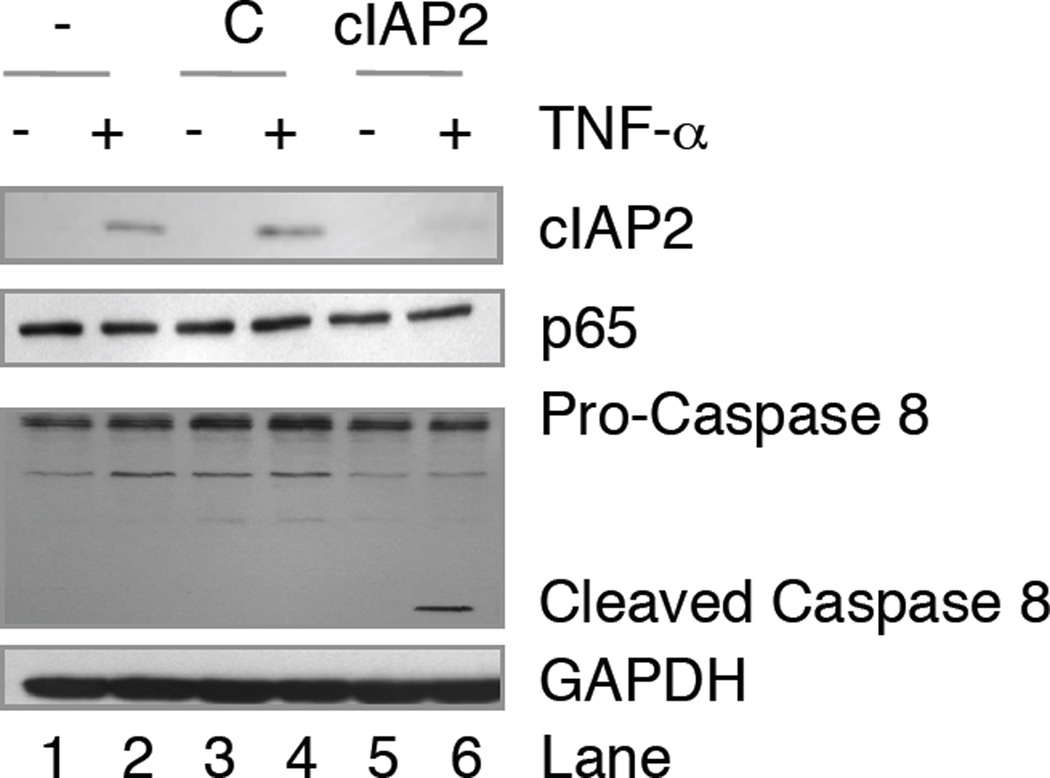
Our data suggest that in gliomas, TNF-α fails to induce apoptosis, in part through NF-κB-induced cIAP2 expression. Indeed, when NF-κB p65 is removed from glioma cells, cIAP2 fails to be expressed, and these glioma cells undergo apoptosis when challenged with TNF-α. To demonstrate that these results are dependent on cIAP2 expression, parental U251-MG cells, which express endogenous p65, were left untransduced (−), transduced with a control lentivirus (C), or a lentivirus expressing shRNA molecules specific for cIAP2 (cIAP2). These cells were allowed to recover for four days, grown in the absence or presence of TNF-α for 24 h, and then p65 and cIAP2 levels assessed by immunoblotting. As shown in Fig. 6, U251-MG cells containing NF-κB p65 do not express cIAP2 unless challenged with TNF-α (compare lanes 1 and 2), and this was not affected by the introduction of control lentiviral molecules (lanes 3 and 4). However, in the presence of lentiviral expressed cIAP2 shRNA molecules, TNF-α induced cIAP2 levels were significantly reduced (compare lanes 2 and 6). To assess whether the reductions in cIAP2 levels were sufficient to induce caspase activation, we evaluated the levels of pro-caspase 8 and cleaved caspase 8 in these samples. In U251-MG cells, TNF-α did not induce caspase 8 activation in untransduced cells or cells transduced with control lentivirus (Fig. 6, lanes 2 and 4). However, in U251-MG cells transduced with lentivirus expressing cIAP2 shRNA, TNF-α induced caspase 8 cleavage (lane 6). These data suggest that TNF-α activates NF-κB and induces cIAP2 to prevent apoptosis in glioma cells.
