SUMMARY
Type 2 diabetes is characterized by insulin resistance and pancreatic β cell dysfunction, the latter possibly caused by a defect in insulin signaling in β cells. Inhibition of class IA phosphatidylinositol 3-kinase (PI3K), using a mouse model lacking the pik3r1 gene specifically in β cells and the pik3r2 gene systemically (βDKO mouse), results in glucose intolerance and reduced insulin secretion in response to glucose. β cells of βDKO mice had defective exocytosis machinery due to decreased expression of soluble N-ethylmaleimide attachment protein receptor (SNARE) complex proteins and loss of cell-cell synchronization in terms of Ca2+ influx. These defects were normalized by expression of a constitutively active form of Akt in the islets of βDKO mice, preserving insulin secretion in response to glucose. The class IA PI3K pathway in β cells in vivo is important in the regulation of insulin secretion and may be a therapeutic target for type 2 diabetes.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is characterized by peripheral insulin resistance and β cell dysfunction (Kahn et al., 2006). Previous studies have suggested that the latter can develop by impairing insulin signaling in β cells. Indeed, it has been shown that β cell-specific insulin receptor (IR) knockout (βIRKO) or insulin-like growth factor-1 receptor (IGFR) knockout (βIGFRKO) mice demonstrate a defect in glucose-stimulated insulin secretion (GSIS), especially in the early phase, with decreased β cell mass only in βIRKO mice (Kulkarni et al., 1999, 2002). Double knockout of IR and IGFR in β cells results in severe diabetes by hypoinsulinemia with a marked β cell loss and a defect in insulin secretion (Ueki et al., 2006). Insulin receptor substrate 2 (IRS2), one of the substrates for IR and IGFR, mainly mediates the signal to regulate β cell mass (Kubota et al., 2004; Lin et al., 2004). Furthermore, phosphatidylinositol-dependent kinase 1 (PDK1) and its downstream Akt are important in the regulation of β cell growth and function (Bernal-Mizrachi et al., 2001; Hashimoto et al., 2006; Tuttle et al., 2001). Class IA phosphatidylinositol 3-kinase (PI3K) is activated by growth factor receptors and plays a crucial role in growth and metabolism (Engelman et al., 2006; Vanhaesebroeck et al., 2010). It is likely that class IA PI3K downstream of the IR(IGFR)/IRS proteins transmits the signal to regulate β cell function and mass through the PDK1/Akt signaling pathway, although the mechanism remains poorly understood.
The class IA PI3K molecule comprises a regulatory and a catalytic subunit, with the holoenzyme catalytically producing phosphatidylinositol (3,4,5)-trisphosphate (PIP3) thereby transmitting the signals for metabolism, proliferation, and antiapoptosis. The regulatory subunits per se have dual roles in these signals: (1) a positive role stabilizing the catalytic subunit and bridging between the catalytic subunit and phosphotyrosyl proteins (Yu et al., 1998) and (2) a negative role interfering with binding of the holoenzyme to phosphotyrosyl proteins as a monomer and transmitting negative signals to activate Akt by an unexplored mechanism (Taniguchi et al., 2006c, 2007; Ueki et al., 2003). The regulatory subunits are derived from three different genes, pik3r1, pik3r2, and pik3r3, which result in multiple isoforms of the regulatory subunits, such as p85α, its splicing variants, p55α and p50α, and p85β. Among the regulatory subunits, p85α represents over 70% of the total, with a further 20% represented by p85β (Ueki et al., 2002a). Thus, these two molecules are the major regulatory subunits of class IA PI3K.
The deletion of regulatory subunit(s) leads to improved insulin sensitivity (Fruman et al., 2000; Terauchi et al., 1999). This is presumably because of a reduction in the negative effects of the regulatory subunits on the downstream signals. In contrast, tissue-specific knockout of pik3r1 on the whole-body knockout of pik3r2 was expected to almost completely shut down the actions mediated by PI3K and to be a suitable system for evaluating the role of class IA PI3K in specific tissues. The important roles of class IA PI3Ks in heart, skeletal muscle, liver, and proopiomelanocortin (POMC) neurons have been revealed by researchers using this method (Hill et al., 2008; Luo et al., 2005; Taniguchi et al., 2006b).
This study has explored the function of class IA PI3Ks in the regulation of insulin secretion in β cells by using β cell-specific knockout mice. Together with the findings using diabetic db/db mice, it is likely that decreased class IA PI3K activity in β cells contributes to the impaired β cell function in diabetes, and enhancing this pathway would be a therapeutic strategy for diabetes.
RESULTS
Insulin Signaling in db/db Islets
In humans, insulin secretion has been suggested to be transiently increased during insulin-resistant prediabetic state but progressively decline once diabetes develops (Rhodes, 2005). Similarly, it is known that in the db/db mouse, an obese and diabetic animal model, insulin secretion and β cell mass are transiently increased against severe peripheral insulin resistance induced by obesity, but thereafter β cells progressively fail to compensate, leading to marked hyperglycemia (Hummel et al., 1972; Lee et al., 1996). Indeed, circulating insulin levels gradually declined as the mice aged, despite severe obesity and hyperglycemia (Figures 1A–1C). Interestingly, expression of insulin/IGF-1 signaling molecules, including class IA PI3K subunits, except irs1, was transiently increased in db/db islets during the markedly hyperinsulinemic period, then declined along with insulin secretion (Figures 1C and 1D). This is consistent with the previous findings that irs2, but not irs1, plays a crucial role in compensatory β cell hyperplasia in response to peripheral insulin resistance (Araki et al., 1994; Kubota et al., 2000; Tamemoto et al., 1994). We hypothesized, based on these data, that class IA PI3K would play a pivotal role in the regulation of insulin secretion downstream of IR(IGFR)/IRS2.
Figure 1. Decreased Expressions of Insulin Signaling Molecules in db/db Mice in Accordance with Age-Dependent Attenuated Insulin Secretion.

(A–C) Profiles of body weight (A), blood glucose levels (B), and plasma insulin concentrations (C) in db/db and +/+ mice at 6, 10, and 14 weeks of age (n = 5–6).
