Abstract
Long known as a coat system that generates small transport vesicles from the endoplasmic reticulum (ER), the COPII coat also drives ER export of cargo proteins that are too large to be contained within these canonical carriers. With crystal and cryo-EM structures giving an atomic level view of coat structure, current advances in the field have focused on understanding how the coat adapts to the different geometries of the underlying cargo. Combined with a growing appreciation for the specific roles of individual COPII paralogs in diverse aspects of mammalian physiology, the field is poised to understand how coat assembly and post-translational modification permits structural rigidity but geometric flexibility to handle the diverse cargoes that exit the ER.
Introduction
Vesicles that bud from the endoplasmic reticulum (ER) to initiate intracellular transport of lipid and protein cargoes are generated by a set of cytoplasmic coat proteins known as the COPII coat. Building on a catalog of yeast mutants [1] and in vitro reconstitution of ER-Golgi tranpsort events [2], the COPII coat was initially defined almost two decades ago [3]. Since that time, our understanding of COPII function has been deepened by ever more minimal reconstitution systems [4,5], crystal structures of the individual proteins [6–8] and cryo-EM reconstructions of the multi-protein assemblies that ultimately drive vesicle formation [9–11]. With the coat machinery well-defined, current challenges lie in understanding (i) the underlying physical principles that govern vesiculation when the coat proteins assemble on the membrane, and (ii) how the coat is regulated to adapt to the specific physiological needs of different cells. The recent appreciation of the role of COPII function in human disease and development has provided exciting new tools to further explore both of these aspects and promises a new era of understanding the flexibility of ER exit.
Biophysics of COPII-mediated vesicle formation
The canonical COPII coat comprises five soluble cytoplasmic proteins that assemble in a hierarchical manner on the ER membrane (Figure 1). Coat assembly is initiated by the small GTPase, Sar1, which becomes membrane-associated when loaded with GTP, an event facilitated by its guanine nucleotide exchange factor, Sec12, an ER resident membrane protein [12]. GTP-binding by Sar1 causes a conformational change that exposes an amphipathic -helix that embeds shallowly in the ER membrane. Activated Sar1 in turn binds the dimeric cargo adaptor platform, Sec23/Sec24. Sec24 serves as the primary site of cargo interaction [13], recognizing specific ER export signals on diverse proteins [14–16]. Sec23 modulates the GTP cycle of Sar1, acting as its GTPase activating protein (GAP) by contributing essential catalytic residues [6]. Finally, the tetrameric Sec13/Sec31 complex is recruited. Sec31 potentiates the GAP activity of Sec23 by optimizing amino acid positions around the catalytic pocket [17,18]. Sec31 also drives vesicle formation by polymerizing into a polyhedral cage structure [9]. The role of Sec13 in this event seems to be to provide structural rigidity to the cage such that the assembled polymer has sufficient force to exert shape changes on the underlying membrane [19]. Most organisms express multiple isoforms of each COPII component, although the physiological significance of this diversification is still being elucidated (described more fully below).
Figure 1. Structure and assembly of the COPII coat.
The guanine nucleotide exchange factor, Sec12 (4H5I [8]) catalyzes GTP loading on Sar1, which switches from a cytosolic GDP-bound form (1F6B [59]) to a membrane-associated GTP-bound form (1M2O [6]) through exposure of an N-terminal amphipathic -helix. Membrane-associated Sar1 recruits Sec23/Sec24 (1M2V [6]). Sec24 provides cargo-binding function by directly interacting with sorting signals on transmembrane clients. The Sar1/Sec23/Sec24 “pre-budding” complex in turn recruits Sec13/Sec31 (2PM6 and 2PM9 [7]). Sec13/Sec31 self-assembles into a polyhedral cage (inset, adapted by permission from Macmillan Pulishers Ltd: Nature [9], copyright 2006) that at least in part drives membrane curvature and contributes to vesicle scission. Sec23 is the GTPase-activating protein for Sar1, with Sec31 further contributing to hydrolysis via a proline-rich domain that extends across the surface of Sec23/Sar1. Sec16 is a peripheral component that binds to Sec13 (3MZK [40]), modulates GTPase activity by preventing Sec31 action and otherwise contributes to vesicle formation in poorly understood ways.