Figure 6. TNF-α Induces Apoptosis in the Absence of cIAP2.
U251-MG cells were left untransduced (−), transduced with control lentivirus (C) or lentivirus expressing shRNA molecules specific for cIAP2 (cIAP2) and allowed to recover for 4 days. Cells were then left untreated (−) or treated (+) with TNF-α for 24 h. Whole cell lysates were collected and the levels of cIAP2, p65, pro-caspase 8, cleaved caspase 8 and GAPDH were analyzed using the antibodies specified. Representative of two experiments.
DISCUSSION
Although neither a classical tumor suppressor nor an oncogene, the role for NF-κB in tumors is quickly becoming the focus of much attention. Increasing evidence supports a role for NF-κB and inflammation in mediating several facets of tumor behavior, including cell growth, migration, invasion, angiogenesis and resistance to apoptosis [1, 44]. In gliomas, we and others have shown that NF-κB is constitutively activated, and that the levels of many NF-κB regulated genes are elevated [11, 12, 45]. At present, the exact mechanism(s) underlying NF-κB activation remains poorly understood, but may be in part explained by the tumor microenvironment, the loss of negative regulators and/or the presence of elevated levels of positive regulators. For example, in the CNS, TNF-α is secreted by microglia, astrocytes and some neurons [46]. Although TNF-α can signal through two receptors, in gliomas, the levels of TNFR1 are elevated compared to normal brain tissues [47, 48]. Therefore, in gliomas, TNF-α signaling through TNFR1 favors NF-κB activation and promotes tumor growth and development [49–51]. NF-κB may also be activated by numerous growth factors or signaling pathways that are dysregulated in gliomas. NF-κB is activated by epidermal growth factor (EGF), which is frequently amplified, and/or its receptor, EGFR, which is often mutated and constitutively activated [7]. Many gliomas also show allelic loss of PTEN, a tumor suppressor and negative regulator of the AKT pathway. In the absence of PTEN, AKT is constitutively active and can activate NF-κB [7]. We have recently shown that ING4, a negative regulator of NF-κB, is expressed at very low levels or is mutated in GBMs, and that the lack of ING4 enables NF-κB to remain constitutively active [12]. Conversely, Pin1, a positive regulator of NF-κB, is overexpressed in GBMs [52, 53], and also contributes to NF-κB activation [52]. Therefore, there is a strong correlation between constitutive NF-κB activation and gliomagenesis.
Human gliomas are notoriously resistant to apoptosis. In the present study, we investigated what role, if any, a single NF-κB family member, p65, plays in mediating apoptotic resistance to TNF-α. We focused on p65 as our studies showed that this protein is overexpressed and constitutively activated in human gliomas compared to non-glioma samples (Fig. 1). Additionally, we determined that the levels of cIAP2, Survivin, Bcl-2 and Bcl-xL were also elevated in gliomas. Each of these genes can be regulated by NF-κB and can inhibit apoptosis. Therefore, because NF-κB is known to be constitutively activated in gliomas and can induce these genes, we hypothesized that NF-κB and one or more of these gene(s) were responsible for suppressing TNF-α induced apoptosis in gliomas. To address this, we engineered human glioma cells that inducibly expressed shRNA molecules specific for p65 (U251-TR/sh-p65) so that we could regulate the levels of endogenous p65 protein, and thus measure the cellular response to TNF-α (Fig. 2). Interestingly, Survivin, Bcl-2 and Bcl-xL were constitutively expressed in U251-MG cells and their expression was unchanged by the absence or presence of p65 or TNF-α. In contrast, cIAP2 expression was absent in U251-MG cells. In the presence of p65, TNF-α increased the levels of cIAP2, and this was impaired in cells that lacked p65 (Fig. 3). Using RT-PCR and ChIP assays, we confirmed that cIAP2 is a direct transcriptional target of NF-κB p65, and that in the absence of p65, TNF-α fails to sufficiently induce cIAP2 expression or recruit NF-κB p65 to the cIAP2 promoter. Thus, our data confirm that cIAP2 is a direct transcriptional target of NF-κB p65 and is induced in response to TNF-α.
