Abstract
BACKGROUND & AIMS
Inflammatory bowel disease (IBD) is associated with increased apoptosis of intestinal epithelial cells (IECs). Mutations in the tumor suppressor p53 appear during early stages of progression from colitis to cancer. We investigated the role of p53 and its target, p53-upregulated modulator of apoptosis (PUMA), in inflammation-induced apoptosis of IECs.
METHODS
Apoptosis was induced in mouse models of mucosal inflammation. Responses of IECs to acute, T-cell activation were assessed in wild-type, p53−/−, Bid−/−, Bim−/−, Bax3−/−, Bak−/−, PUMA−/−, and Noxa−/− mice. Responses of IECs to acute and chronic colitis were measured in mice following 1 or 3 cycles of dextran sulfate sodium (DSS), respectively. Apoptosis was assessed by TUNEL staining and measuring activity of caspases 3 and 9; levels of p53 and PUMA were assessed in colon tissue from patients with and without ulcerative colitis.
RESULTS
Apoptosis of IECs occurred in the lower crypts of colitic tissue from humans and mice. Colitis induction with anti-CD3 or 3 cycles of DSS increased apoptosis and protein levels of p53 and PUMA in colonic crypt IECs. In p53−/− and PUMA−/− mice, apoptosis of IECs was significantly reduced but inflammation was not. Levels of p53 and PUMA were increased in inflamed mucosal tissues of mice with colitis and in patients with UC, compared with controls. Induction of PUMA in IECs of p53−/− mice indicated that PUMA-mediated apoptosis was independent of p53.
CONCLUSIONS
In mice and humans, colon inflammation induces apoptosis of IECs via p53-dependent and -independent mechanisms; PUMA also activates an intrinsic apoptosis pathway associated with colitis.
Keywords: IBD, UC, Crohn’s Disease, Cell Death, Signaling
Maintenance of intestinal barrier integrity requires regulation of epithelial proliferation, differentiation, and death. For homeostasis of the gastrointestinal tract, proliferation occurring in crypt bases is matched by the number of cells lost by apoptosis.1 In the uninflamed colon, apoptosis occurs in differentiated cells on the surface plateau and at very low rates within proliferative regions.2–4 Epithelial cell apoptosis increases within crypt proliferative zones in both ulcerative colitis (UC) and Crohn’s disease.5,6 In fact, excessive apoptosis with insufficient proliferation has been proposed as a mechanism for mucosal ulceration in inflammatory bowel disease (IBD).7–9
There are 2 pathways signaling cell death by apoptosis: the intrinsic and extrinsic pathway.10,11 Activation of the extrinsic pathway occurs by ligand-induced cell surface receptor (ie, tumor necrosis factor receptor 1 [TNFR1], Fas, and death receptor 5) activation. Cellular death domains of these receptors activate a signaling cascade that recruits and cleaves procaspase-8. Caspase-8 activates effector caspases 2, 3, 6, and 7 that ultimately cause apoptosis by cleaving various cellular substrates. Under some conditions, caspase 8 can activate the intrinsic mitochondrial apoptosis pathway by cleaving BH3 interacting domain death agonist (Bid), a BH3-only member of the B-cell lymphoma 2 (Bcl-2) family.12 Cleaved Bid (tBid) associates with Bcl-2-associated X protein (Bax) and Bcl-2 homologous antagonist killer (Bak) causing the dissociation of apoptotic protease activating factor 1 (Apaf-1) from the anti-apoptotic proteins Bcl-2 and B-cell lymphoma-extra large (Bcl-xL) leading to mitochondrial membrane depolarization and cytochrome c release. The apoptosome, consisting of Apaf-1, cytochrome c, and caspases 3 and 9, then activates the caspase cascade causing eventual DNA fragmentation. The intrinsic pathway is activated with growth factor deprivation, oncogene activation, or when DNA damage is detected by cellular censors such as ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related (ATR), and tumor protein 53 (p53).
The tumor suppressor gene p53 is a central factor for cell fate decisions and prevents the expansion of damaged and mutated cells.13,14 Probably the best of these examples can be found in radiation-induced intestinal injury where p53 was found to be required for IEC apoptosis.15–17 Studies showed that p53-mediated radiation-induced IEC apoptosis requires expression of p53 up-regulated modulator of apoptosis (PUMA) and p53.18,19 Several groups have shown that p53 mutations are an early event in chronic colitis and dysplasia.20–24 One theory is that cells with dysfunctional (mutated) p53 fail to enact cell cycle arrest and apoptosis predisposing to survival of cells harboring DNA mutation.25,26 Studies show that p53 loss of function is an early event in colitis-induced cancer.20–24,27,28 p53 Mutation is also associated with Crohn’s disease progression to dysplasia.29 Finally, in the dextran sulfate sodium (DSS) mouse model of colitis, loss of p53 was found to exacerbate the incidence and multiplicity of dysplasia and cancer found.30,31 However, the involvement of p53 in immune-mediated apoptosis has not been fully explored.
In this study, we test the notion that p53 and PUMA are required for inflammation-induced IEC apoptosis. Using both acute and chronic mouse colitis models, we show that p53 utilizes the intrinsic apoptosis pathway. Surprisingly, our data indicate that PUMA also mediated apoptosis, but through a p53-independent pathway, and that both p53 and PUMA knockout mice had similar levels of inflammation compared with wild-type (WT) mice given 3 rounds of DSS. Thus, these data differ from radiation models and suggest that IEC apoptosis in IBD occurs through p53 and PUMA.