(D) mRNA levels of insulin signaling molecules. Expressions of the indicated genes were determined by quantitative RT-PCR of total RNA isolated from islets of db/db and +/+ mice at 6, 10, 14, and 18 weeks of age (n = 4–6). Data are presented as the means ± SEM.
Loss of PI3K Subunits Led to Impaired PI3K Signaling
To inhibit PI3K signaling in β cells, we generated β cell-specific pik3r1 knockout (βPik3r1KO) mice using rat insulin promoter-Cre transgenic mice (RIP-Cre mice) and mice homozygous for pik3r1 floxed alleles (Flox mice), as described in the Experimental Procedures. However, pik3r1 deletion alone has been reported to activate downstream of PI3K signaling in the liver (Taniguchi et al., 2006b). Thus, to almost completely shut down PI3K signaling, we also generated mice lacking both pik3r1 and pik3r2 genes in β cells with loss of the pik3r2 gene alone in other tissues (βDKO mice) using the systemic pik3r2 knockout mice (Pik3r2KO mice) and βPik3r1KO mice as described in the Experimental Procedures. We assessed the role of PI3K in β cells by comparing between βPik3r1KO mice and the controls (RIP-Cre mice or Flox mice) or between βDKO mice and the controls (Pik3r2KO mice or Flox mice).
To confirm the deletion of class IA PI3K, we performed immunostaining with a specific antibody for p85α or the antibody for all regulatory subunits (pan-p85) and found that all regulatory subunits were diminished in most of the islet cells of both βPik3r1KO and βDKO mice, and to a greater degree in βDKO mice (Figures 2A and 2B). Western blot analysis also revealed that the intensity of bands corresponding to p85α and p85β were significantly decreased in islets of both βPik3r1KO and βDKO mice, although an increased ratio of non-β to β cells in the islets of βDKO mice appeared to mask further reduction in the band intensity owing to the lack of p85β (Figure 2C). Due to a leak in Cre expression (Kubota et al., 2004), p85α expression was decreased in the hypothalami of βPik3r1KO and βDKO mice (Figure 2C), although this did not affect the body weight, food intake, or insulin sensitivity in either group (Figures 3A, 3D, and S1).
Figure 2. Pancreatic β Cell-Specific Deletion of the pik3r1 Gene and Inhibition of PI3K Signaling in β Cells in βPik3r1KO and βDKO Mice.

(A and B) Immunohistochemical analysis using pancreas sections from 8-week-old male indicated genotypes of mice. Staining by p85α-specific antibody using pancreas sections of RIP-Cre and βPik3r1KO mice is shown in (A) (scale bar = 100 μm). Staining by pan-p85 (p85α and p85β) antibody using DAB substrate on pancreas sections of Flox, βPik3r1KO, Pik3r2KO, and βDKO mice is shown in (B) (scale bar = 100 μm).
(C) Protein expression of p85 regulatory subunits in islets, liver, skeletal muscle, and hypothalami in 8-week-old male mice assessed by western blotting.
(D) Decreased PI3K activity detected by accumulation of PIP3 in response to glucose stimulation. Staining for PIP3 using pancreatic sections of Flox, RIP-Cre, and βPik3r1KO mice is shown (scale bar = 100 μm).
(E and F) Subcellular localization of FoxO1 in pancreatic β cell. Immunofluorescence staining for FoxO1 is shown (E) (scale bar = 50 μm). Quantified analysis FoxO1 intensity in the nucleus of pancreatic β cells of Flox, βPik3r1KO, Pik3r2KO, and βDKO mice is also shown (F). FoxO1 intensity in nuclei was normalized by FoxO1 intensity in total islet. Data are presented as the means ± SEM.
Figure 3. Metabolic Features of βPik3r1KO and βDKO Mice.
(A) Profiles of body weight in male βPik3r1KO and βDKO mice with their controls at the indicated ages (n = 9–11).
(B and C) Blood glucose levels after an i.p. injection of glucose (1.5 g/kg BW) in 8-week-old male RIP-Cre, Flox, βPik3r1KO (B) (n = 9–11), Pik3r2KO, and βDKO (C) (n = 10) mice with their controls.
(D) Insulin tolerance test (0.75 U/kg BW) in 8-week-old male Flox, βPik3r1KO, Pik3r2KO, and βDKO mice with their controls (n = 9–11).
(E) Serum insulin concentrations after i.p. injection of glucose (3 g/kg BW) in 12-week-old male Flox, βPik3r1KO, Pik3r2KO, and βDKO mice (n = 5–8).
(F) Static incubation study of islets from 8-week-old Flox, βPik3r1KO, Pik3r2KO, and βDKO mice. Results are shown as an insulin secretion ratio to total insulin content (n = 5–10). Data are presented as the means ± SEM.
To assess how the deletion of pik3r1 affected the downstream events of PI3K, we examined the production of PIP3 and the localization of Forkhead box O1 (FoxO1) in β cells by immunostaining. Glucose stimulation resulted in marked impairment of PIP3 production in the islets of βPik3r1KO mouse compared to the controls (Figure 2D). As a consequence, FoxO1 was localized in the nucleus of β cells of βPik3r1KO and βDKO mice, as shown by quantitative analysis of FoxO1 staining (Figures 2E and 2F). These data suggest that in β cells, the loss of p85α diminished PI3K-dependent signaling and that the disruption of all the major regulatory subunits further shut down the signal in β cells.
βPik3r1KO and βDKO Mice Exhibited Glucose Intolerance
Both βPik3r1KO and βDKO mice grew normally (Figure 3A), and all groups displayed normoglycemia in the ad libitum feeding state (Figure S2A), with similar levels of plasma insulin concentration (Figure S2B).