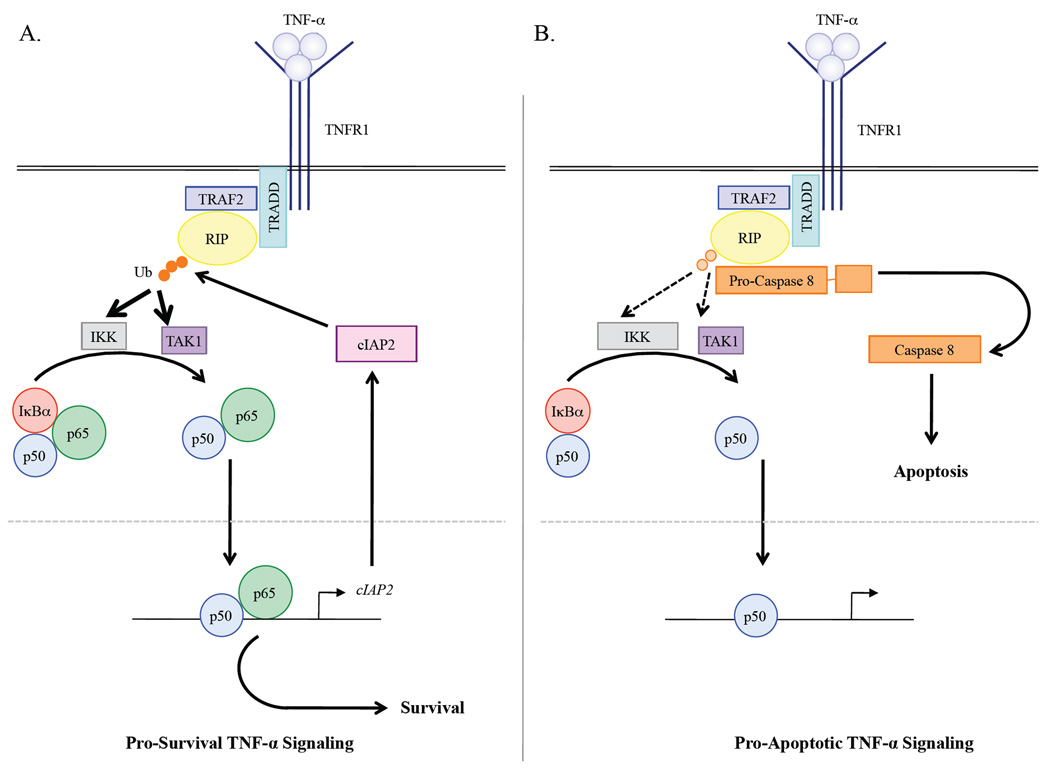
cIAP2 contains an E3 ubiquitin ligase domain and can target RIP1 for K63-linked ubiquitination [29] (Fig. 7A). When ubiquitinated, RIP1 associates with IKK and TAK1, which activate NF-κB [29]. In the absence of ubiquitination, RIP1 associates with and activates pro-caspase 8, which leads to caspase 3 activation and apoptosis [29] (Fig. 7B). Therefore, the ubiquitination status of RIP1 is critical in determining whether TNF-α signaling is pro-survival or pro-apoptotic. Thus far, our data suggest that gliomas inevitably favor NF-κB activation, and consequently, NF-κB ensures the levels of cIAP2 are elevated. We provide data demonstrating that TNF-α stimulation rapidly increases the levels of RIP1 ubiquitination in a manner dependent on NF-κB p65 (Fig. 4), since the loss of endogenous p65 dramatically reduced the levels of RIP1 ubiquitination. Presently, we are working to address the status of RIP1 ubiquitination in human gliomas, since our data would suggest that as a consequence of NF-κB activation and elevated cIAP2 levels, RIP1 is likely to be hyper-ubiquitinated. Moreover, we would speculate that if RIP1 is hyper-ubiquitinated in gliomas, then TNF-α signaling is, by default, pro-survival in gliomas.
Figure 7. NF-κB p65 and cIAP2 are Key in Determining the Outcome of TNF-α Signaling in Glioma Cells.