Materials and Methods
Mice and Treatments
C57BL/6, p53−/−, Bax−/−, and Bid−/− mice on the B6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). Experiments with PUMA−/− mice on C57BL/6 background were done in Dr. Lin Zhang’s laboratory at the University of Pittsburgh. Bak−/−, Bim−/−, and Noxa−/− mice were kind gifts from Drs. Craig Thompson and Andreas Strasser. Villin-Cre/IkkβF/F mice were used with permission from Michael Karin (University of California, San Diego, CA). Mice were maintained under specific pathogen-free conditions in the Northwestern University Medical School Animal Care Facility in accordance with guidelines of the Northwestern University Animal Care and Usage Committee. Mice were given intraperitoneal injection of 0.2 mg anti-CD3 monoclonal antibody (mAb) (145-2C11) or control hamster mAb (UC8-IB9) and killed at different time points after the injection as specified in results. Antibodies were purified from culture supernatant over a protein G column (Amersham Pharmacia Biotech, Piscataway, NJ). For all mAbs, endotoxin levels are tested using the limulus amebocyte assay (Biowhittaker Company, Walkersville, MD). B6, p53−/−, and PUMA−/− mice were fed an AIN-76A diet (Research Diets, New Brunswick, NJ) with 1.5% DSS (MP Biomedicals, Solon, OH) in their drinking water for 1 week followed by 2 weeks of regular water.
Immunohistochemical Localization of Apoptotic Cells
Formalin-fixed, paraffin-embedded sections were stained for apoptotic cells by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) method with an in situ cell death detection kit according to the manufacturer’s instructions (Roche, Indianapolis, IN). Standard immunohistologic methods were used to stain for p53 (Santa Cruz Biotechnology, Santa Cruz, CA).
The number of TUNEL-positive epithelial cells were divided by the total number of epithelial cells to yield the apoptotic index as follows: (number of positively stained epithelial cells/number of total epithelial cells) × 100 = apoptotic index. A minimum of 10 crypts were counted for each mouse. The data are presented as the mean ± standard error of mean. Two-tailed Student t test was used to evaluate differences between the control and experimental groups. Differences were considered statistically significant when P < .05.
Epithelial Cell Isolation
Dissected mouse colons were opened lengthwise and held at 4°C in Ca2+- and Mg+-free Hank’s balanced salt solution (HBSS) (CMF-HBSS). The tissue was then transferred to 5 mL CMF-HBSS containing 10 mmol/L dithiothreitol (1:100; Sigma, St. Louis, MO) and 50 nmol/L calyculin A (1:200; Wako, Richmond, VA) and incubated for 30 minutes at 4°C. After incubation, the tube was shaken briefly and the tissue transferred to a fresh tube containing 5 mL CMF-HBSS with 1 mmol/L EDTA and 50 nmol/L calyculin A. After incubation at 4°C for 1 hour, epithelial cells were dislodged by vigorous shaking for 10–15 minutes. Large pieces of tissue were removed from the tube and discarded, and epithelial cells were harvested by centrifugation at 300 rpm for 5 minutes. Supernatant was decanted, and cells were snap frozen in liquid nitrogen. Flow cytometry confirmed purity of the isolated epithelial cells with less than 2% CD45-positive contaminating cells.
Western Blotting
The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using precast gels (Lonza, Rockland, ME) and transferred to polyvinylidene difluride membrane (Millipore, Billerica, MA) using semidry electrotransfer apparatus (Bio-Rad, Hercules, CA). The membranes were blocked with protein-free T20 blocking buffer (Pierce, Rockford, IL) and incubated with primary antibodies specific for p53 (Santa Cruz Biotechnology), cleaved caspase 3 and 9 (Cell Signaling, Danvers, MA), caspase 8 p20 (Abcam, Cambridge, MA), and β-actin (Sigma) as a loading control followed by corresponding anti-mouse or anti-rabbit secondary antibody at room temperature. Proteins were detected by chemiluminescence (West Pico or West Dura kits; Pierce).
Human Colonic Specimens
Biopsy samples were obtained from patients 18 years or older undergoing diagnostic or surveillance colonoscopy or surgical resections. No patients were pregnant, had a history of intestinal surgery, bleeding diathesis, or coagulopathy. No control or UC patients were on colitis treatment. Untreated active UC patients underwent colonoscopy for increased symptoms (mean Ulcerative Colitis Disease Activity Index score of 9.8 ± 2.34). All patient materials were approved by Northwestern University Office for the Protection of Human Subjects.
Results
p53 Is a Critical Inducer of Apoptosis Following T-Cell Activation
Because T-cell activation is known to induce IEC apoptosis,7 we examined the role of p53 in IEC apoptosis following T-cell activation by anti-CD3 mAb. p53 Levels were assessed in protein lysates from purified epithelial cells analyzed by Western blot. Data in Figure 1A show that, within 3 hours of T-cell activation, IEC p53 levels increased significantly over baseline and remained elevated for at least 12 hours. By 18 hours after anti-CD3 treatment, p53 levels returned to baseline (Supplementary Figure 1A). Thus, these data were consistent with the notion that T-cell activation stabilizes colonic epithelial p53 levels. As shown later, we find this in human colitis as well.
