Abstract
We present a case of a 72-year-old man who presented with fluctuating right-sided weakness and numbness. This was characterised by episodic sudden onset weakness with resolution of symptoms in between. His symptoms and signs were becoming persistent despite the addition of dual antiplatelet therapy. The history we describe is classical of capsular warning syndrome. The patient went on to have further definitive neuroimaging which revealed a pontine infarct, rather than the expected capsular infarct. We discuss the importance of capsular warning syndrome, the proposed pathophysiological mechanisms and different locations of infarction in previous cases of capsular warning syndrome.
We also discuss the lack of consensus (within the literature) in treatment options which are used to try and prevent a completed stroke occurring in cases of capsular warning syndrome.
Background
Capsular warning syndrome (CWS) can be a dramatic occurrence in the clinical setting, which is important to recognise.
Despite its typical history and clinical course, the exact location of infarcts can vary from case to case. As in our case, instead of the internal capsule, the location of the infarct was in fact within the pons.
Our case highlights that CWS can be difficult to manage and try prevent a completed stroke. In the literature there is a lack of conclusive evidence on attempting to prevent a completed stroke in CWS. Our case highlights this further, and provides a case for further research that could provide specific treatment options for CWS.
Case presentation
A 72-year-old right-handed man presented with fluctuating neurological signs. He had a medical history of hypertension, type 2 diabetes and he was an exsmoker of 35 pack-years.
He presented with right leg weakness and numbness, which he noticed on waking in the early hours of the morning. This initial episode lasted 10 min, before returning back to normal. He woke later in the morning to discover that he was unable to mobilise. He had developed weakness and numbness affecting both the right arm and leg. He had developed mild right-sided facial droop and dysarthria. The second episode lasted for 60 min before full resolution. Nine hours later after the onset of the first episode he developed further symptoms. He developed motor and sensory symptoms affecting the right arm and leg, he was again affected by facial droop and dysarthria. At the time of arrival to our department (after his third episode) his symptoms had improved considerably, and had resolved completely back to normal.
Examination
On examination, he had an elevated blood pressure (BP) of 198/86, the rest of his observations were within normal limits. He had a normal cranial nerve examination and there was no evidence of dysarthria. There was no evidence of cortical or any cerebellar signs.
Examination of the peripheral neurological system was normal. Examination of tone and power in all four limbs was normal. He had symmetrical reflexes and bilateral down going plantars. There was no evidence of limb ataxia and examination of the sensory system in all modalities was normal. His gait was normal; his National Institutes of Health Stroke Scale (NIHSS) was scored as 0.
Investigations
His investigations revealed that full blood count, urea and electrolytes and coagulation screen were all normal.
His ECG revealed sinus rhythm.
His initial CT of the head did not reveal any acute ischaemic changes. His initial CT angiogram was normal. It revealed normal carotids and vertebrobasilar system; it did show a left vertebral artery of reduced calibre. This is due to the vessel being congenitally smaller, suggesting a non-dominant vessel. This is a normal variant. The vessel itself was patent throughout its course. There was no evidence of intravascular thrombus.
Clinical progression/management
He was started with aspirin 300 mg once daily, simvastatin 40 mg once daily and was given a dose of amlodipine 5 mg (in view of his BP).
During the course of his admission he developed further symptoms the following morning, which were similar to his initial presentation. This episode lasted for approximately 30 min, with complete resolution again. Following this episode he was started on dual antiplatelet therapy and was given a stat dose of clopidogrel 300 mg.
In the early hours of the second day of his admission he developed further symptoms which were more severe in terms of deficit and duration. At this stage he developed weakness in the right face, arm and leg with profound dysarthria. He had 0/5 power in the right upper limb and 3+/5 in the right lower limb with a right upper motor neuron seventh cranial nerve weakness. Sensory examination (assessing all modalities) was normal. There was no evidence of any cortical or cerebellar signs after this deterioration.
He further had a CT head at the time of his deterioration; this revealed an area of low density in the left pons consistent with an acute infarct. Similar to the admission CT angiogram, the second CT angiogram was normal.
Later that day he underwent an MRI and contrast-enhanced magnetic resonance angiography (CEMRA), the images confirmed left pontine infarction, with evidence of restricted diffusion (figure 1), which corresponded with the apparent diffusion coefficient (ADC) map (figure 2).
Figure 1.