A. Pro-survival TNF-α signaling. Secreted TNF-α molecules bind to the death receptor TNFR1. Associated with the cytoplasmic portion of TNFR1 are several adaptor molecules including TRADD, TRAF2 and the serine/threonine kinase RIP1. Upon TNF-α stimulation, RIP1 is poly-ubiquitinated (orange circles) by cIAP2, which enables RIP1 to interact with and activate the kinases IKK and TAK1. These kinases then phosphorylate the inhibitory protein IκBα, which targets it for ubiquitin-mediated degradation. In the absence of IκBα, the newly released NF-κB molecule, p65/p50, is free to move into the nucleus and induce the expression of genes that mediate pro-survival. In particular, NF-κB upregulates the expression of cIAP2, which poly-ubiquitinates RIP1 during pathophysiological conditions to ensure that TNF-α signaling promotes a pro-survival pathway. B. Pro-apoptotic TNF-α signaling. TNF-α binds to TNFR1, which interacts with TRADD, TRAF2 and RIP1. Immediately before and after TNF-α binding, RIP1 remains poly-ubiquitinated, and perhaps competent to activate IKK and/or TAK1, since IκBα still undergoes proteolytic degradation. However, in the absence of p65 and a transcriptionally active NF-κB complex, cIAP2 fails to be expressed. As a consequence of this, the levels of poly-ubiquitinated RIP1 rapidly diminish (light orange circles) and pro-caspase 8 interacts with and becomes activated by hypo-ubiquitinated RIP1. Once activated, caspase 8 activates additional caspases, which execute the ensuing phases of apoptosis.
Moreover, our data suggest that NF-κB-induced cIAP2 expression is a key upstream event that promotes cell survival, and that reductions in cIAP2 expression, either indirectly, through reductions in p65 levels (Fig. 5), or directly, by preventing cIAP2 expression (Fig. 6), can re-route TNF-α signaling towards an apoptotic end (Fig. 7). Therefore, we propose that in gliomas, constitutively activated NF-κB mediates resistance to apoptotic stimuli by increasing or stabilizing the levels of cIAP2 in order to promote a pro-survival signaling pathway (Fig. 7A).
What does this mean in terms of therapeutic potential for gliomas? These findings underscore the importance of targeting NF-κB and/or cIAP2 and potentially other cIAP family members (as discussed below) as therapeutically relevant strategies for gliomas and other cancers. Herein, we show that NF-κB is constitutively activated in human gliomas and that reductions in NF-κB p65 levels can re-sensitize glioma cells to the cytotoxic effects of TNF-α. This is consistent with other studies that have also shown increased cell death in glioma models by chemotherapies and/or radiation if NF-κB activity is simultaneously blocked [54–61]. However, since inhibiting global NF-κB activity may be undesirable and/or specifically targeting NF-κB activity in gliomas may be difficult, we propose that identifying and targeting those genes that mediate particular facets of NF-κB behavior might be a more reasonable goal.
Members of the IAP family regulate whether the cell lives or dies in response to numerous stresses and insults. This is because the IAP proteins are the only endogenous proteins thus far identified that can regulate the activity of both initiator and effector caspases [62]. As such, IAP proteins have garnered attention lately, specifically as pharmacological targets in the development of therapeutic strategies in various cancers. In vivo, second mitochondria-derived activator of caspases, direct IAP-binding protein with low pI (Smac/DIABLO) is a mitochondrial protein. In the presence of apoptotic signals, Smac/DIABLO is cleaved and released into the cytoplasm, where it antagonizes the activity of IAP proteins [62]. Thus far, Smac/DIABLO appears to inhibit all IAP proteins except NAIP, and minimally requires the first four amino terminal residues of the cleaved cytoplasmic protein (Ala-Val-Pro-Ile) in order to inhibit IAP proteins and activate caspases [62]. Studies based on how this tetrapeptide of Smac/DIABLO recognizes and binds to IAP proteins have lead to the development of small molecules and prototypical drugs that would function analogously to cytoplasmic Smac/DIABLO [62]. Thus far, these compounds have shown very promising results in reducing tumor burden and prolonging survival time in numerous studies both in vitro and in vivo [62]. Indeed, several pharmaceutical and biotechnology companies have currently active programs developing Smac mimetics, with some compounds already in clinical trials [62]. While data from these studies are sparse, increasing interest in the NF-κB family and IAP members continue to reinforce our belief that these proteins are key targets with therapeutic potential in fighting apoptotic resistance in gliomas, and perhaps other types of neoplasia.