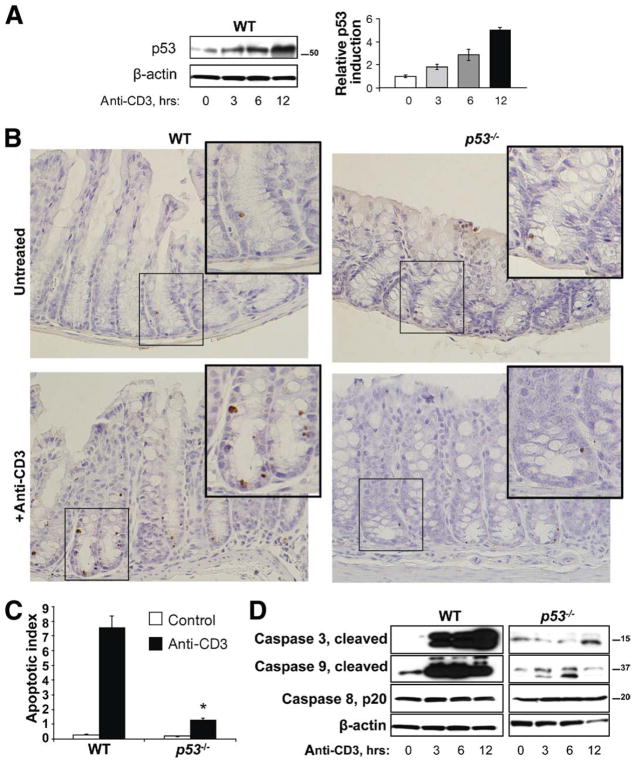
Figure 1.
p53 Stabilization is induced by T-cell activation and required for colonic crypt cell apoptosis. (A) Western blot for p53 in isolated colonic crypts of untreated versus anti-CD3 treated wild-type (WT) mice killed 3, 6, and 12 hours after injection. Densitometry data shows p53 induction over time with T-cell activation relative to control untreated mice and normalized to actin. (B) TUNEL staining of colonic sections of untreated and anti-CD3 treated WT and p53−/− mice. Anti-CD3 treated mice were killed 24 hours after injection. Arrows point to TUNEL-positive apoptotic epithelial cells. (C) Bar graph of apoptotic index (see Materials and Methods section) is shown. (D) Western blots for cleaved caspases 3 and 9 and caspase 8, p20. Isolated colonic IECs from WT and p53−/− mice were analyzed at 0, 3, 6, and 12 hours after anti-CD3 injection. β-actin was used as a loading control.
To examine the requirement for p53 in immune-mediated epithelial apoptosis, tissues from C57/BL6 WT and p53 knockout (p53−/−) mice were examined by staining for TUNEL and cleaved caspase 3. Immunohistochemical (IHC) and Western blot data from anti-CD3 treated mice revealed that IEC apoptosis accelerated at times when IEC p53 protein increased (Figure 1A and D, and Supplementary Figure 1B). IHC data show numbers of apoptotic IECs increased in lower crypts, both in stem cell and transit-amplifying (mid-crypt) regions (Figure 1B and C). There was little change in IEC apoptosis observed at the surface of the crypts (Figure 1B, and Supplementary Figure 1B). While apoptosis may be occurring in leukocyte populations (eg, T cells or B cells), only apoptotic epithelial cells were counted. Therefore, immune cell activation induced apoptosis specifically in colonic crypt epithelial cells. In p53−/− mice, numbers of apoptotic cells decreased 82% compared with WT anti-CD3-treated mice (Figure 1B and C). Western blot analysis of purified IEC in WT and p53−/− mice indicated T-cell activation induced caspase 3 and 9 cleavage through p53-dependent mechanisms. Analysis of mucosal cytokine levels indicated that tumor necrosis factor (TNF), interferon γ, interleukin (IL)-6, and IL-1β levels were increased by T-cell activation more in p53−/− mice, where IEC apoptosis was less, than in WT mice where there were more apoptotic cells observed (Supplementary Figure 2). These data suggest that p53-mediated epithelial apoptosis in the colon is largely mediated via the intrinsic pathway. Interestingly, even in p53-deficient mice, there was still increased IEC apoptosis albeit far less than in WT mice.
PUMA Is a Major Contributor to T-Cell Activation-Induced Crypt Cell Apoptosis
To explore which mediators were involved in inducing the intrinsic pathway of apoptosis by immune cell stimulation, intestinal TUNEL staining was assessed in Bcl-2 family and BH3-only gene knockout mice. Analysis of apoptotic indices of isolated deficiencies revealed that singular deficiency of Bid, Bim, Bak, Bax3, or Noxa had no effect on IEC apoptosis (Figure 2A). In contrast, IEC apoptosis was greatly reduced in PUMA-deficient compared with WT mice (Figure 2A). T-cell activation induced PUMA and NOXA expression in WT and to a lesser extent in p53−/− mice (Figure 2B). It was previously shown that basal levels of TNF, IL-1, IL-6, and IL-1β are elevated in p53−/− mice (Komarova et al32). Thus, elevated expression of Noxa and PUMA at time 0 may reflect heightened inflammatory cytokine stimulation in these mice. Because the Fas and perforin are both implicated in IEC apoptosis,33 we examined whether these apoptotic factors were dependent on p53. Data in Figure 2B show T-cell activation induced epithelial Fas and perforin in WT mice. By comparison, results indicate that p53 deletion abrogated Fas and perforin induction (Figure 2B). Taken together, these data suggest Fas and perforin are likely downstream of p53 and implicate PUMA as a mediator in p53-independent IEC apoptosis.
