Abstract
Gynecologic cancer confers a large burden among women in the United States. Several evidence-based interventions are available to reduce the incidence, morbidity, and mortality from these cancers. The National Comprehensive Cancer Control Program (NCCCP) is uniquely positioned to implement these interventions in the US population. This review discusses progress and future directions for the NCCCP in preventing and controlling gynecologic cancer.
Gynecologic Cancer in the United States
Approximately 84,000 new cases are diagnosed and about 28,000 deaths occur each year from gynecologic cancer among women in the United States.1 Five cancers account for the vast majority of gynecologic cancer cases: cervical, ovarian, uterine, vaginal, and vulvar. Uterine cancer diagnoses are common; uterine is the fourth highest incident cancer among women in the US after breast, lung, and colorectal cancers.1 Ovarian cancer is the eighth most common cancer diagnosed; however, it is the fifth leading cause of cancer death among US women. Cervical, vaginal, and vulvar cancers are relatively less common than uterine and ovarian cancers; however, diagnoses and deaths from these three cancers still number in the thousands each year.1 The economic burden of gynecologic cancer is substantial in the US. In a single state (California) during a 1-year period, cervical, ovarian, and uterine cancers accounted for $624 million in direct health care costs and lost productivity due to premature death.2 Ovarian cancer was the most costly ($292 million), followed by cervical cancer ($206 million) and uterine cancer ($126 million).2
There are several evidence-based interventions available to reduce gynecologic cancer incidence and mortality; however, there is variable uptake of these services by women in the United States. Nearly all of cervical and 40%–70% of vaginal and vulvar cancers are associated with the human papillomavirus (HPV).3 The Advisory Committee on Immunization Practices currently recommends routine vaccination against the HPV virus in females and males 11–12 years of age.4,5 In 2011, only 35% of females aged 13–17 years had received the recommended three doses of HPV vaccine.6 The Pap test prevents and detects cervical cancer at early stages. Recent data show that 83.0% of women reported guideline-consistent Pap testing within the past 3 years,7 significantly less than the Healthy People 2020 target of 93.0%. Pap Testing rates are significantly lower among Asians (75.4%), and a small but significant downward trend was observed in the number of women who reported receiving guideline-consistent Pap testing over the last decade.7 Ovarian and uterine cancers are linked to genetic syndromes; mutations in the BRCA tumor suppressor gene increase risk for ovarian cancer and mutations associated with Lynch syndrome increase risk for both ovarian and uterine cancers.8 Genetic testing is available for BRCA mutations; however, several studies have shown low testing rates, even when a mutation has been previously found within the family.9 A study in Pennsylvania reported that although offered free genetic counseling and testing, only 57% of individuals with a positive BRCA1/2 family mutation status participated in testing.10 A similar percentage of individuals (51%) underwent genetic testing for Lynch syndrome when presented with a positive family mutation status.11 Following diagnosis with a gynecologic cancer, several organizations—Centers for Disease Control and Prevention (CDC), the National Institutes of Health, American College of Obstetricians and Gynecologists, the Society of Gynecologic Oncologists, and the National Comprehensive Cancer Network—all recommend receiving treatment from a gynecologic oncologist.12 Survival time among patients treated by these subspecialists is much improved, especially for ovarian cancer.12