ACKNOWLEDGEMENTS
This work was supported in part by Public Service Grants CA-97247 from the National Cancer Institute (E. N. B. and L. B. N.), NS-50665 from the National Institute of Neurological Disorders and Stroke (E. N. B.), CA-13148-35 from the National Cancer Institute (E. N. B.), 5P30CA013149-38 from the UAB Comprehensive Cancer Center (E. N. B.), IRG-60-001-47 from the American Cancer Society (S. N.), CA-13148-31 from the National Cancer Institute (S. N.), and funding from the Southeastern Brain Tumor Foundation (S. N.).
We thank Dr. Xinbin Chen for providing the pBABE-HI-TetO plasmid, Dr. Tong Zhou for providing us antibodies specific for Bcl-2 and Bcl-xL, and Dr. G. Yancey Gillespie and the UAB Brain Tumor Tissue Core for providing us the brain tissue samples used herein. We also thank Maria G. Salazar of the CFAR/CCC DNA Sequencing Core at UAB for expert advice and technical assistance.
ABBREVIATIONS
- NF-κB
Nuclear factor-kappaB
- cIAP2
cellular inhibitor of apoptosis 2
- TNF-α
Tumor necrosis factor-alpha
- Bcl-2
B-cell leukemia/lymphoma-2
- RIP1
Receptor interacting protein 1
- TAD
transactivation domain
- IκBα
Inhibitor of NF-κB alpha
- IKK
IκB kinase
- CNS
central nervous system
- cIAP1
cellular inhibitor of apoptosis 1
- XIAP
X-linked inhibitor of apoptosis
- BRUCE
BIR-containing ubiquitin-conjugating enzyme
- CARD
caspase recruitment domain
- BIR
baculovirus IAP repeat
- TNFR1
TNF receptor 1
- ChIP
Chromatin immunoprecipitation
- Tet
Tetracycline
- Ub
Ubiquitin
- EGF
Epidermal growth factor
- EGFR
EGF Receptor
- PTEN
Phosphatase and tensin homologue on chromosome ten protein
- ING4
Inhibitor of growth 4
- GBM
Glioblastoma
- Smac
Second mitochondria-derived activator of caspases
- DIABLO
Direct IAP-binding protein with low pI
REFERENCES
- 1.Naugler WE, Karin M. NF-κB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meffert MK, Baltimore D. Physiological functions for brain NF-κB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Widera D, Kaus A, Kaltschmidt C, Kaltschmidt B. Neural stem cells, inflammation and NF-κB: basic principle of maintenance and repair or origin of brain tumours? J Cell Mol Med. 2008;12:459–470. doi: 10.1111/j.1582-4934.2007.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widera D, Mikenberg I, Kaltschmidt B, Kaltschmidt C. Potential role of NF-κB in adult neural stem cells: the underrated steersman? Int J Dev Neurosci. 2006;24:91–102. doi: 10.1016/j.ijdevneu.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore T. 2008 www.NF-κB.org. [Google Scholar]
- 6.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 7.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 8.Reifenberger G, Collins VP. Pathology and molecular genetics of astrocytic gliomas. J Mol Med. 2004;82:656–670. doi: 10.1007/s00109-004-0564-x. [DOI] [PubMed] [Google Scholar]
- 9.van den Bent MJ. Adjuvant treatment of high grade gliomas. Ann Oncol. 2006;17(Suppl 10):x186–x190. doi: 10.1093/annonc/mdl258. [DOI] [PubMed] [Google Scholar]
- 10.Schiff D. Temozolomide and radiation in low-grade and anaplastic gliomas: temoradiation. Cancer Invest. 2007;25:776–784. doi: 10.1080/07357900701509403. [DOI] [PubMed] [Google Scholar]