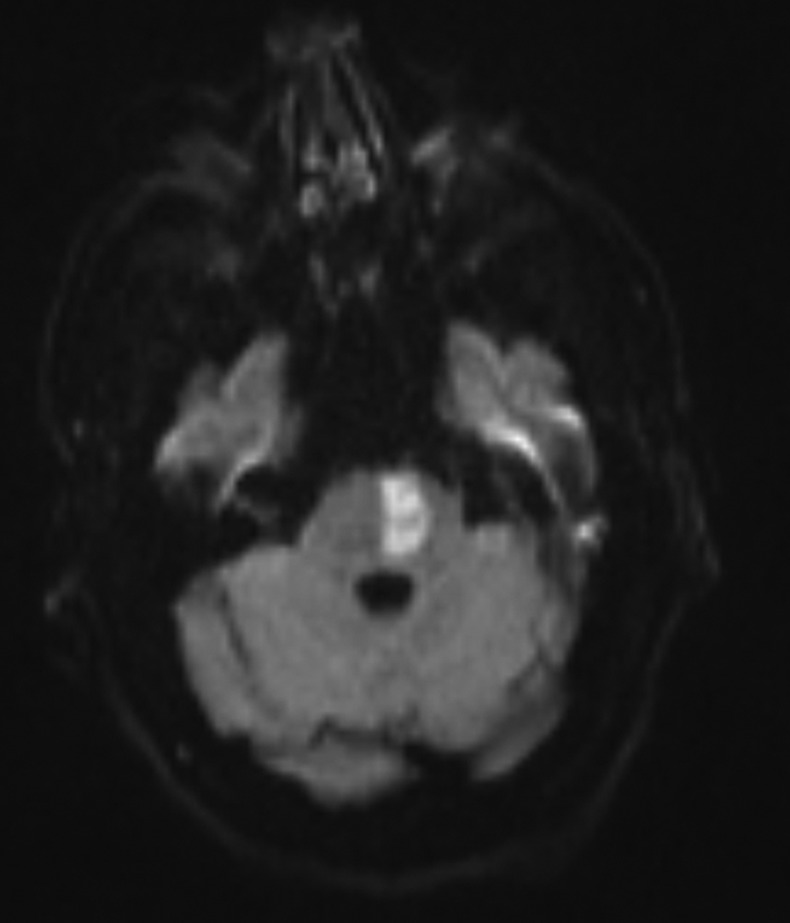
Image showing the left pontine infarct on diffusion-weighted images.
Figure 2.
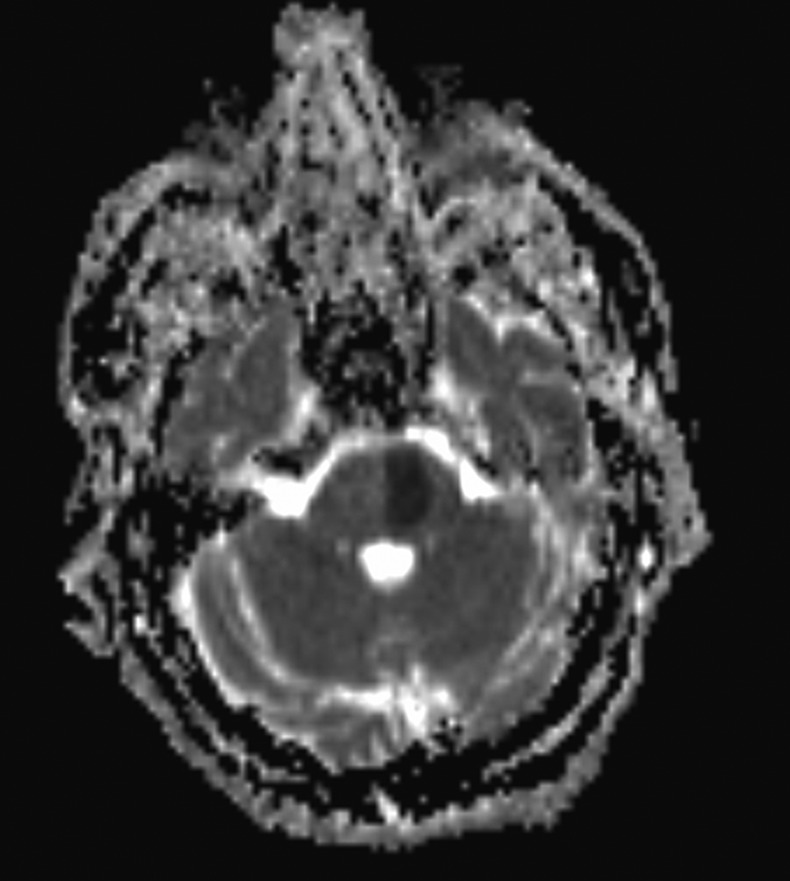
Image showing the corresponding MRI apparent diffusion coefficient changes.