To investigate metabolic features, we performed glucose tolerance tests (GTTs) using 8-week-old male mice. There are some reports that RIP-Cre mice have glucose intolerance due to toxicity of the Cre expression (Lee et al., 2006). Therefore, RIP-Cre mice as well as Flox mice were used as controls for βPik3r1KO mice. βPik3r1KO mice exhibited glucose intolerance compared to the controls with no difference in glucose levels during GTT between RIP-Cre and Flox mice (Figure 3B). Thus, Flox mice were hereafter used as controls for βPik3r1KO mice. We also found that βDKO mice appeared to show more prominent glucose intolerance than that seen in βPik3r1KO mice, even though pik3r2 systemic knockout mice exhibited an insulin-sensitive phenotype (Figure 3C) (Ueki et al., 2002b). Similar glucose intolerance in βPik3r1KO and βDKO mice was continuously observed at 12 and 16 weeks of age (data not shown). Insulin levels of βPik3r1KO and βDKO mice were significantly decreased during GTT compared to those of controls (Figure S2C). Insulin tolerance tests (ITTs) were also performed on 8-week-old male βPik3r1KO and βDKO mice, with no difference in insulin sensitivity found compared to the controls (Figure 3D). These data suggested that deletion of the pik3r1 gene in β cells caused glucose intolerance due to a defect in insulin secretion.
A GSIS test in vivo using 12-week-old male βPik3r1KO and βDKO mice revealed decreased GSIS in these mice compared to the controls (Figure 3E). In particular, early-phase insulin secretion was significantly impaired in βDKO mice (Figure 3E). Consistent with the in vivo findings, both 8-week-old βPik3r1KO and βDKO islets exhibited significantly decreased insulin secretion upon higher concentration of glucose or 50 mM KCl stimulation (Figure 3F). Pancreas perfusion tests were also performed on 12-week-old male βPik3r1KO and βDKO mice; results indicated attenuated GSIS in both groups (Figure S2D). These data suggest that both βPik3r1KO and βDKO mice exhibit impaired GSIS, with a greater degree in βDKO mice, and at least one of the defects is located at a step downstream of Ca2+ influx.
Deletion of Class IA PI3K Decreased β Cell Mass
Because disruption of IR/IGFR signaling in β cells induced progressive and robust β cell loss with increased apoptosis and decreased proliferation (Ueki et al., 2006), we examined the effect of class IA PI3K deletion, one of the signal transducers downstream of IR/IGFR, on islet mass. Immunofluorescence analysis revealed that the morphology of islets was generally maintained in βPik3r1KO and βDKO mice compared to the controls, with a slight increase in non-β cells in βDKO islets (Figure 4A), and that the ratio of islet area to total pancreas did not differ among all genotypes at 8 weeks of age (Figure 4B). However, this ratio was decreased in βPik3r1KO and βDKO mice at 32 weeks of age (Figures 4C and S3). Previous studies showed that in pancreatic β cells, insulin signaling is indispensable for antiapoptosis (Ueki et al., 2006). We found significantly increased TUNEL-positive β cells of βPik3r1KO and βDKO mice at 8 weeks of age (Figure 4D), when a reduction in the islet area was not evident in either group. Surprisingly, however, the number of bromodeoxyuridine (BrdU)-positive β cells was significantly increased in βPik3r1KO and βDKO mice at the same age (Figure 4E). Insulin/IGF-1 signaling mediates the signals via two major downstream pathways, the PI3K/Akt and Ras/Erk pathways (Taniguchi et al., 2006a). The PI3K/Akt pathway mediates a variety of insulin's actions, such as glucose metabolism and antiapoptosis, while the Ras/Erk pathway mainly regulates cell proliferation, partly in cooperation with the PI3K/Akt pathway (Burns et al., 2000). In the islets of βPik3r1KO and βDKO mice where PI3K/Akt signaling was blunted, Erk1/2 phosphorylation was markedly enhanced compared to that seen in the controls (Figures 4F and 4G). This upregulation of the Erk pathway, which activates cell proliferation, may compensate for the increased apoptosis by suppressing PI3K and hence may maintain the β cell mass of younger βPik3r1KO and βDKO mice.
Figure 4. Alterations in β Cell Mass, Apoptosis, and Cell Proliferation in Pancreas with Upregulated Phosphorylation of Erk1/2 in βPik3r1KO and βDKO Islets.
(A) Staining with antibodies to insulin (green) and glucagon (red) and nuclear stain DAPI using pancreatic sections from 8-week-old male mice of indicated genotypes (magnification: 20×).
(B) Histological analysis of pancreatic β cell area in Flox, βPik3r1KO, Pik3r2KO, and βDKO mice at 8 weeks of age.
(C) Histological analysis of pancreatic β cell area in Flox, βPik3r1KO, Pik3r2KO, and βDKO mice at 32 weeks of age.
(D) TUNEL staining of pancreatic sections at 8 weeks of age.
(E) BrdU incorporation into β cell nuclei of 8-week-old male Flox, βPik3r1KO, Pik3r2KO, and βDKO mice.
(F) Western blotting by phospho-Erk1/2 and total Erk in islets isolated from each genotype at 8 weeks of age.
(G) Quantified by densitometry of the western blotting. Data are presented as the means ± SEM.
βDKO Mice Showed Dysfunctions in Insulin Secretion
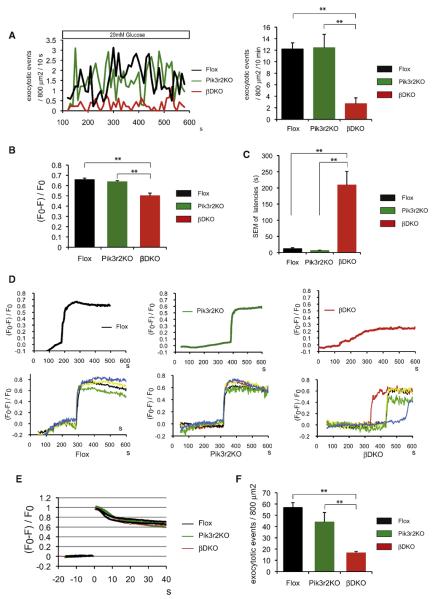
To explore the mechanism underlying the impaired GSIS by PI3K deletion, we directly analyzed the islets by two-photon excitation imaging, which allowed us to observe exocytotic events in the insulin granule and Ca2+ influx in intact islets in 8-week-old male βDKO mice, which appeared to have more profound defects than did the βPik3r1KO mice (Takahashi et al., 2002).
The number of exocytotic events associated with high glucose stimulation was significantly decreased in βDKO islets within 10 min (Figure 5A). Levels of Ca2+ influx in individual β cells were also assessed using the Ca2+ indicator, fura-2. In β cells of βDKO mice, the levels of Ca2+ influx upon glucose stimulation were significantly decreased compared to those of Flox or Pik3r2KO mice (Figure 5B), suggesting a defect between the glucose transport and Ca2+ influx steps.