- 11.Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- 12.Nozell S, Laver T, Moseley D, Nowoslawski L, De Vos M, Atkinson GP, Harrison K, Nabors LB, Benveniste EN. The ING4 tumor suppressor attenuates NF-κB activity at the promoters of target genes. Mol Cell Biol. 2008;28:6632–6645. doi: 10.1128/MCB.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFκB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 14.Angileri FF, Aguennouz M, Conti A, La Torre D, Cardali S, Crupi R, Tomasello C, Germano A, Vita G, Tomasello F. NF-κB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112:2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 15.Korkolopoulou P, Levidou G, Saetta AA, El-Habr E, Eftichiadis C, Demenagas P, Thymara I, Xiromeritis K, Boviatsis E, Thomas-Tsagli E, Panayotidis I, Patsouris E. Expression of nuclear factor-κB in human astrocytomas: relation to pI kappa Bα, vascular endothelial growth factor, Cox-2, microvascular characteristics, and survival. Hum Pathol. 2008;39:1143–1152. doi: 10.1016/j.humpath.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Robe PA, Bentires-Alj M, Bonif M, Rogister B, Deprez M, Haddada H, Khac MT, Jolois O, Erkmen K, Merville MP, Black PM, Bours V. In vitro and in vitro activity of the nuclear factor-κB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res. 2004;10:5595–5603. doi: 10.1158/1078-0432.CCR-03-0392. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka G, Nagaya T, Saito K, Mizuno M, Yoshida J, Seo H. Inhibition of NF-κB activation confers sensitivity to tumor necrosis factor-alpha by impairment of cell cycle progression in human glioma cells. Cancer Res. 1999;59:4446–4452. [PubMed] [Google Scholar]
- 18.Moriuchi S, Glorioso JC, Maruno M, Izumoto S, Wolfe D, Huang S, Cohen JB, Yoshimine T. Combination gene therapy for glioblastoma involving herpes simplex virus vector-mediated codelivery of mutant IκBα HSV thymidine kinase. Cancer Gene Ther. 2005;12:487–496. doi: 10.1038/sj.cgt.7700816. [DOI] [PubMed] [Google Scholar]
- 19.Weaver KD, Yeyeodu S, Cusack JC, Jr, Baldwin AS, Jr, Ewend MG. Potentiation of chemotherapeutic agents following antagonism of NF-κB in human gliomas. J Neurooncol. 2003;61:187–196. doi: 10.1023/a:1022554824129. [DOI] [PubMed] [Google Scholar]
- 20.Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NF-κB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamini B, Yu X, Dolan ME, Wu MH, Kufe DW, Weichselbaum RR. Inhibition of NF-κB activity by temozolomide involves O6-methylguanine induced inhibition of p65 DNA binding. Cancer Res. 2007;67:6889–6898. doi: 10.1158/0008-5472.CAN-06-4496. [DOI] [PubMed] [Google Scholar]
- 22.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 23.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varfolomeev E, Vucic D. (Un)expected roles of c-IAPs in apoptotic and NFκB signaling pathways. Cell Cycle. 2008;7:1511–1521. doi: 10.4161/cc.7.11.5959. [DOI] [PubMed] [Google Scholar]
- 25.Cao L, Wang Z, Yang X, Xie L, Yu L. The evolution of BIR domain and its containing proteins. FEBS Lett. 2008;582:3817–3822. doi: 10.1016/j.febslet.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 26.Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 27.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-κB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002;22:724–736. doi: 10.1128/MCB.22.3.724-736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nozell S, Laver T, Patel K, Benveniste EN. Mechanism of IFN-beta-mediated inhibition of IL-8 gene expression in astroglioma cells. J Immunol. 2006;177:822–830. doi: 10.4049/jimmunol.177.2.822. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, Qin H, Benveniste EN. Transcriptional suppression of matrix metalloproteinase-9 gene expression by IFN-gamma and IFN-beta: critical role of STAT-1alpha. J Immunol. 2001;167:5150–5159. doi: 10.4049/jimmunol.167.9.5150. [DOI] [PubMed] [Google Scholar]
- 35.Nozell S, Chen X. p21B, a variant of p21(Waf1/Cip1), is induced by the p53 family. Oncogene. 2002;21:1285–1294. doi: 10.1038/sj.onc.1205191. [DOI] [PubMed] [Google Scholar]