The National Comprehensive Cancer Control Program
CDC established the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) in 1991 to administer low-cost cervical cancer screening and diagnostic services to low income, uninsured, and underinsured women aged 18–64 years.13 In 1992, CDC's National Program of Cancer Registries (NPCR) was established to expand the collection and reporting of data on the occurrence of cancer, including the type, stage, and initial treatment for the United States.14 Recognizing the need for a coordinated approach to the prevention and control of cancer, CDC began a pilot program in 1998 that provided funding for five states and one tribal health board that evolved into the National Comprehensive Cancer Control Program (NCCCP).15 For the last several years, the NCCCP has provided seed funding to 65 programs in all 50 states, the District of Columbia, 7 tribal governments and organizations, and 7 territories and US-associated Pacific Island jurisdictions to support the development and implementation of evidence-based initiatives to prevent and control cancer in their populations.15 These initiatives align with the NCCCP priorities to promote the primary prevention of cancer, assist with coordination of secondary prevention activities, address the public health needs of cancer survivors, and reduce health disparities.16 The NCCCP provides the evidence for, and evaluation of, policy, systems, and environmental change strategies used to achieve these priorities.16
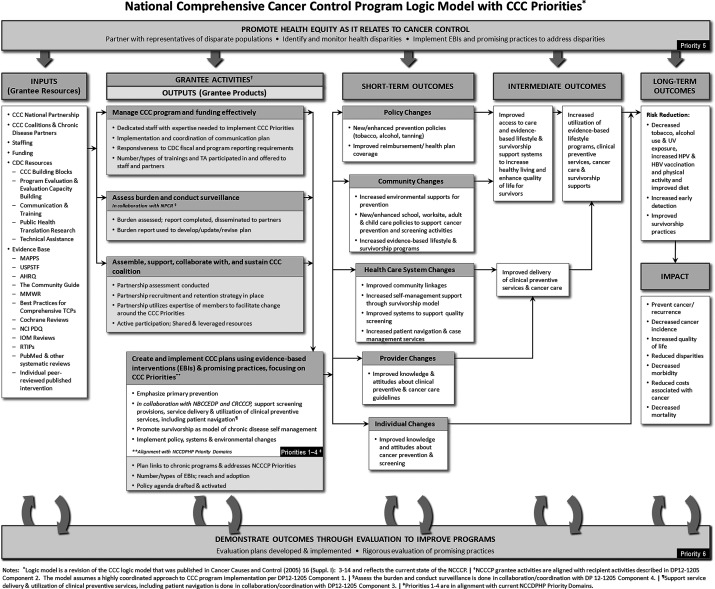
NCCCP grantees in each state convene a coalition of relevant partners that leverages resources from multiple sources to implement cancer prevention and control activities. The model through which NCCCP operates to reduce cancer incidence, morbidity, and mortality is shown in Fig. 1. The inputs reflect the different partnerships that programs develop or are guided by, as well as the evidence-based resources used. The outputs include the strong association with other CDC programs (NPCR for assessing the burden of cancer within the population and NBCCEDP for coordinating secondary prevention activities), and alignment of activities with NCCCP priorities. Outcomes are policy, systems, or environmental changes at the individual, provider, and community levels, with the ultimate impact being the reduction of cancer burden. This model guides program activities and progress, and the impact of these activities is monitored regularly by CDC.
FIG. 1.
Logic model for the National Comprehensive Cancer Control Program (NCCCP) (available at www.cdc.gov/cancer/ncccp/pdf/NCCCPLogicModel.pdf). †NCCCP grantee activities are aligned with recipient activities in the current funding agreement. §Assessment of the burden and conduct surveillance is done in collaboration/coordination with the National Program of Cancer Registries (NPCR). ¶Support of service delivery & utilization of clinical preventive services, including patient navigation, is done in collaboration/coordination the National Breast and Cervical Cancer Early Detection Program (NBCCEDP). ‡Priorities 1–4 are from reference 16. CCC, comprehensive cancer control. MAPPS, Media, Access, Point of decision information, Price, and Social support/services; USPSTF, United States Prevention Services Task Force; AHRQ, Agency for Healthcare Research and Quality; MMWR, Morbidity and Mortality Weekly Report; TCPs, Tobacco Control Programs; PDQ, Physician Data Query; IOM, Institute of Medicine; RTIPS, Research-Tested Interventions Program.