Figure 5. Glucose-Stimulated Exocytotic Events Estimated by Two-Photon Microscopy Imaging.
(A) Left: Glucose-stimulated exocytotic events averaged for 5–6 islets in each genotype, normalized by an arbitrary area (800 mm2) of islets. Right: Glucose-stimulated exocytotic events in 10 min.
(B) Glucose-stimulated Ca2+ influx in single β cell measured by Ca2+ indicator fura-2. Fura-2 fluorescence (F) was normalized by the resting fluorescence (F0). F0 and F stand for resting and poststimulation fluorescence, respectively.
(C and D) Defect of cell-cell synchronization in βDKO islets (n = 5–6) estimated by two-photon microscopy imaging. Data were acquired from islets from 8-week-old male Flox, Pik3r2KO, and βDKO mice. SEM of the Ca2+ influx latencies of all cells in an islet were measured in Flox, Pik3r2KO, and βDKO mice (C). Each trace showed the alterations of the Ca2+ concentrations in a whole islet of indicated genotype (D, upper). The alterations of the cytosolic Ca2+ concentrations of single β cell from one islet of Flox, Pik3r2KO, and βDKO mice are shown in the lower panel of (D). Each trace showed the alteration of the cytosolic Ca2+ concentrations recorded from single β cell in one islet.
(E and F) Exocytotic events triggered by photolysis of a caged-Ca2+ compound. Alteration of cytosolic Ca2+ concentrations provoked by caged-Ca2+ stimulation is shown in (E). Each trace showed the cytosolic Ca2+ concentration recorded from a single islet. Exocytotic events upon caged-Ca2+ stimulation, normalized by an arbitrary area (800 mm2) of islets, are shown in (F). Data are presented as the means ± SEM.
To explore the defect responsible for impaired glucose-induced Ca2+ influx, we conducted perforated whole-cell clamp experiments. The results showed that conductance densities of ATP-sensitive K+ (KATP) channels, resting membrane potentials, and latencies of KATP channel responsiveness to glucose were not different between Pik3r2KO and βDKO at the single β cell level (Figures S4A–S4D). At the whole-islet level, ATP production in response to glucose was slightly, but not significantly, decreased in βDKO mice, with only modest reductions in some of the mitochondria-related gene expressions (Figures S4E and S4F).
On the other hand, we found that both in Flox and in Pik3r2KO islets, Ca2+ influx of almost all the β cells occurred simultaneously (Figures 5C and 5D and Movies S1 and S2). In contrast, in βDKO islets, β cells lost synchronicity in secreting insulin in response to an intracellular Ca2+ rise, and individual β cells secreted insulin after various latencies (Figures 5C and 5D and Movie S3). As a consequence, total Ca2+ influx in the whole islet exhibited a blunted curve in βDKO islets (Figure 5D, upper panel), similar to that of βIRKO islets (Otani et al., 2004).
Insulin exocytosis was investigated when triggered with a large and rapid increase in Ca2+ influx induced by photolysis of a caged-Ca2+ compound, which was artificial but allowed us to determine a defect downstream of Ca2+ influx (Takahashi et al., 2004). Even though the same levels of Ca2+ influx were provoked (Figure 5E), the exocytosis events were markedly reduced in βDKO islets (Figure 5F), suggesting that βDKO mice also have a defect downstream of the Ca2+ influx in their exocytosis machinery. Taken together, these results suggest that defects in exocytosis machinery and cell-cell synchronization may be causally involved, at least partly, in the impaired GSIS in βDKO islets.
β Cell Functions Were Regulated by PI3K Signaling
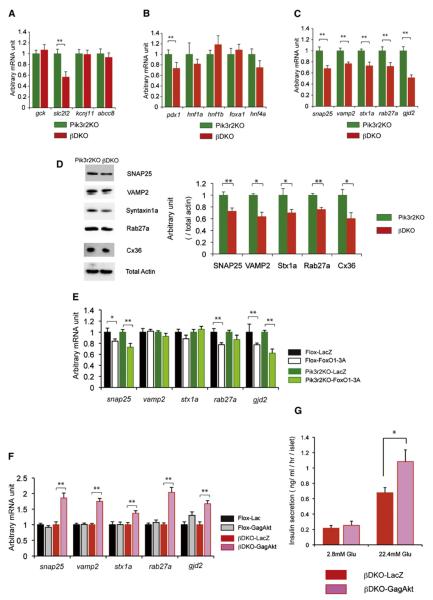
To explore the molecular mechanism causing the impaired GSIS associated with deletion of class IA PI3K, we extensively examined the expression levels of genes regulating β cell functions by quantitative real-time PCR in 8-week-old male βDKO mice and their controls. Deletion of class IA PI3K did not affect the expression of gck (Gck), kcnj11 (Kir6.2), or abcc8 (Sur1) (Figure 6A). In contrast, the expression of slc2l2 (Glut2) was significantly reduced in βDKO mice (Figure 6A), although it is unclear whether this contributes to the decreased level of Ca2+ influx in βDKO mice. The expression level of pdx1 was reduced in βDKO mice compared to that seen in the controls (Figure 6B), consistent with the increase in nuclear FoxO1. Exocytosis from the insulin granules of β cells is largely dependent on soluble N-ethylmaleimide attachment protein receptor (SNARE) complex proteins, including SNAP25, VAMP2, Syntaxin 1a, and Rab27a (Kasai et al., 2005; Nagamatsu et al., 1999; Takahashi et al., 2004). We found that the cluster of SNARE complex genes was decreased significantly, as estimated by mRNA and protein levels in βDKO mice (Figures 6C and 6D). The reduced expression of these genes may account for an aggravated alteration in exocytosis and vesicular movement in class IA PI3K-deficient β cells. On the other hand, pancreatic β cells communicate with each other thorough gap-junction channels, which are composed of Connexin36 protein hexamer (Nlend et al., 2006). Indeed, deletion of gjd2 encoding Connexin36 results in loss of synchronization in Ca2+ oscillations and impaired GSIS (Ravier et al., 2005). Consistent with these findings, the mRNA and protein expressions of gjd2 in βDKO islets were significantly decreased compared to the controls (Figures 6C and 6D). Similar changes in expressions of these genes were also observed in βPik3r1KO islets (data not shown).