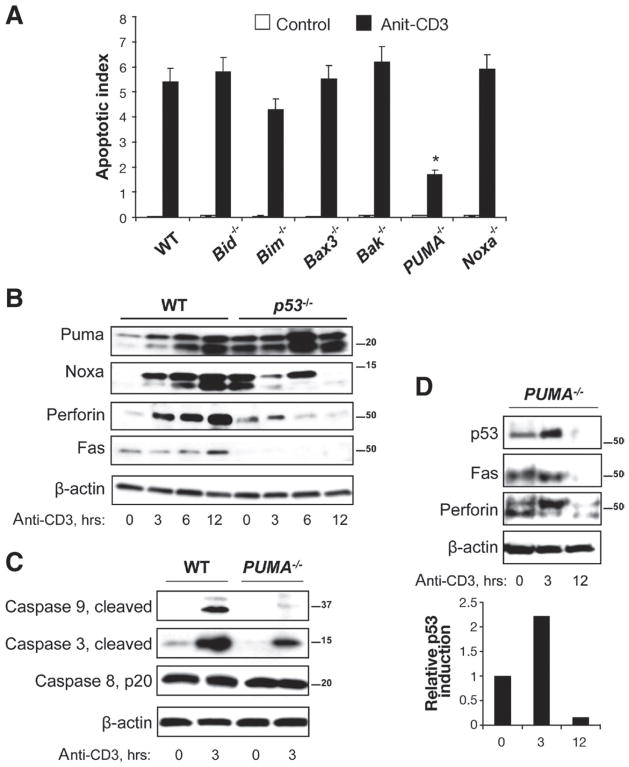
Figure 2.
PUMA is required for T cell-activation induced crypt cell apoptosis. (A) Apoptotic indeces determined from TUNEL staining of colon tissue from untreated and anti-CD3 injected BID−/−, BIM−/−, Bax−/−, Bak−/−, PUMA−/−, and Noxa−/− mice 24 hours after treatment. (B) Western blotting for Puma, Noxa, Perforin, and Fas in WT and p53−/− IECs at the indicated time after anti-CD3 injection. (C) Western blotting for activated caspases 3, 8, and 9 in isolated IECs from control and anti-CD3 treated WT and PUMA−/− mice. (D) Western blotting for p53, Noxa, Fas, and Perforin in PUMA−/− mice at 0, 3, and 12 hours after treatment. Densitometry data shows p53 induction over time with T-cell activation relative to control untreated mice and normalized to actin. β-actin was used as a loading control.
To examine directly the role of PUMA in IEC apoptosis, the induction of apoptotic effector proteins were examined in WT and PUMA−/− mice. PUMA deficiency attenuated activation of the caspases 3 and 9 relative to WT mice (Figure 2C). Caspase 8 activation remained unaffected in all conditions, suggesting that extrinsic pathways of apoptosis were not involved. Furthermore, PUMA deficiency did not abrogate the p53 induction seen in WT mice (Figures 1A and 2D, see densitometry data). Noxa was also induced in PUMA−/− (Supplementary Figure 1C). The enhanced induction of Noxa in PUMA−/− mice relative to WT may reflect compensation for PUMA deficiency; however, Figure 2A suggests that Noxa is not required for inflammation-induced IEC crypt apoptosis. PUMA deficiency attenuated Fas and perforin induction. These data indicate that caspase 3 and 9 activation, Fas and perforin induction, but not p53 stabilization were dependent on PUMA.
To investigate a mechanism for p53-independent PUMA induction, villin-Cre/IkkβF/F mice were examined. Previous data suggested that nuclear factor (NF)-κ B contributes to PUMA-mediated IEC apoptosis.34 These results differ from observations in Ikkβ-deficient mice where loss of NF-κB signaling increased colitis-induced IEC apoptosis.35 Data here show that Ikkβ deficiency failed to attenuate T cell-induced IEC apoptosis and p53 stabilization (Supplementary Figure 3). Interestingly, PUMA induction was blunted in Ikkβ-deficient mice. These data suggest that epithelial NF-κB does not play a role in IEC apoptosis despite apparent regulation of PUMA expression.
Apoptosis Is Differentially Induced in Plateau vs Crypt IECs in Acute and Chronic DSS-Treated Mice
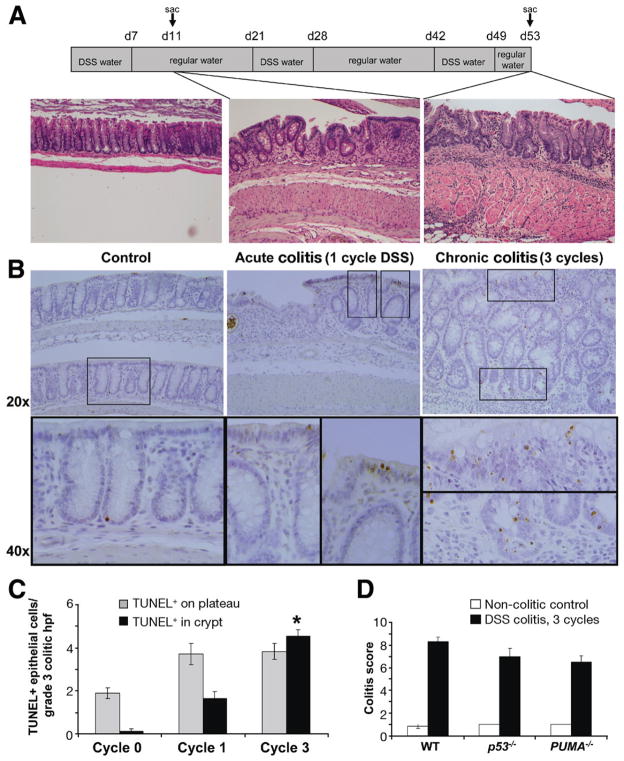
Although mucosal ulceration is seen in acute and chronic disorders of mucosal inflammation, chronic architectural distortion is a hallmark of epithelial responses in human IBD.36 To examine mechanisms of IEC apoptosis in the DSS mouse model of colitis, we compared morphologic features after 1 and 3 cycles of 1.5% DSS in the drinking water to determine during which interval histologic changes most mimic human IBD (Figure 3A). Analysis of tissue histology from mice following the first round of DSS treatment indicated changes with acute disease. Acute colitis was evidenced by acute inflammatory infiltrates, cryptitis, and superficial ulceration (Figure 3A, center panel). Notably absent were signs of goblet cell depletion or crypt branching, both signs of chronic inflammation. After 3 cycles of DSS, there was crypt architectural distortion with goblet cell dropout, crypt branching, and separation of crypts from the hypertrophied muscularis by chronic infiltrates (Figure 3A, right panel). TUNEL staining for apoptotic cells in these tissues show that, during acute phases of disease, increased numbers of apoptotic cells predominate on the epithelial surface. In contrast, increased lower crypt IEC apoptosis predominated in tissue with chronic inflammation (Figure 3B and C). Overall, these data suggest that chronic inflammation preferentially induces IEC apoptosis in lower crypt populations, a finding we later show correlates to human IBD (Figure 5).