Recent National Comprehensive Cancer Control Gynecologic Cancer Successes
Several NCCCP grantees have reported success of interventions that specifically address cervical and ovarian cancers. In 2011, the Alabama Comprehensive Cancer Control Program collaborated with their state NBCCEDP to create and implement a campaign to promote HPV vaccination, called “Third Time's the Charm.”17 The campaign emphasized the importance of getting the recommended three doses of HPV vaccine to prevent cervical cancer to Alabama parents, college students, and physicians. Approximately 8,000 informational cards and pamphlets were mailed to Alabama residents, and further collaborations within the state were initiated as a result of the campaign. The Michigan Comprehensive Cancer Control Program, in collaboration with the University of Michigan Health System, organized a Pap Test Screening clinic for cervical cancer.17 They recruited volunteer doctors, nurses, and pathologists, and promoted the clinic to women aged 21 years and over without medical coverage. During the 3-hour session, 103 women between the ages of 25 and 59 were screened for cervical cancer with the Pap Test. About half of the women screened were black. As a result of screening, 7 women were found to have abnormal results and were contacted by a doctor or social worker for follow-up.17
In 2010, the California Comprehensive Cancer Control Program held a statewide ovarian cancer conference in collaboration with several physicians and advocacy groups in the state.17 A total of 38 ovarian cancer survivors attended the conference and reported that they appreciated the opportunity to be in touch with other survivors and share information. Florida, New York, and West Virginia engaged in ovarian cancer provider education, utilizing the Ovarian Cancer National Alliance's Survivors Teaching Students (STS): Saving Women's Lives program.18 This partnership brought ovarian cancer survivors into medical and nursing student classrooms to share survivor stories and key information on the disease. In Florida in one year, approximately 277 students were educated about ovarian cancer symptoms and the psychosocial issues related to an ovarian cancer diagnosis by trained ovarian cancer survivors. Florida reported that students' “basic understanding of ovarian cancer” increased from 61% to 98% after the STS presentation. Furthermore, about 97% of students responded that the program was “effective,” and 47% of students responded that they now “know what to look for and educate patients to be aware of (early) symptoms and signs.”18
Current National Comprehensive Cancer Program Gynecologic Cancer Initiatives
In 2012, the NCCCP began a new 5-year period of CDC funding. In this new award period, NCCCP programs developed new activities that align with their cancer plan and began reporting action plans for these activities to CDC in early 2013. A review of current NCCCP action plans indicates there is continued substantial engagement in gynecologic cancer. A total of 46% (n=30 of 65) of programs currently reference cervical cancer in their activities, with 60% of those (n=18 of 30) reporting a measurable goal related to the reduction of cervical cancer burden (Table 1). For ovarian cancer, 14% (n=9 of 65) of programs reference this cancer with 44% of those (n=4 of 9) having measurable goals. Only a few programs make reference to uterine (1), vaginal (2), or vulvar (1) cancers in their action plans, and measurable goals are not indicated for these cancers.
Table 1.
National Comprehensive Cancer Control Program Initiatives in Gynecologic Cancer, 2013
| Gynecologic cancer type | Programs that reference cancer type* n (%) | Programs with measurable goals for cancer type** n (%) |
|---|---|---|
| Cervical | 30 (46) | 18 (60) |
| Ovarian | 9 (14) | 4 (44) |
| Uterine | 1 (1.5) | 0 (—) |
| Vaginal | 2 (3) | 0 (—) |
| Vulvar | 1 | 0 (—) |
Based on a keyword search for the specific gynecologic cancer type. The total number of funded programs (n=65) was used as the denominator in percentage calculations.
Based on a search of all objectives listed in action plans. The total number of programs that reference cancer type was used as the denominator in percentage calculations.
Examples of measurable goals for cervical and ovarian cancers are listed in Table 2. For cervical cancer, current goals tend to focus on primary and secondary prevention, while those for ovarian cancer tend to focus on education. Most cervical cancer goals indicate provision of measurable increases in HPV vaccination and Pap testing. Ovarian cancer goals relate to the reduction of late-stage disease, and a number of goals focused on public and provider education.
Table 2.