Figure 6. Analysis of Gene Expressions in the Islets of 8-Week-Old Male Pik3r2KO and βDKO Mice and Effects of Constitutively Active FoxO1 (FoxO1-3A) or Akt (GagAkt) on the Gene Expression and Insulin Secretion.
(A) The expression levels of gck, kcnj11, abcc8, and slc2l2 in Pik3r2KO and βDKO islets (n = 5–6).
(B) The expression levels of mature onset diabetes of the young (MODY) genes in Pik3r2KO and βDKO islets.
(C) The expression levels of SNARE complex genes, snap25, vamp2, stx1a, rab27a, and gjd2.
(D) Protein expression of SNARE complex and Connexin36 in Pik3r2KO and βDKO islets assessed by western blotting (n = 4).
(E) Effects of constitutively active FoxO1 (FoxO1-3A) in the islet isolated from 8-week-old male Pik3r2KO mice on the mRNA levels of the indicated genes.
(F) Effects of constitutively active Akt (GagAkt) in the islet isolated from 8-week-old male βDKO mice on the mRNA levels of the indicated genes.
(G) Effects of constitutively active Akt (GagAkt) in the islets isolated from 8-week-old male βDKO mice on glucose-stimulated insulin secretion estimated by the static incubation. Data are presented as the means ± SEM.
To assess whether the loss of PI3K signaling in β cells directly affected the reduced expression in SNARE complex genes and gjd2, we conducted in silico analyses of the promoter regions of these genes and found that they all have potential FoxO1 binding sites. Given that FoxO1 is preferentially localized in the nucleus in the β cells of βDKO mice due to the blunted Akt activity, it is possible that FoxO1 directly binds to the promoters of these genes, suppressing their expression. The forced activation of Akt would exclude FoxO1 from the nucleus and restore the expression of these genes. To assess this possibility, we expressed a constitutively active form of FoxO1 (FoxO1-3A) adenovirus in 8- to 10-week-old islets. Indeed, expression of FoxO1-3A resulted in a significant reduction in expression of gjd2 and snap25, of which putative FoxO1 binding sites are conserved across species, human, mouse, and rat (Figure 6E). In contrast, the expression of a constitutively active form of Akt (GagAkt) in the isolated islets of 8- to 10-week-old male βDKO mice significantly increased the expression of all SNARE complex genes and gjd2 compared to lacZ infection (Figure 6F). Moreover, the expression of GagAkt in the isolated islets of 8- to 10-week-old male βDKO mice significantly augmented GSIS in βDKO islets (Figure 6G).
Impaired Exocytosis and Synchronization in db/db
To evaluate the contribution of these mechanisms to obesity-induced diabetes, we also observed the db/db islets by using two-photon microscopy. In the isolated islets of 10-week-old male db/db mice, exocytosis was found to decrease after glucose stimulation (Figure 7A) and caged-Ca2+ stimulation (Figure 7B). Moreover, a defect in cell-cell synchronization in terms of Ca2+ influx similar to that seen in βDKO islets was observed (Figures 7C and 7D and Movie S4). Furthermore, the expression levels of gjd2 and SNARE complex genes in db/db islets progressively declined to much lower levels compared to the age-matched controls (Figure 7D). This suggested that PI3K/Akt signaling may have been downregulated in the islets of these mice. Indeed, immunohistochemistry using phospho-Akt antibody revealed that Akt activity was almost abolished in the islets of db/db mice at 10 weeks of age (Figure 7E).
Figure 7. Analyses of Exocytotic Events of the Islets of db/db Mice by the Two-Photon Microscopy Imaging and Gene Expressions.
(A) Left: analysis of glucose-stimulated exocytotic events averaged for five islets, normalized by an arbitrary area (800 mm2) of islets. Right: measurements of exocytotic events in 10 min.
(B) Exocytotic events upon caged-Ca2+ stimulation, normalized by an arbitrary area (800 mm2) of islets.
(C) SEM of the Ca2+ influx latencies upon glucose stimulation (n = 5).
(D) Upper: Each trace showed the alterations of the Ca2+ concentrations in a whole islet. Lower: Representative recordings from two-photon microscopy imaging show defects of cell-cell synchronization in db/db islets (n = 5–7). Each trace showed the changes of the cytosolic Ca2+ concentration of a single cell from one islet of +/+ mice and db/db mice.
(E) mRNA expressions of gjd2 and SNARE complex genes estimated by quantitative real-time PCR in the islets of db/db and +/+ mice at 6, 10, and 14 weeks of age (n = 5–6).
(F) Immunohistochemical analysis of pancreas sections from 10-week-old db/db and +/+ mice; staining for phosphorylation of Akt in the db/db islets. Data are presented as the means ± SEM.
DISCUSSION
In T2DM patients, insulin signaling, especially PI3K/Akt signaling, is known to be diminished in muscle and liver owing to insulin resistance and/or decreased circulating insulin, leading to a reduction in glucose metabolism and subsequent hyperglycemia (Bandyopadhyay et al., 2005). We and others have previously shown that β cells also possess insulin signaling systems that play a critical role in the regulation of β cell mass and function (Kulkarni et al., 1999, 2002; Leibiger et al., 2001, 2008; Ueki et al., 2006). It is possible that decreased insulin signaling in β cells, resulting from either insulin resistance or decreased insulin secretion in these cells, may cause loss of β cell function and mass, leading to hypoinsulinemia and subsequent hyperglycemia.
We asked whether insulin signaling in the β cell in db/db mice, an obese diabetic animal model, would play a role in the compensatory hyperinsulinemia in response to insulin resistance and the exacerbated hyperglycemia seen after decompensation. Indeed, as db/db mice grow older and obese, hyperglycemia and defects in insulin secretion become evident, and phosphorylated Akt levels significantly decrease in β cells, indicating impaired insulin signaling within these cells. In accordance with the age-dependent impairment of insulin secretion and decreased β cell mass, expressions of insulin signaling molecules, such as class IA PI3K, decline. In addition to the reductions in expression of these molecules, posttranslational modulation of signaling molecules by increased oxidative stress, ER stress, and inflammation may further attenuate insulin signaling (Evans et al., 2003; Hotamisligil, 2006). These results suggest the possibility that insulin signaling in β cells plays a crucial role in the maintenance of β cell mass and functions in response to insulin resistance through the class IA PI3K pathway. Thus, we have tried to explore the roles of class IA PI3K in maintaining β cell functions and mass using the mice lacking all the major regulatory subunits of class IA PI3K specifically in β cells.