CEMRA did not reveal any abnormality within the vasculature.
Treatment
He was started with aspirin 300 mg once daily, simvastatin 40 mg once daily, and was given a dose of amlodipine 5 mg (in view of his BP).
Following further clinical deterioration he was started on dual antiplatelet therapy and was given clopidogrel 75 mg once daily, after receiving an initial stat dose of 300mg clopidogrel.
Outcome and follow-up
He was transferred to another stroke unit for ongoing rehabilitation. He was subsequently discharged home with early supported discharge where he has been recovering.
On review 6 months after his initial presentation, he has had some restoration of power and function in his right upper limb, with power 3/5 proximally and 2/5 distally. He has recovered almost full power in his right lower limb with power 4+/5. He is able to walk independently with the aid of a stick. He is able to use the right arm for certain functional tasks, such as holding the paper and his signature.
Discussion
CWS was first described by Donnan et al1 2 in the 1980s. CWS describes a clinical pattern of transient ischaemic attacks (TIAs). CWS comprises of recurrent episodes of stereotyped TIAs which are due to ischaemia within the region of the internal capsule.1–3 Hence the inclusion of the word “capsular” within the name of the syndrome (CWS).
CWS is comprised of a cluster of TIAs, which tend to occur within proximity of each other in time. CWS is defined as consisting of three or more events within a 24 h time period.2 3 Our case had seven distinct episodes within 36 h of each other.
CWS is characterised by an abrupt onset of symptoms, with one study showing a mean duration of each event being 6.1 min.3 CWS is typified by brief dramatic episodes of hemiplegia followed by complete resolution between further events.3
The clinical features that are seen in CWS are unilateral motor and/or sensory deficits that involve at least two of the three, face, arm or leg.3 The commonest presentation being pure motor hemiparesis affecting the face, arm and leg.3 To make a diagnosis of CWS there should not be any evidence of cortical signs.3 4
This syndrome is particularly important because it has a high risk of developing ischaemic stroke with a permanent deficit.2 3 It has been reported in population studies that the 7-day stroke risk following CWS is as high as 60%.5
The exact pathophysiology has yet to be fully determined, and various hypotheses have been proposed. Authors have suggested that CWS is most likely to be ischaemia due to in situ small-penetrating vessel disease.3 One proposed theory suggests that this syndrome could be due to haemodynamic changes within the territory of the penetrating arteries. These haemodynamic changes could become particularly important when there is a structural arterial change within a penetrating vessel, such as atheroma and or lipohyalinosis.3
These haemodynamic changes could lead to critical hypoperfusion within single penetrating arteries, subsequently leading to infarction.6 This particular hypothesis could be relevant to our case. It is possible that the antihypertensive medication given on admission played a role in the patient developing an infarction. The administration of antihypertensive medication would have lead to BP reduction, which may have produced a reduction in perfusion to the ischaemic lesion. It is proposed that BP reduction in CWS may have deleterious effects and increases the chances of the patient developing a stroke.6
Other theories that have been proposed are vasospasm affecting the vessel concerned, and emboli of arterial or cardiac source.7
While CWS was coined to describe a distinct syndrome caused by ischaemia affecting the region of the internal capsule, it is now clear that these repeated TIA could occur in other areas, such as the brain stem and are not limited to the internal capsule. It has been suggested that some cases of CWS could be due to ischaemia affecting the corticospinal tracts below the level of the internal capsule, perhaps in areas supplied by single penetrators from the basilar artery (such as the pons).3 An early study reviewed 50 cases of pure motor hemiplegia, with nine of these cases being studied later pathologically through autopsy.8 Of these nine cases it was found on autopsy that six were due to an infarct within the internal capsule. The other three cases showed evidence of infarction within the basis pontis.8 Therefore it is not entirely unsurprising that as in our case, the initial label of CWS may be misleading when it comes to localising the final lesion.