Examples of National Comprehensive Cancer Program Grantee-Specific Measureable Goals Related to Gynecologic Cancer Initiatives, 2013
| Cervical cancer |
|---|
| Incidence |
| Decrease the number of cervical cancer cases diagnosed from 6.20 to 4.20 by June 2017. |
| Mortality |
| Decrease the percent of cervical cancer mortality rates from 3.10% to 2.60% by June 2017. |
| Screening: General |
| Increase the percent of breast and cervical cancer screening rates from 45% to 70% by June 2017. |
| Increase the percent of women receiving cervical cancer screenings based on the most recent guidelines from 84.10% to 88.30% by June 2017. |
| Increase the number of cervical cancer screenings using evidence-based strategies from 793 to 872 by June 2017. |
| Increase the percent of cervical cancer screening among women 21–65 years older from 83% to 85% by June 2017. |
| Screening: Pap test |
| Increase the percent of women who had their Pap Test done from 68% to 73% by June 2017. |
| Increase the percent of women reporting having had a pap test in the past 3 years from 78.40% to 93% by June 2017. |
| Human papillomavirus vaccination |
| Increase the percent of children and adolescent between 11 and 18 years who complete HPV vaccination (3 doses) from 7% to 40% by June 2017. |
| Increase the number of school nurses trained in the prevention of cervical cancer and the HPV vaccine from 404 to 504 by June 2017. |
| Ovarian cancer |
|---|
| Mortality |
| Decrease the rate of late stage ovarian cancers from 7.7 to 6.9 by June 2017. |
| Prevention education |
| Increase the number of health professionals/students educated from 765 to 876 by June 2014. |
| Increase the number of healthcare professionals who received education on ovarian cancer risk factors, signs, and symptoms from 0 to 100 by June 2013. |
| Increase the number of health education materials distributed from 3000 to 3150 by June 2013. |
| Increase the number of trainings from 0 to 7 by June 2013. |
There are several positive aspects of the current NCCCP gynecologic cancer initiatives. A large proportion (almost half) of NCCCP state programs are engaged in activities that aim to reduce cervical cancer incidence and mortality. Additionally, large percentages (between 44% and 60%) of programs indicate clear, measurable objectives that specifically relate to their population needs. Importantly, all the NCCCP gynecologic cancer initiatives reflect the evidence-base for these cancers, focusing on prevention for cervical cancer and reduced morality and education for ovarian cancer. However, there is room for improvement, especially given the relatively large gynecologic cancer burden in the US.
Current Gynecologic Cancer Mortality Rates and Potential Future Directions for the National Comprehensive Cancer Program
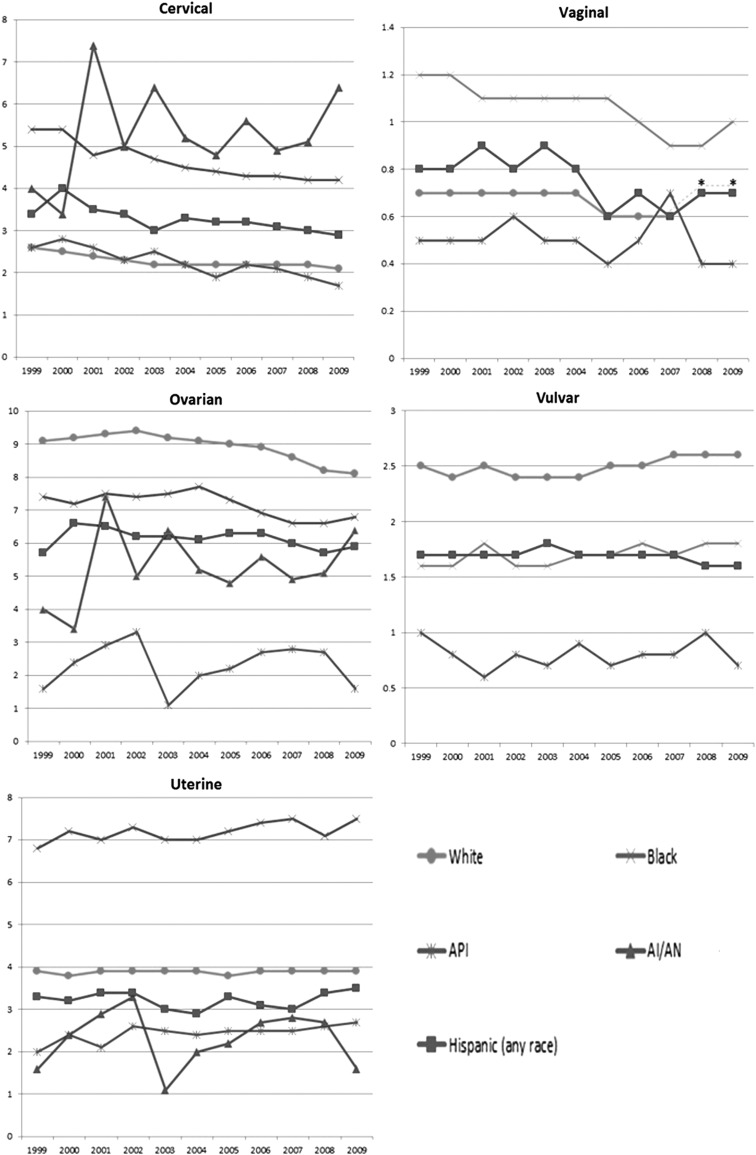
Recent mortality data suggest that gynecologic cancer death rates are not decreasing among all US populations uniformly (Fig. 2).19 While cervical cancer death rates have generally decreased in the most recent 10-year period, especially among black women, rates remain high among American Indian/Alaska Native women and appear to be increasing. Ovarian mortality appears to be decreasing among black and white women, but not in women of other racial and ethnic populations. Uterine cancer death rates have generally been stable in recent years, and appear to be increasing among black, Hispanic, and Asian/Pacific Islander women. Although rare, vulvar cancer death rates appear stable and are increasing among white women. Considering these data, there are several potential directions of future activity for NCCCP grantees.