In β cells, unlike in hepatocytes, deletion of pik3r1 markedly suppresses downstream signaling, such as Akt activation, and results in impaired glucose tolerance with a defect in GSIS. Double knockout of pik3r1 and pik3r2 in β cells (βDKO), which further shuts down the downstream signaling, causes an even greater defect in GSIS and decreased β cell mass. These defects are caused by severely impaired insulin secretion and a robust increase in apoptosis. Interestingly, βDKO mice do not develop overt diabetes, unlike insulin/IGF-1 receptor double knockout mice in which both PI3K and the Erk pathways are suppressed, even considering the fact that pik3r2 null mice exhibit a modest insulin-sensitive phenotype (Ueki et al., 2002b, 2006).
The PI3K/Akt pathway can negatively affect IR/IGFR signaling via multiple mechanisms, such as serine phosphorylation of IRS1 and Raf (Um et al., 2004). Abrogation of this signal may enhance tyrosine phosphorylation of IRS proteins and reduce serine phosphorylation of Raf, ultimately increasing the signaling to the Raf/Erk pathway. Indeed, βDKO islets exhibit significantly increased phosphorylation of Erk1/2 as well as increased BrdU incorporation. Thus, βDKO mice can preserve β cell mass presumably at a level necessary to maintain normoglycemia, despite a marked increase in apoptosis and impaired insulin secretion.
The current study has revealed that class IA PI3K plays a crucial role in maintaining β cell function through regulating cell-cell synchronization associated with Ca2+ influx and exocytosis machinery. Previous studies have reported that gap-junction channels composed of Connexin36 hexamer regulate the cell-cell synchronization in β cells through direct exchanges of metabolites and ions and that these communications are important for proper GSIS and oscillations (Nlend et al., 2006; Ravier et al., 2005). In βDKO islets, the expression of gjd2 is significantly reduced. Moreover, db/db islets also show impaired cell-cell synchronization similar to that seen in the βDKO islets, with decreased expression of gjd2 in accordance with decreased insulin secretion. Thus, the impaired Ca2+ influx can be accounted for, at least in part, by the impaired cell-cell synchronization.
On the other hand, ATP production and the expressions of slc2l2 and mitochondria-related genes are slightly decreased in βDKO islets, consistent with the recent report that the expression of slc2l2 and mitochondrial functions are regulated by insulin signaling in β cells (Assmann et al., 2009; Liu et al., 2009). Although electrophysiological experiments fail to indicate significant impairment of the steps upstream of Ca2+ influx, at least at the single β cell level, these defects might also contribute to the impairment of GSIS by deletion of class IA PI3K. Meanwhile, the mRNA expression of gck shows no reduction in βDKO islets, although gck expression is reduced both in βIRKO and in βIGFRKO islets. Recently, it has been reported that class II PI3K (PI3K-C2α) may play an important role in GSIS in pancreatic β cells downstream of the IR (Leibiger et al., 2010). In this report, impairment of class II PI3K signaling has been shown to result in the reduction of gck expression. Thus, the expression of gck might be regulated through class II PI3K signaling, not through class IA PI3K signaling downstream of IR.
Insulin secretion occurs to a much lesser extent in βDKO islets compared to Pik3r2KO islets, even when using caged-Ca2+ stimulation, suggesting another defect downstream of Ca2+ influx, such as in the exocytosis machinery (Takahashi et al., 2004). Indeed, expressions of SNARE complex genes are markedly reduced in the islets of βDKO mice, and these reductions can contribute to defects in insulin secretion downstream of Ca2+ channels.
Are these insulin secretion defects directly caused by ablating PI3K signaling? Expression of FoxO1-3A in the Pik3r2KO islets reduces the expression of snap25 and gjd2, both of which have the putative FoxO1 binding sites conserved across species in their promoter region, suggesting that FoxO1 binds to the promoter regions and inhibits the transcription of these genes. Thus, activation of PI3K/Akt appears to promote nuclear excursion of FoxO1, thereby increasing the expression levels of these genes. Indeed, expression of GagAkt normalizes the expression of these genes with other SNARE complex genes, such as vamp2, stx1a, and rab27a. These data also suggest that other transcription factor(s) may regulate the expression of other SNARE complex genes through Akt activation, independently of FoxO1. Importantly, restoration of the expression of these genes by Akt activation almost completely normalizes GSIS in vitro.
T2DM patients show decreased islet mRNA and protein expressions of SNAP25, Syntaxin 1a, and VAMP2 compared to nondiabetic subjects (Ostenson et al., 2006). Moreover, Connexin36 is also expressed in human islets, and it has been suggested that this may play a role in insulin secretion (Serre-Beinier et al., 2009). Taken together, these findings suggest that these SNARE complex proteins and Connexin36, whose expression is regulated by the PI3K/Akt pathway downstream of insulin signaling, may control the function of the human β cell. In addition, downregulation of these proteins by impaired PI3K/Akt activity may play a crucial role in the deteriorated GSIS, one of the characteristics of T2DM.
Similar to db/db mice in the current study, human obese T2DM patients show hyperinsulinemia with a defect in early-phase GSIS at the early stage of T2DM and then develop severe diabetes with hypoinsulinemia and reduced β cell mass in the advanced stage (Butler et al., 2003; Rhodes, 2005). It is possible that insulin resistance in β cells inhibits PI3K activity by some mechanisms, such as decreased expressions of insulin signaling molecules, including class IA PI3K. As shown in βDKO mice, decreased PI3K activity in β cells can impair insulin secretion by suppressing glucose metabolism, cell-cell communication, and SNARE proteins and by increasing apoptosis. However, β cell mass can be maintained by enhanced Erk signaling induced by decreased PI3K activity as long as input of the insulin signal to activate IRS/Erk pathway is preserved by hyperinsulinemia in response to peripheral insulin resistance. Meanwhile, at an advanced stage of diabetes, severely impaired IR activation due to further reductions in expression levels of IR/IRS2/PI3K and relative hypoinsulinemia may abolish both PI3K/Akt and Erk activity. This leads to exacerbation of impaired insulin secretion, decreased β cell mass, and subsequent hypoinsulinemia, making a vicious cycle.