Figure 3.
Three-cycle DSS treatment resembles changes seen in UC. (A) Schematic of the DSS feeding protocol and the accompanying H&E-stained colon tissue from mice killed 4 days after either 1 or 3 cycles of 1.5% DSS. (B) TUNEL staining of colons from untreated WT mice or 1 or 3 cycle DSS-treated mice. 20X and 40X Magnifications are shown. (C) TUNEL-positive cells on the plateau vs in the crypt base after 0, 1, or 3 cycles of DSS were quantified per grade 3 colitic high-powered fields (hpf). The asterisk indicates that the mean TUNEL-positive crypt IECs in cycle 3 are significantly (P< .05) higher than in cycle 1, as determined by t test. Five mice were analyzed in each group and a minimum of 10 hpf per mouse were counted. (D) Colitis scores were determined by analyzing H&E-stained sections from WT, p53−/−, and PUMA−/− untreated relative to 3 cycle DSS-treated mice.
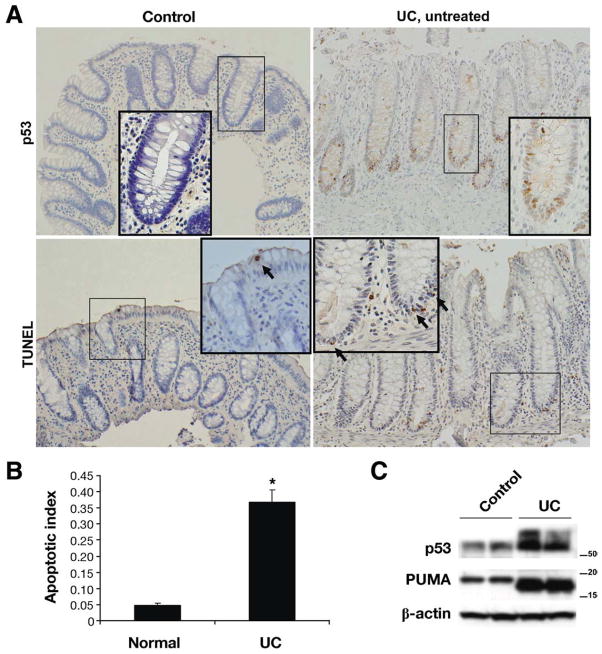
Figure 5.
p53 And PUMA are both induced in UC patients. (A) Colonic biopsy specimens from normal control study patients and untreated UC patients were stained for p53 and TUNEL. (B) The apoptotic index was determined in the human patient samples analyzed. Biopsy specimens from 5 normal and 6 UC patients were analyzed. Asterisk indicates p<.005 relative to normal patients. (C) p53 and PUMA protein levels were analyzed by Western blot analysis of protein extracts from normal control biopsy specimens relative to those from UC patients. β-actin was used as a loading control.
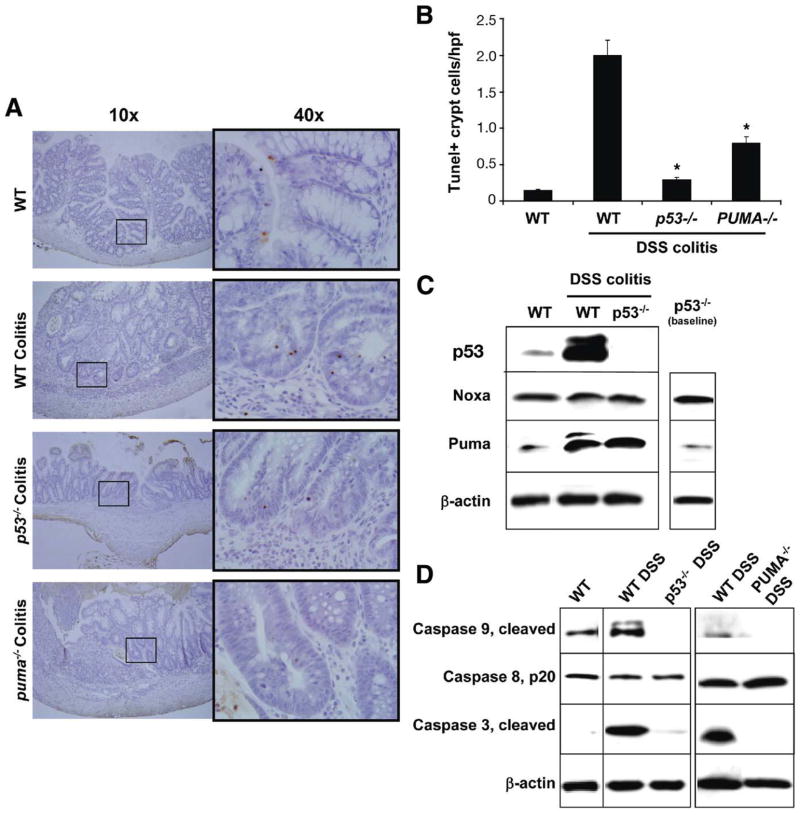
p53 Mediates Epithelial Cell Apoptosis in DSS Colitis
Given that chronic (ie, after 3 cycles) DSS colitis most resembled human IBD, this DSS disease model was utilized to assess the role of p53 and PUMA in colitis-induced IEC apoptosis. TUNEL staining for apoptotic cells in WT, p53−/−, and PUMA−/− mice was compared. By day 53, IEC apoptosis was markedly reduced in p53−/− mice, as compared with colitic WT mice (Figure 4A and B). Western blotting of lysates from colon epithelial cells showed that, during chronic colitis, just as with anti-CD3 treatment, p53 protein levels increased relative to noncolitic (WT) mice (Figure 4C). Furthermore, expression of PUMA, but not Noxa, was elevated (Figure 4C). PUMA, however, was also induced in the epithelial cells of p53−/− just as in WT mice by chronic disease indicating a possible p53-independent pathway for inducing PUMA during chronic colitis.