FIG. 2.
Recent trends in gynecologic cancer mortality in the United States, 1999–2009. Mortality is displayed as death rates per 100,000 women; all rates are age-adjusted to the 2000 US standard. Hispanic ethnicity is not mutually exclusive from race. Rates for vaginal and vulvar cancer among American Indian/Alaska Native women are suppressed due to fewer than 16 deaths. *Vaginal cancer rates among white and Hispanic populations are the same for 2008 and 2009. API, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native.
An area that can be expanded is inclusion of activities related to genomics, as inherited mutations increase risk for ovarian and uterine cancers in some individuals. Increased knowledge and understanding of this increased risk, may lead to risk-reducing behaviors among individuals that may ultimately result in decreases in incidence. A survey of state cancer planners indicated that while planners believed there is increased awareness of genomics, no respondents felt that genomics related-activities were high priority for their programs.20 Some respondents identified that increases in funding, stronger partnerships with health insurance companies, managed care agencies, researchers, healthcare workers, and academia, as well as examples of successful programs that implement genomics concepts may facilitate increases in genomic content in NCCCP activities.20 Recently, CDC has provided funding to three states to increase appropriate counseling and testing for BRCA1/2 mutations, increase insurance coverage of BRCA 1/2 mutation testing and related clinical interventions for appropriate women. This funding will also assist to develop educational programs to increase the public and healthcare provider knowledge about family history, risk assessment, and the appropriateness of BRCA 1/2 counseling and testing. Increased collaboration within states between NCCCP and these genomics grantees may assist with promoting the inclusion of genomics content and initiatives. This could also lead to the existence of successful model programs that can serve as an example for other NCCCP grantees wanting to enact genomics initiatives.
Increased education about uterine cancer symptoms is another potential future direction for the NCCCP. Uterine cancer death rates are almost twice as high among black women compared to white women, even though incidence rates are similar,1, and black women are significantly more likely to present with late-stage uterine cancer.21 Early-stage uterine cancer has an excellent prognosis and an increase in early diagnoses will likely help alleviate some of the racial disparities that have long been observed in uterine cancer survival.21 In 2008, CDC initiated the Inside Knowledge: Get the Facts About Gynecologic Cancer campaign, with a main objective to increase education of the signs and symptoms of gynecologic cancers, including uterine.22 Materials freely available from the Inside Knowledge website include uterine cancer fact sheets, and a symptoms diary that clearly indicates symptoms of uterine cancer and allows women to track any symptoms experienced over a 2-week period (www.cdc.gov/cancer/knowledge). Informational symptoms cards are also available for providers. CDC recently began a pilot study to promote collaboration between these two initiatives by having a small number of NCCCP grantees distribute these materials through formal educational sessions held in their populations and measure gains in knowledge among women and providers. Material distribution will be aimed toward populations experiencing disparities. This study will yield best practices for education that NCCCP grantees can potentially adopt in their populations.