Taken together, class IA PI3K in β cells is indispensable for normal insulin secretion mainly through controlling intracellular Ca2+ levels, maintaining cell-cell synchronization, and regulating SNARE complex proteins. It is also important for antiapoptosis and regulates β cell mass, in cooperation with cell proliferation controlled by the Erk pathway. Thus, enhancing the class IA PI3K pathway, not only in peripheral tissues but also in β cells, may provide a therapeutic strategy for T2DM.
EXPERIMENTAL PROCEDURES
Animals
The pik3r1 floxed mice, pik3r2 knockout mice, and RIP-Cre transgenic mice have all been described previously (Luo et al., 2005). Mice were maintained on a 129Sv-C57BL/6-FVB mixed background. Animals were housed on a 12 hr light-dark cycle. Animal care and experimentations were approved by the Animal Care and Use Committee of the University of Tokyo. All experiments in this study were performed on male mice. By mating pik3r1 heterozygous floxed mice carrying a RIP-Cre transgenic allele with heterozygous pik3r1 floxed mice, we generated βPik3r1KO mice and the controls (Flox mice and RIP-Cre mice) in same littermates. Alternatively, by mating pik3r1 homozygous floxed mice carrying RIP-Cre transgenic allele with pik3r1 homozygous floxed mice, we generated βPik3r1KO mice and the control Flox mice. Mating pik3r1 homozygous floxed mice carrying a RIP-Cre transgenic allele (with pik3r2 homozygous knockout alleles) with pik3r1 homozygous floxed mice (with pik3r2 homozygous knockout alleles) brought βDKO mice and the control Pik3r2KO mice. We confirmed the Cre-mediated deletion of pik3r1 by PCR using DNA and by quantitative real-time PCR using RNA extracted from βPik3r1KO islets and βDKO islets and detected the remaining wild-type gene products to some extent, presumably due to the contamination of non-β cells.
Metabolic Studies
GTT and ITT were performed as described previously (Ueki et al., 2002b). For GTT, mice were fasted for 16 hr, and blood samples were obtained at indicated time points after intraperitoneal (i.p.) injection of D-glucose (WAKO; Osaka, Japan). For ITT, mice were injected with the insulin solution (HumalinR, Eli Lilly; Indianapolis, IN) intraperitoneally. Blood glucose levels were checked at indicated time points (Glutest Pro, Sanwa Kagaku Kenkyusho; Mie, Japan). Serum insulin levels were measured using a radioimmunoassay insulin kit (Institute of Isotopes, Budapest) in accordance with the manufacturer's instructions. For first-phase GSIS testing, mice were fasted for 16 hr, and blood samples were obtained at indicated time points after i.p. injection of D-glucose (3.0 g/kg BW).
Islet Isolation and Measurement of Insulin Content
Islets were isolated by Liberase RI (Roche) pancreatic perfusion and subsequent digestion for 24 min at 37°C (Kulkarni et al., 1999). Islets were picked manually and then immediately used for secretion studies or were maintained in Hank's balanced salt solution (HBSS) (Sigma) buffer supplemented with 10% FCS and 25 mM HEPES buffer.
Batch Incubation Experiments and Measurement of ATP Production
Freshly isolated islets were maintained in Krebs-Ringer Bicarbonate (KRB) buffer (129 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 5 mM NaHCO3, and 10 mM HEPES [adjusted to pH 7.4]) containing 0.2% bovine serum albumin supplemented with 2.8 mM glucose for 30 min at 37°C. The islets were incubated for 30 min in the same buffer containing indicated stimulants. For measurement of insulin content, islets were extracted by the acid ethanol overnight incubation at –20°C.
Quantitative RT-PCR Analysis
Quantitative RT-PCR was performed by PRISM 7900HT (ABI; Carlsbad, CA). For RT-PCR quantification of islets, all mice were killed in ad libitum state, and islets were processed as described earlier. Total RNA was extracted from islets using an RNeasy kit (QIAGEN). cDNA was prepared from total RNA using a High Capacity cDNA Reverse Transcription Kit (ABI). Gene expression levels were normalized to the expression of cyclophilin in each sample. All primers and probes of genes were purchased from ABI.
Immunostaining and Morphometric Analysis of Islets
Immunohistochemical and morphometric analyses of pancreas sections were performed using methods described earlier (Ueki et al., 2006) with a slight modification. Four or five mice of each genotype at 8 and 32 weeks of age were subjected to morphometric analysis. Sections were stained with antibodies to insulin (DAKO; Glostrup, Denmark), glucagon (DAKO), phospho-Akt (Ser473) (Cell Signaling; Danvers, MA), FoxO1 (Cell Signaling and homemade cocktail), p85 (N-SH domain) (Upstate; Charlottesville, VA), p85α (MBL; Woburn, MA), PIP3 (MBL), phospho-Erk (Cell Signaling), and β-catenin (BD Biosciences Pharmingen; San Diego, CA). For morphometric analysis, images of islets were traced manually and analyzed with the use of ImageJ software (NIH). Glucose stimulation for PIP3 staining was conducted after mice were fasted for 16 hr and then sacrificed 10 min after i.p. injection of D-glucose (3 g/kg BW in saline) (WAKO). Cell proliferation was detected by DNA replication using BrdU. BrdU incorporation was analyzed as previously described (Kubota et al., 2004). The TUNEL staining was performed using the Roche Cell Death Detection Kit.
Two-Photon Excitation Imaging
Two-photon excitation imaging of islets was performed as previously described (Hatakeyama et al., 2007; Takahashi et al., 2004). Exocytotic events were counted in a region of interest with an area of 3200–5200 mm2 and were normalized to an area of 800 mm2. Ca2+ measurements were performed using fura-2 as the Ca2+ indicator. Fura-2 fluorescence (F) was normalized by the resting fluorescence (F0). The increase of Ca2+ concentration is calculated by (F0 – F) / F0, where F0 and F stand for resting and poststimulation fluorescence, respectively. Islets loaded with 30 μM NP-EGTA-AM (Invitrogen) for 30 min were used for experiments.