- 36.Nozell S, Wu Y, McNaughton K, Liu G, Willis A, Paik JC, Chen X. Characterization of p73 functional domains necessary for transactivation and growth suppression. Oncogene. 2003;22:4333–4347. doi: 10.1038/sj.onc.1206470. [DOI] [PubMed] [Google Scholar]
- 37.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Edelstein LC, Gelinas C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen F, Demers LM, Vallyathan V, Lu Y, Castranova V, Shi X. Involvement of 5'-flanking κB-like sites within bcl-x gene in silica-induced Bcl-x expression. J Biol Chem. 1999;274:35591–35595. doi: 10.1074/jbc.274.50.35591. [DOI] [PubMed] [Google Scholar]
- 41.Tracey L, Perez-Rosado A, Artiga MJ, Camacho FI, Rodriguez A, Martinez N, Ruiz-Ballesteros E, Mollejo M, Martinez B, Cuadros M, Garcia JF, Lawler M, Piris MA. Expression of the NF-κB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206:123–134. doi: 10.1002/path.1768. [DOI] [PubMed] [Google Scholar]
- 42.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM, Olson JJ, Mikkelsen T, Lehman N, VandenBerg S, Berger M, Prados M, Muzny D, Morgan M, Scherer S, Sabo A, Nazareth L, Lewis L, Hall O, Zhu Y, Ren Y, Alvi O, Yao J, Hawes A, Jhangiani S, Fowler G, San Lucas A, Kovar C, Cree A, Dinh H, Santibanez J, Joshi V, Gonzalez-Garay ML, Miller CA, Milosavljevic A, Donehower L, Wheeler DA, Gibbs RA, Cibulskis K, Sougnez C, Fennel T, Mahan S, WIlkinson J, Ziaugra L, Onofrio R, Bloom T, Nicol R, Ardlie K, Baldwin J, Gabriel S, Lander ES, Ding L, Fulton RS, McLellan MD, Wallis J, Larson DE, Shi X, Abbot R, Fulton L, Chen K, Koboldt DC, Wendl MC, Meyer R, Tang Y, Lin L, Osborne JR, Dunford-Shore BH, Miner TL, Delehaunty K, Markovic C, Swift G, Courtney W, Phol C, Abbott S, Hawkins A, Leong S, Haipek C, Schmidt H, Wiechert M, Vickery T, Scott S, Dooling DJ, Chinwalla A, Weinstock GM, Mardis ER, Wilson RK, Getz G, Winckler W, Verhaak RGW, Lawrence MS, O'Kelly M, Robinson J, Alexe G, Beroukhim R, Carter S, Chiang D, Gould J, Gupta S, Korn J, Mermel C, Mesirov J, Monti S, Nguyen H, Parkin M, Reich M, Stransky N, Weir BA, Garraway L, Golub T, Meyerson M, Chin L, Protopopov A, Zhang J, Perna I, Aronson S, Sathiamoorthy N, Ren G, Yao J, Wiedemeyer WR, Kohane IS, Seidman J, Park PJ, Kucherlapati R, Laird PW, Cope L, Herman JG, Weisenberger DJ, Pan F, Van Den Berg D, Van Neste L, Yi JM, Schuebel KE, Baylin SB, Absher DM, Li JZ, Southwick A, Brady S, Aggarwal A, Chung T, Sherlock G, Brooks JD, Myers RM, Spellman PT, Purdom E, Jakkula LR, Lapuk AV, Marr H, Dorton SC, Choi YG, Han J, Ray A, Wang V, Durinck S, Robinson M, Wang NJ, Vranizan K, Peng V, Van Name E, Fontenay GV, Ngai J, Conboy JG, Parvin B, Feiler HS, Speed TP, Gray JW, Brennan C, Socci ND, Olshen A, Taylor BS, Lash A, Schultz N, Reva B, Antipin Y, Stukalov A, Gross B, Cerami E, Wang WQ, Qin L, Seshan VE, Villafania L, Cavatore M, Borsu L, Viale A, Gerald W, Sander C, Ladanyi M, Perou CM, Hayes DN, Topal MD, Hoadley KA, Qi Y, Balu S, Shi Y, Wu J, Penny R, Bittner M, Shelton T, Lenkiewicz E, Morris S, Beasley D, Sanders S, Kahn A, Sfeir R, Chen J, Nassau D, Feng L, Hickey E, Zhang J, Weinstein JN, Barker A, Gerhard DS, Vockley J, Compton C, Vaught J, Fielding P, Ferguson ML, Schaefer C, Madhavan S, Buetow KH, Collins F, Good P, Guyer M, Ozenberger B, Peterson J, Thomson E. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Cai L, Chen H, Zhang Q, Zhang S, Wang Y, Dong Y, Cheng H, Qi J. Inhibitor of growth 4 induces growth suppression and apoptosis in glioma U87MG. Pathobiology. 2009;76:181–192. doi: 10.1159/000218334. [DOI] [PubMed] [Google Scholar]