With the use of MRI, it has been shown that the exact location of ischaemia with CWS can vary. Pontine infarction (rather than capsular) has been shown with MR diffusion-weighted imaging in a patient fulfilling the clinical criteria for CWS.9
MR diffusion-weighted imaging was used in a case series of eight patients presenting with CWS, four of whom subsequently developed a fixed stroke. Among these four patients, three had infarction involving the corona radiata.4 The development of permanent paresis in these three cases was due to the involvement of the pyramidal tract in the corona radiata.4 The fourth patient had an infarction in the brain stem (pontomesencephalic junction). This area would also directly involve the pyramidal tract.4
As one would expect when the pathophysiology of a syndrome is still debated, there is no consensus on an effective treatment for CWS. Despite various treatments being available and used, it is unclear whether these treatments alter the natural course of the syndrome. Antiplatelets, heparin and measures to elevate BP (such as vasopressors), have been used to treat patients with CWS, it remains uncertain whether any of these therapies are able to change the progression of the syndrome.10 It is thought that by elevating BP, one will be able to reduce distal vessel hypoperfusion, and thereby improving perfusion to the affected areas.11 There have been case series suggesting that dual antiplatelets (aspirin and clopidogrel) may be beneficial, similar to the effect seen in acute coronary syndromes. In a case series including two patients with CWS, it is reported that following the start of dual antiplatelets, there was no progression of symptoms.7
In a case series of four patients, three of the patients who received alteplase were discharged with NIHSS of 0 with no areas of restricted diffusion on MRI. The authors report a possible benefit of thrombolysis in CWS, although they were not able to exclude spontaneous recanalisation in these cases.11
There are various hypotheses related to pathophysiology of CWS, and consequentially no general consensus on treatment options to alter disease progression. There needs to be more research focused on the treatment options which target the various proposed aetiologies.7
Until there are randomised trials it remains unclear whether dual antiplatelet treatment will be of any benefit.
Learning points.
Capsular warning syndrome (CWS) describes recurrent stereotyped lacunar transient ischaemic attacks clustered within a short period of time and is associated with a high risk of developing a completed stroke.
While the clinical picture of CWS (and the nomenclature) might suggest an internal capsule involvement, CWS may be caused by an infarct affecting the corticospinal tracts below the internal capsule, such as within the pons.
It is not clear whether antiplatelets, heparin or thrombolysis affect the outcome of a completed stroke in patients with CWS.
Footnotes
Contributors: The case was chosen by TA. The case history and introduction were written by VN. TA contributed part of the discussion and all of the learning points.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Donnan GA, Bladin PF. The capsular warning syndrome: repetitive hemiplegic events preceding capsular stroke (abstract). Stroke 1987;2013:296 [Google Scholar]
- 2.Donnan GA, Tress BM, Bladin PF. A prospective study of lacunar infarction using computerized tomography. Neurology 1982;2013:49–56 [DOI] [PubMed] [Google Scholar]
- 3.Donnan GA, O'Malley HM, Quang L, et al. The capsular warning syndrome: pathogenesis and clinical features. Neurology 1993;2013:957–62 [DOI] [PubMed] [Google Scholar]
- 4.Staaf G, Geijer B, Lindgren A, et al. Diffusion weighted MRI findings in patients with capsular warning syndromes. Cerebrovasc Dis 2004;2013:1–8 [DOI] [PubMed] [Google Scholar]
- 5.Paul NL, Simoni M, Chandratheva A, et al. Population based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;2013:1356–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey JL. Capsular warning syndrome. Neurology 1994;2013:195–6 [DOI] [PubMed] [Google Scholar]
- 7.Asil T, Ir N, Karaudman F, et al. Combined antithrombotic treatment with aspirin and clopidogrel for patients with capsular warning syndrome: a case report. Neurologist 2012;2013:68–9 [DOI] [PubMed] [Google Scholar]
- 8.Fisher CM, Curry HB. Pure motor hemiplegia of vascular origin. Arch Neurol 1965;2013:30–44 [DOI] [PubMed] [Google Scholar]
- 9.Benito-Leon J, Alvarez-Linera J, Porta-Etessam J. Detection of acute pontine infarction by diffusion weighted MRI in capsular warning syndrome. Cerebrovasc Dis 2001;2013:350–1. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Albers GW, Marks MP, et al. Capsular warning syndrome caused by middle cerebral artery stenosis. J Neurol Sci 2010;2013:115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivanco-Hidalgo RM, Rodriguez-Campello A, Ois A, et al. Thrombolysis in capsular warning syndrome. Cerebrovasc Dis 2008;2013:508–10 [DOI] [PubMed] [Google Scholar]