Figure 4.
Crypt epithelial apoptosis in DSS colitis is p53 and PUMA dependant. (A) TUNEL staining for apoptotic IEC in 3-cycle 1.5% DSS treated WT, p53−/−, and PUMA−/− mice. (B) Apoptotic index indicating relative levels of apoptotic IECs in DSS-treated WT, p53−/−, and PUMA−/− mice. Asterisk indicates P<.05 relative to DSS treated WT mice. (C) Western blots for p53, PUMA, and Noxa levels in DSS-treated WT and p53−/− mice relative to untreated WT controls. (D) Western blotting for activated caspases 3, 8, and 9 in isolated colon IECs of WT and 3 cycle 1.5% DSS-treated WT, p53−/−, or PUMA−/− mice. β-actin was used as a loading control.
Finally, it was determined which caspases become activated during DSS colitis. Caspases 3 and 9 became activated in WT mice as there was an increase in the cleaved forms (Figure 4D). In both p53−/− and PUMA−/− mice, there was a significant reduction in the amounts of cleaved forms of caspases 3 and 9 detected. These data indicate that p53 and PUMA are important regulators of intrinsic apoptosis during chronic colitis.
p53 and PUMA Are Induced in Human Chronic UC
Biopsy samples taken during colonoscopy were examined for p53 stabilization and apoptosis in epithelial cells as a result of human UC. Specimens from noninflamed patients have very few p53-positive and apoptotic epithelial cells (Figure 5A). Furthermore, apoptosis of the crypt epithelial cells is even rarer in control tissue. In tissue from untreated, inflamed UC patients, however, many more p53-positive and TUNEL positive colon epithelial cells were detected, especially in lower to midcrypt regions (Figure 5A). Consistent with our mouse models, Western blotting revealed that p53 and PUMA levels were elevated in biopsy material from UC patients relative to control patients. Therefore, data in human tissue were consistent with mouse data that indicate IEC apoptosis is induced via p53- and PUMA-dependent mechanisms during chronic colitis.
Discussion
Much remains unknown about the molecular mechanisms for IEC apoptosis during chronic inflammation. To address the mechanisms for colitis-induced IEC apoptosis, we sought to identify suitable mouse models representative of observations from human disease. In patient samples, we found increased IEC apoptosis localized to lower crypt regions within areas of crypt architectural distortion. Mouse models of anti-CD3-induced apoptosis resembled features of crypt-based apoptosis. Chronic (3 cycles), but not acute (1 cycle), DSS colitis most mimicked human disease based on histologic and biochemical observations. Using these mouse models of mucosal inflammation, we found that IEC apoptosis involves induction of the intrinsic apoptosis pathway associated with caspase 9, rather than caspase 8, cleavage. Mechanistically, we found p53-dependent and p53-independent PUMA-mediated apoptosis of IECs during chronic inflammation. These novel findings allowed us to interrogate whether inflammation was affected in mice with attenuated IEC apoptosis. In p53 and PUMA deficient mice, IEC apoptosis was significantly reduced, but tissues were equivalently inflamed compared with WT. Taken together, these studies support the model that chronic inflammation induced robust IEC apoptosis in lower crypts is mediated by the intrinsic mitochondrial pathway.
Data presented here suggest inflammation invokes distinct apoptotic mechanisms than those observed during normal homeostasis. Apoptosis during normal regulation of intestinal homeostasis is largely only observed in non-proliferative regions of the small bowel and colon.1 Cells shed into the lumen from the luminal surface at the end of their life cycle die by a anoikis, detachment-induced cell death.37 Data presented here show that immune activation most significantly increased the number of apoptotic IECs in the proliferative lower crypt regions both in mouse inflammatory models and during human IBD. These observations are of note because this implies that the apoptotic stimuli from inflammation induced by T-cell activation and IBD most affect IECs in the stem cell and transit-amplifying niches.