- 46.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45.41–45.13. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi S, Yamamoto M, Ueno Y, Ikeda K, Ohshima K, Soma G, Fukushima T. Expression of nuclear factor-κB, tumor necrosis factor receptor type 1, and c-Myc in human astrocytomas. Neurol Med Chir (Tokyo) 2001;41:187–195. doi: 10.2176/nmc.41.187. [DOI] [PubMed] [Google Scholar]
- 48.Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006;78:281–293. doi: 10.1007/s11060-005-9097-6. [DOI] [PubMed] [Google Scholar]
- 49.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 50.Sudheerkumar P, Shiras A, Das G, Jagtap JC, Prasad V, Shastry P. Independent activation of Akt and NF-κB pathways and their role in resistance to TNF-alpha mediated cytotoxicity in gliomas. Mol Carcinog. 2008;47:126–136. doi: 10.1002/mc.20372. [DOI] [PubMed] [Google Scholar]
- 51.Wilson J, Balkwill F. The role of cytokines in the epithelial cancer microenvironment. Semin Cancer Biol. 2002;12:113–120. doi: 10.1006/scbi.2001.0419. [DOI] [PubMed] [Google Scholar]
- 52.Atkinson GP, Nozell SE, Harrison DK, Stonecypher MS, Chen D, Benveniste EN. The prolyl isomerase Pin1 regulates the NF-κB signaling pathway and interleukin-8 expression in glioblastoma. Oncogene. 2009;28:3735–3745. doi: 10.1038/onc.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol. 2004;164:1727–1737. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNF-α-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Transfection with anti-p65 intrabody suppresses invasion and angiogenesis in glioma cells by blocking NF-κB transcriptional activity. Clin Cancer Res. 2007;13:2178–2190. doi: 10.1158/1078-0432.CCR-06-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuboi Y, Kurimoto M, Nagai S, Hayakawa Y, Kamiyama H, Hayashi N, Kitajima I, Endo S. Induction of autophagic cell death and radiosensitization by the pharmacological inhibition of nuclear factor-κB activation in human glioma cell lines. J Neurosurg. 2009;110:594–604. doi: 10.3171/2008.8.JNS17648. [DOI] [PubMed] [Google Scholar]
- 57.Kuwayama K, Matsuzaki K, Mizobuchi Y, Mure H, Kitazato KT, Kageji T, Nakao M, Nagahiro S. Promyelocytic leukemia protein induces apoptosis due to caspase-8 activation via the repression of NFκB activation in glioblastoma. Neuro Oncol. 2009;11:132–141. doi: 10.1215/15228517-2008-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng M, Morgan-Lappe SE, Yang J, Bockbrader KM, Pamarthy D, Thomas D, Fesik SW, Sun Y. Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008:7570–7578. doi: 10.1158/0008-5472.CAN-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma V, Tewari R, Sk UH, Joseph C, Sen E. Ebselen sensitizes glioblastoma cells to Tumor Necrosis Factor (TNFalpha)-induced apoptosis through two distinct pathways involving NF-κB downregulation and Fas-mediated formation of death inducing signaling complex. Int J Cancer. 2008;123:2204–2212. doi: 10.1002/ijc.23771. [DOI] [PubMed] [Google Scholar]
- 60.Jiang W, Cazacu S, Xiang C, Zenklusen JC, Fine HA, Berens M, Armstrong B, Brodie C, Mikkelsen T. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-κB signaling pathway. Neoplasia. 2008;10:235–243. doi: 10.1593/neo.07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ichiyama T, Nishikawa M, Lipton JM, Matsubara T, Takashi H, Furukawa S. Thiopental inhibits NF-κB activation in human glioma cells and experimental brain inflammation. Brain Res. 2001;911:56–61. doi: 10.1016/s0006-8993(01)02672-5. [DOI] [PubMed] [Google Scholar]
- 62.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]