While a substantial number of programs remain engaged in activities related to HPV vaccination for cervical cancer, enhanced communication by NCCCP grantees on the benefits of this vaccination for vaginal and vulvar cancers as well could potentially serve to increase the number of adolescents and women who receive the vaccination. Although the incidence of vaginal and vulvar cancers is low compared to other gynecologic cancers, the surgical treatment of these cancers is often mutilating and traumatic for women.23,24 Additionally, the prognosis for vaginal cancer patients is very poor.23 A large population-based study estimated that wide implementation of HPV vaccination would prevent approximately one half of vulvar carcinomas in women younger than aged 56 and approximately two-thirds of the intraepithelial precursor lesions in the lower genital tract.24 There are several related areas in which NCCCP grantees can readily engage in to assist with this. It is suggested that educating healthcare workers about the importance of provider recommendations for parents may be the single most important way to increase HPV vaccination among children. 25 Additionally, programs that educate parents themselves about the importance of HPV vaccination as an anticancer vaccine (not limited to cervical cancer only) may also help. And systems changes, such as the implementation of automatic electronic reminders (for receipt of the recommended three doses) are also likely to be important in increasing vaccination.25
Finally, in terms of survivorship, an intervention that should be advanced by the NCCCP in gynecologic cancer patients and survivors is to help ensure patients receive treatment and clinical care from gynecologic oncologists. Studies have consistently demonstrated that gynecologic oncologists, subspecialists specifically trained to perform gynecologic cancer surgery and administer chemotherapy, more often adhere to standard treatment guidelines resulting in increased survival from gynecologic cancers.12,26,27 Receipt of care from a gynecologic oncologist is consistent with national guidelines, and several recommendations have been made to ensure that US women receive this standard of care. These include the emphasis of public education (direct consumer information) as well as professional education; and partnering with local, state, and national patient advocacy groups to ensure optimal treatment for gynecologic cancers.28 CDC has funded several research projects to examine the extent and locations of gynecologic oncologist providers in the US.12 Providing education to their local populations that incorporates these resources may increase the number of women who receive guidelines-based care, potentially resulting in increased survival of gynecologic cancer patients.
Conclusion
There are currently several evidence-based mechanisms available for reducing the incidence, morbidity, and mortality of the gynecologic cancers in the United States. Grantees of the NCCCP are uniquely positioned to implement these activities, and while several successes have been recorded by these grantees, some areas can be augmented. In particular, genomics and uterine cancer symptom education initiatives would assist with reducing the burden of these cancers, and align with the Healthy People 2020 objectives to decrease uterine cancer death rates and increase receipt of genetic counseling among those with a family history of ovarian cancer.29 It is recognized that funding and resources are necessary for grantees to enhance activities. However, the development and maintenance of strong partnerships, including those with non-governmental organizations, state and local agencies and other CDC-funded programs such as the NPCR, NBCCEDP, Inside Knowledge: Get the Facts About Gynecologic Cancer campaign, and Vaccines for Children Program can enable even more productive use of combined resources. In addition to the leveraging of resources, collaborations between programs at the local level may also have the benefit of streamlining efforts and reducing duplication. More research is needed to develop additional prevention and control methods for gynecologic cancer. In the meantime, more widespread application of the evidence-based methods available by the NCCCP—especially to populations in need—will likely lessen the gynecologic cancer burden in the United States.
Disclosure Statement
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. No competing financial interests exist.