Consistent with the above, we and others report that p53 is stabilized in crypt IEC in human UC.38 Increased p53 in IBD tissue is a physiologic consequence of oxidative stress.23 Inflammation-induced reactive oxygen species and nitric oxide leads to p53 stabilization and accumulation, thereby leading to p53 activation in, and elimination of, damaged cells.25,39 We also detected enhanced p53 stabilization following acute activation of T cells in mice with anti-CD3 treatment (Figure 1). Examination of p53 levels after T-cell activation revealed that levels returned to baseline after the inflammatory stimulus abated, indicating p53 stabilization was not associated with mutation or loss of heterozygosity (data not shown). In murine model indicative of a significant role for p53 in response to immune stimulation, fewer apoptotic IECs were detected in p53−/− mice after anti-CD3 injection or after 3 cycles of DSS colitis (Figures 1 and 4). Together, these data suggest that p53 plays an integral role in inducing cell death in IECs during chronic inflammation. Excess production of nitric oxide, however, can induce p53 gene mutations. Additionally, cells lacking functional p53 are less sensitive to nitric oxide-induced apoptosis.25,26 This may explain why loss-of-function p53 mutations are found early in the progression of colitis-induced dysplasia to cancer. The notion that mutated p53 is procarcinogenic in IBD is supported by the finding that tumorigenicity is enhanced in p53−/− mouse models of colitis.30,31 Thus, given that chronic inflammation enhances DNA damage,25 without p53-induced apoptosis, aberrant cells are not eliminated and cancer ensues.
We detected increased PUMA levels following anti-CD3 treatment and DSS colitis in WT and p53−/− mice. These data are consistent with the IEC response to ischemia reperfusion where PUMA expression and apoptosis were also unchanged in p53−/− mice.40 These data need to be reconciled with findings that PUMA is required for virtually all p53-mediated apoptosis.41 PUMA can be up-regulated by other transcription factors other than p53 including c-myc, FoxO3a, p73, and E2F1.41 Thus, several candidates exist that may explain PUMA induction by T-cell activation or chronic colitis as detected in p53−/− mice. The data here therefore suggest that PUMA regulates survival and repair of the epithelial barrier during colitis.
We show that IEC apoptosis in colitis increases specifically in crypt bases and not among differentiated cells on the surface plateau region (Figures 3–5). The findings presented here differ from previous reports in cell lines and following in vivo infusion of exogenous TNF injection or inducing ischemia reperfusion.40,42–44 These models largely detected apoptosis in differentiated epithelial cell populations such as cells on the villous surface. Apoptosis was found to be independent of p53.40,43 We propose that mechanisms operating during colitis utilize p53- and PUMA-dependent mechanisms to selectively induce apoptosis through mitochondrial pathways, caspase 3 and 9-mediated, within crypt populations. Other reports have implicated death domain containing receptors such as Fas, death receptor 5, and TNFR1 in IEC apoptosis.33,43,45 These results suggested that extrinsic pathways of apoptosis, caspase 8 mediated, predominate during inflammation. Our results suggest a different interpretation. Our detection of caspases 3 and 9, but not 8, activation is consistent with the model that inflammation induces IEC apoptosis through direct intrinsic rather than indirect extrinsic signaling. Therefore, we predict that apoptosis would be attenuated in Bax/Bak double knockouts, which were not examined here.46 In fact, multiple other systems have been described where signaling through death domain surface receptors (TNFR1 and FasL) induce apoptosis through mitochondrial mechanisms.47 Taken together, these data present new insight into the mechanisms of IEC apoptosis in colitis. We suspect that mechanisms will be “context-dependent,” thus villous enterocytes are susceptible to apoptosis by tissue ischemia or high-dose TNF (which may induce ischemia), whereas crypt base enterocytes are susceptible to inflammatory apoptotic signals.
Both p53 and PUMA protein levels were increased in IECs from UC patients (Figure 5). Our results from mouse models further enhance our understanding of these events as they suggest that increased IEC apoptosis in IBD is largely due to p53 and PUMA. In areas of increased WT p53 and PUMA, these proteins cooperate to induce cell death in cells with DNA damage. In areas with mutated p53, we predict that WT PUMA plays a larger role. We suspect both p53 and PUMA play key roles in deleting IEC from pools of rapidly proliferating cells. It seems likely that epithelial progenitors harboring DNA errors function less well and threaten the integrity of crypt structures. In these cases, induction of WT p53 and PUMA may promote a normal epithelial reaction to an abnormal mucosal inflammatory response. These mechanisms would also function to protect against survival of mutant progenitor cells with dysplastic potential. In the setting of “gain of function” mutations, p53 may induce excessive apoptosis and impair mucosal healing. In crypts harboring IEC with “loss of function” mutations, deficient p53-mediated apoptosis may allow mutant IEC clones to persist and accumulate other mutations on their way to cancer. Thus, the results have direct relevance to both mechanisms of IEC apoptosis in non-neoplastic colitis as well as in areas of neoplastic transformation.
Supplementary Material
Acknowledgments
Funding
Supported by the National Institutes of Health (R01DK-54778 and R01AI061701; to T.A. Barrett), CA106348 (to L. Zhang), and UO1DK085570 (to J. Yu) 5PO1 HL071643-08 to Navdeep Chandel.