Statistical Analyses
Data are presented as mean ± SEM and analyzed by Student's t test or ANOVA followed by post hoc comparisons. The null hypothesis is rejected at p < 0.05. Statistical significance is displayed as *p < 0.05 or **p < 0.01.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Nakae for providing the FoxO1-3A and FoxO1-ADA adenoviruses and F. Takahashi, R. Hoshino, Y. Kanto, E. Hirata, and H. Chiyonobu for their excellent technical assistance. This work was supported by a grant-in-aid for Scientific Research in Priority Areas (B) (to K.U.) and a grant-in-aid for Scientific Research in Priority Areas (S) (to T. Kadowaki) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. It was also supported by a grant for TSBMI from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to T. Kadowaki) and Health Science Research grants (Research on Human Genome and Gene Therapy) from the Ministry of Health and Welfare (to T. Kadowaki). K.K. is a Research Fellow of Japan Society for the Promotion of Science.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, Supplemental References, four figures, and four movies and can be found with this article online at doi:10.1016/j.cmet.2010.11.005.
REFERENCES
- Araki E, Lipes MA, Patti ME, Brüning JC, Haag B, 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol. Cell. Biol. 2009;29:3219–3228. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54:2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, Squires PE, Persaud SJ. Signaling through the p38 and p42/44 mitogen-activated families of protein kinases in pancreatic beta-cell proliferation. Biochem. Biophys. Res. Commun. 2000;268:541–546. doi: 10.1006/bbrc.2000.2179. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 α. Nat. Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat. Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse beta-cells. J. Physiol. 2007;582:1087–1098. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Coleman DL, Lane PW. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem. Genet. 1972;7:1–13. doi: 10.1007/BF00487005. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J. Clin. Invest. 2005;115:388–396. doi: 10.1172/JCI22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Tobe K, Yano W, Suzuki R, Ueki K, Takamoto I, Satoh H, Maki T, Kubota T, et al. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J. Clin. Invest. 2004;114:917–927. doi: 10.1172/JCI21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat. Genet. 2002;31:111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J. Biol. Chem. 2006;281:2649–2653. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- Leibiger B, Leibiger IB, Moede T, Kemper S, Kulkarni RN, Kahn CR, de Vargas LM, Berggren PO. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol. Cell. 2001;7:559–570. doi: 10.1016/s1097-2765(01)00203-9. [DOI] [PubMed] [Google Scholar]
- Leibiger IB, Leibiger B, Berggren PO. Insulin signaling in the pancreatic beta-cell. Annu. Rev. Nutr. 2008;28:233–251. doi: 10.1146/annurev.nutr.28.061807.155530. [DOI] [PubMed] [Google Scholar]
- Leibiger B, Moede T, Uhles S, Barker CJ, Creveaux M, Domin J, Berggren PO, Leibiger IB. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB J. 2010;24:1824–1837. doi: 10.1096/fj.09-148072. [DOI] [PubMed] [Google Scholar]
- Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J. Clin. Invest. 2004;114:908–916. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Okada T, Assmann A, Soto J, Liew CW, Bugger H, Shirihai OS, Abel ED, Kulkarni RN. Insulin signaling regulates mitochondrial function in pancreatic beta-cells. PLoS ONE. 2009;4:e7983. doi: 10.1371/journal.pone.0007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol. Cell. Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S, Furukawa H, Ishida H. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999;48:2367–2373. doi: 10.2337/diabetes.48.12.2367. [DOI] [PubMed] [Google Scholar]
- Nlend RN, Michon L, Bavamian S, Boucard N, Caille D, Cancela J, Charollais A, Charpantier E, Klee P, Peyrou M, et al. Connexin36 and pancreatic beta-cell functions. Arch. Physiol. Biochem. 2006;112:74–81. doi: 10.1080/13813450600712019. [DOI] [PubMed] [Google Scholar]
- Ostenson C-G, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonsky KS. Reduced beta-cell mass and altered glucose sensing impair insulin-secretory function in betaIRKO mice. Am. J. Physiol. Endocrinol. Metab. 2004;286:E41–E49. doi: 10.1152/ajpendo.00533.2001. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Güldenagel M, Charollais A, Gjinovci A, Caille D, Söhl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters b-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Serre-Beinier V, Bosco D, Zulianello L, Charollais A, Caille D, Charpantier E, Gauthier BR, Diaferia GR, Giepmans BN, Lupi R, et al. Cx36 makes channels coupling human pancreatic beta-cells, and correlates with insulin expression. Hum. Mol. Genet. 2009;18:428–439. doi: 10.1093/hmg/ddn370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hatakeyama H, Okado H, Miwa A, Kishimoto T, Kojima T, Abe T, Kasai H. Sequential exocytosis of insulin granules is associated with redistribution of SNAP25. J. Cell Biol. 2004;165:255–262. doi: 10.1083/jcb.200312033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006a;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006b;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Tran TT, Kondo T, Luo J, Ueki K, Cantley LC, Kahn CR. Phosphoinositide 3-kinase regulatory subunit p85alpha suppresses insulin action via positive regulation of PTEN. Proc. Natl. Acad. Sci. USA. 2006c;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Aleman JO, Ueki K, Luo J, Asano T, Kaneto H, Stephanopoulos G, Cantley LC, Kahn CR. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol. Cell. Biol. 2007;27:2830–2840. doi: 10.1128/MCB.00079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat. Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- Ueki K, Fruman DA, Brachmann SM, Tseng YH, Cantley LC, Kahn CR. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol. 2002a;22:965–977. doi: 10.1128/MCB.22.3.965-977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA. 2002b;99:419–424. doi: 10.1073/pnas.012581799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Fruman DA, Yballe CM, Fasshauer M, Klein J, Asano T, Cantley LC, Kahn CR. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 2003;278:48453–48466. doi: 10.1074/jbc.M305602200. [DOI] [PubMed] [Google Scholar]
- Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, et al. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat. Genet. 2006;38:583–588. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol. Cell. Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.