References
- 1.US Cancer Statistics Working Group. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. [Apr 24;2013 ]. United States cancer statistics: 1999–2009 incidence and mortality web-based report. [Google Scholar]
- 2.Max W. Rice DP. Sung HY, et al. The economic burden of gynecologic cancers in California. Gynecol Oncol. 1998 2003 Feb;88:96–103. doi: 10.1016/s0090-8258(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 3.De Vuyst H. Clifford GM. Nascimento MC, et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males: Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011;2011;60:1705–1708. [PubMed] [Google Scholar]
- 5.Markowitz LE. Dunne EF. Saraiya M, et al. Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years: United States. MMWR Morb Mortal Wkly Rep. 2011;2012;61:671–677. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Cancer screening: United States. MMWR Morb Mortal Wkly Rep. 2010;2012;61:41–45. [PubMed] [Google Scholar]
- 8.Auersperg N. Wong AS. Choi KC, et al. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. Review. [DOI] [PubMed] [Google Scholar]
- 9.Claes E. Evers-Kiebooms G. Boogaerts A, et al. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116A:11–19. doi: 10.1002/ajmg.a.10868. [DOI] [PubMed] [Google Scholar]
- 10.Finlay E. Stopfer JE. Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12:81–91. doi: 10.1089/gte.2007.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadley DW. Jenkins J. Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163:573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- 12.Stewart SL. Rim SH. Richards TB. Gynecologic oncologists and ovarian cancer treatment: Avenues for improved survival. J Womens Health (Larchmt) 2011;20:1257–1260. doi: 10.1089/jwh.2011.3053. [DOI] [PubMed] [Google Scholar]
- 13.Khan K. Curtis CR. Ekwueme DU, et al. Preventing cervical cancer: Overviews of the National Breast and Cervical Cancer Early Detection Program and two US immunization programs. Cancer. 2008;113(10 Suppl):3004–3012. doi: 10.1002/cncr.23765. [DOI] [PubMed] [Google Scholar]
- 14.Wingo PA. Jamison PM. Hiatt RA, et al. Building the infrastructure for nationwide cancer surveillance and control: A comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) program (United States) Cancer Causes Control. 2003;14:175–193. doi: 10.1023/a:1023002322935. [DOI] [PubMed] [Google Scholar]
- 15.Major A. Stewart SL. Celebrating 10 years of the National Comprehensive Cancer Control Program, 1998 to 2008. Prev Chronic Dis. 2009;6:A133. [PMC free article] [PubMed] [Google Scholar]
- 16.Belle Isle L. Plescia M. La Porta M, et al. In conclusion: Looking to the future of comprehensive cancer control. Cancer Causes Control. 2010;21:2049–2057. doi: 10.1007/s10552-010-9666-7. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Atlanta: Centers for Disease Control and Prevention, National Comprehensive Cancer Control Program; 2012. [Apr 24;2013 ]. Stories of success: National Comprehensive Cancer Control Program: Comprehensive cancer control in action. [Google Scholar]
- 18.Stewart SL. Rim SH. Trivers KF. Summary and impact of ovarian cancer research and programmatic activities at the Centers for Disease Control and Prevention. J Womens Health (Larchmt) 2010;19:1427–1432. doi: 10.1089/jwh.2010.2164. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance Research Program, National Cancer Institute. Fast stats: An interactive tool for access to SEER cancer statistics. http://seer.cancer.gov/faststats. [Apr 24;2013 ]. http://seer.cancer.gov/faststats
- 20.Laufman JD. Duquette D. Trepanier A. Evaluation of state comprehensive cancer control plans for genomics content. Prev Chronic Dis. 2012;9:E176. doi: 10.5888/pcd9.120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madison T. Schottenfeld D. James SA, et al. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–2111. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rim SH. Polonec L. Stewart SL, et al. A national initiative for women and healthcare providers: CDC's Inside knowledge: Get the Facts About Gynecologic Cancer campaign. J Womens Health (Larchmt) 2011;20:1579–1585. doi: 10.1089/jwh.2011.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter JS. Downs LS., Jr. Vulvar and vaginal cancer. Obstet Gynecol Clin North Am. 2012;39:213–231. doi: 10.1016/j.ogc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Hampl M. Sarajuuri H. Wentzensen N, et al. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obstet Gynecol. 2006;108:1361–1368. doi: 10.1097/01.AOG.0000245786.86267.80. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A. Simard EP. Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cress RD. Bauer K. O'Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: Results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol. 2011;121:94–99. doi: 10.1016/j.ygyno.2010.12.359. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK. Sherman AE. Kapp DS, et al. Influence of gynecologic oncologists on the survival of patients with endometrial cancer. J Clin Oncol. 2011;29:832–838. doi: 10.1200/JCO.2010.31.2124. [DOI] [PubMed] [Google Scholar]
- 28.Gershenson DM. Why American women are not receiving state-of-the-art gynecologic cancer care. Cancer J. 2001;7:450–457. [PubMed] [Google Scholar]
- 29.Office of Disease Prevention and Health Promotion. Washington, DC: US Department of Health and Human Services; 2020. [Apr 24;2013 ]. Healthy People. [Google Scholar]