Abbreviations used in this paper
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad3-related
- Bak
Bcl-2 homologous antagonist killer
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- Bid
BH3 interacting domain death agonist
- CMF-HBSS
Ca2+- and Mg+-free Hank’s balanced salt solution
- DSS
dextran sulfate sodium
- HBSS
Hank’s balanced salt solution
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cell
- IL
interleukin
- IHC
immunohistochemistry
- mAb
monoclonal antibody
- NF-κB
nuclear factor-κB
- p53
tumor protein 53
- PUMA
p53 up-regulated modulator of apoptosis
- TNF
tumor necrosis factor
- TNFR1
tumor necrosis factor receptor 1
- UC
ulcerative colitis
- WT
wild type
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.05.032.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Hall PA, Coates PJ, Ansari B, et al. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 3.Ijiri K, Potten CS. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer. 1983;47:175–185. doi: 10.1038/bjc.1983.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ijiri K, Potten CS. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987;55:113–123. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto M, Koji T, Makiyama K, et al. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Di Sabatino A, Ciccocioppo R, Luinetti O, et al. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis Colon Rectum. 2003;46:1498–1507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 7.Croitoru K, Zhou P. T-cell-induced mucosal damage in the intestine. Curr Opin Gastroenterol. 2004;20:581–586. doi: 10.1097/00001574-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenberger GS, Flavell RA, Alexopoulou L. Innate immunity and apoptosis in IBD. Inflamm Bowel Dis. 2004;10(Suppl 1):S58–S62. doi: 10.1097/00054725-200402001-00012. [DOI] [PubMed] [Google Scholar]
- 9.Ruemmele FM, Seidman EG, Lentze MJ. Regulation of intestinal epithelial cell apoptosis and the pathogenesis of inflammatory bowel disorders. J Pediatr Gastroenterol Nutr. 2002;34:254–260. doi: 10.1097/00005176-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Ozoren N, El-Deiry WS. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 12.Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10:161–167. doi: 10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- 13.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 14.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 15.Merritt AJ, Allen TD, Potten CS, et al. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after γ-irradiation. Oncogene. 1997;14:2759–66. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 16.Komarova EA, Christov K, Faerman AI, et al. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–3798. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 17.Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 18.Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu W, Leibowitz B, Zhang L, et al. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene. 2010;29:1622–1632. doi: 10.1038/onc.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida T, Mikami T, Mitomi H, et al. Diverse p53 alterations in ulcerative colitis-associated low-grade dysplasia: full-length gene sequencing in microdissected single crypts. J Pathol. 2003;199:166–175. doi: 10.1002/path.1264. [DOI] [PubMed] [Google Scholar]
- 21.Takaku H, Ajioka Y, Watanabe H, et al. Mutations of p53 in morphologically non-neoplastic mucosa of long-standing ulcerative colitis. Jpn J Cancer Res. 2001;92:119–126. doi: 10.1111/j.1349-7006.2001.tb01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogt F, Vortmeyer AO, Goldman H, et al. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29:131–136. doi: 10.1016/s0046-8177(98)90222-2. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SP, Amstad P, Raja K, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 24.Kern SE, Redston M, Seymour AB, et al. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107:420–428. doi: 10.1016/0016-5085(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 25.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman JE, Hofseth LJ, Hussain SP, et al. Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease. Environ Mol Mutagen. 2004;44:3–9. doi: 10.1002/em.20024. [DOI] [PubMed] [Google Scholar]
- 27.Brentnall TA, Crispin DA, Rabinovitch PS, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 28.Harpaz N, Peck AL, Yin J, et al. p53 protein expression in ulcerative colitis-associated colorectal dysplasia and carcinoma. Hum Pathol. 1994;25:1069–1074. doi: 10.1016/0046-8177(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 29.Nathanson JW, Yadron NE, Farnan J, et al. p53 mutations are associated with dysplasia and progression of dysplasia in patients with Crohn’s disease. Dig Dis Sci. 2008;53:474–480. doi: 10.1007/s10620-007-9886-1. [DOI] [PubMed] [Google Scholar]
- 30.Chang WC, Coudry RA, Clapper ML, et al. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375–2381. doi: 10.1093/carcin/bgm134. [DOI] [PubMed] [Google Scholar]
- 31.Fujii S, Fujimori T, Kawamata H, et al. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komarova EA, Krivokrysenko V, Wang K, et al. p53 is a suppressor of inflammatory response in mice. FASEB J. 2005;19:1030–1032. doi: 10.1096/fj.04-3213fje. [DOI] [PubMed] [Google Scholar]
- 33.Merger M, Viney JL, Borojevic R, et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor α in T cell-induced mucosal damage in the mouse intestine. Gut. 2002;51:155–163. doi: 10.1136/gut.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Qiu W, Dudgeon C, et al. PUMA is directly activated by NF-κB and contributes to TNF-α-induced apoptosis. Cell Death Differ. 2009;16:1192–1202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greten FR, Eckmann L, Greten TF, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 37.Grossmann J, Mohr S, Lapentina EG, et al. Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am J Physiol. 1998;274:G1117–G1124. doi: 10.1152/ajpgi.1998.274.6.G1117. [DOI] [PubMed] [Google Scholar]
- 38.Sipos F, Molnar B, Zagoni T, et al. Growth in epithelial cell proliferation and apoptosis correlates specifically to the inflammation activity of inflammatory bowel diseases: ulcerative colitis shows specific p53- and EGFR expression alterations. Dis Colon Rectum. 2005;48:775–786. doi: 10.1007/s10350-004-0831-5. [DOI] [PubMed] [Google Scholar]
- 39.Hussain SP, Harris CC. p53 Biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J Nippon Med Sch. 2006;73:54–64. doi: 10.1272/jnms.73.54. [DOI] [PubMed] [Google Scholar]
- 40.Wu B, Qiu W, Wang P, et al. p53 Independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56:645–654. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(Suppl 1):S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones SA, Butler RN, Sanderson IR, et al. The effect of specific caspase inhibitors on TNF-α and butyrate-induced apoptosis of intestinal epithelial cells. Exp Cell Res. 2004;292:29–39. doi: 10.1016/j.yexcr.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Piguet PF, Vesin C, Guo J, et al. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–3505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 44.Guy-Grand D, DiSanto JP, Henchoz P, et al. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 45.Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–4612. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- 46.Lindsten T, Ross AJ, King A, et al. The combined functions of pro-apoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakibaei M, Schulze-Tanzil G, Takada Y, et al. Redox regulation of apoptosis by members of the TNF superfamily. Antioxid Redox Signal. 2005;7:482–496. doi: 10.1089/ars.2005.7.482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.