As crystal structures of individual COPII sub-complexes have been solved, we have gained an ever more detailed picture of coat architecture. This atomic-level insight has coupled nicely with minimal reconstitution experiments to appreciate the multiple functions of the different coat components with respect to membrane bending [20,21], cargo capture [22,23] and vesicle release [20,21]. Recruitment of full-length Sar1 to synthetic liposomes induces tubulation, suggesting membrane curvature could be initiated by insertion of the amphipathic -helix [20]. Such asymmetric insertion could drive membrane bending by the bilayer couple hypothesis, which posits that expansion of one leaflet of a bilayer will cause compression of the opposing bilayer leading to membrane curvature [24]. Ectopic recruitment of a truncated form of Sar1 that lacks the amphipathic helix reduced the amount of tubulation but still permitted downstream recruitment of Sec23/Sec24 and Sec13/Sec31, which led to distinct curved budding profiles that remained attached to the parent liposome [20]. Similar experiments in permeabilized mammalian cells using GTP-locked forms of Sar1 led to the conclusion that both GTP hydrolysis by Sar1 and the amphipathic helix are required for vesicle release from the membrane [21]. More recent experiments have re-examined the physical effect of protein addition to synthetic liposomes, concluding that steric crowding effects of densely bound proteins are sufficient to drive curvature without helix insertion [25].
The observation that highly curved budding profiles could form independent of helix insertion suggests that the bilayer couple mechanism cannot be entirely responsible for the spherical morphology of vesicles. Instead, additional curvature likely comes from the rest of the COPII coat. The crystal structure of Sec23/Sec24 reveals a concave surface that is thought to be oriented towards the membrane [6]. The high concentration of basic amino acids on this surface could drive electrostatic interactions with acidic phospholipids to generate or capture curvature. Whether Sec23/Sec24 in isolation has the capacity to bend membranes remains to be tested, but the relatively small interface that links the two proteins together has called into question whether the dimer is robust enough to exert force on the membrane [26]. Instead, perhaps the concave structure formed by Sec23/Sec24 acts as a curvature sensor, facilitating recruitment to locally altered regions of the bilayer that are decorated with membrane-associated Sar1. Finally, there seems little doubt that Sec13/Sec31 can also contribute to membrane curvature through the ordered self-assembly of a polyhedral cage [9]. The relatively low resolution of the original cryo-EM structures did not permit accurate modeling of the ensuing crystal structure of the Sec13/Sec31 “edge” element [7]. More recent refined methods have yielded a pseudo-atomic model that permits a more detailed view of the likely interactions that drive cage assembly [11]. This insight may pave the way for a description of the energetics of coat polymerization and more precisely define the force that might be generated by this event. The new cage model also supports the proposal that Sec13 functions to rigidify the COPII coat while still permitting some degree of flexion [19]. This flexibility is probably key in allowing the rigid cage to adopt subtly different geometries that may be driven by the underlying energetic barrier created by the cargo-rich membrane (Figure 2). In this respect, large cargoes like collagen fibers (300 nm) and lipoprotein particles (150–500 nm) probably have unique requirements that dictate the geometry of the cage and thus the dimensions of the vesicle [27].
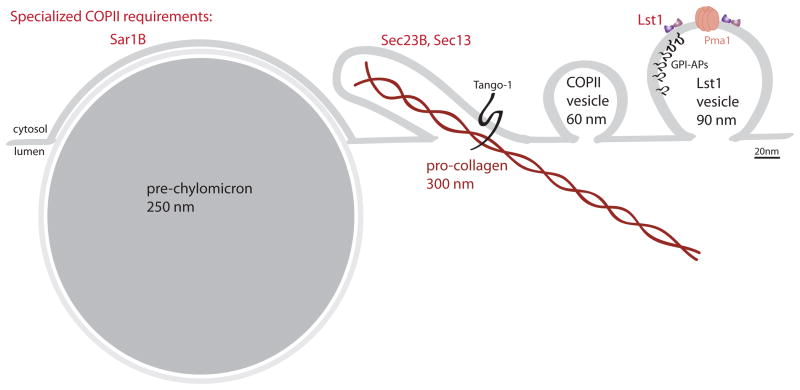
Figure 2. Flexible form and function of COPII vesicles.
The canonical COPII vesicles, as initially characterized in yeast, is 60–80nm in diameter and forms through the action of the minimal COPII coat, Sar1/Sec23/Sec24/Sec13/Sec31, on both complex ER membranes and synthetic liposomes. Different cargo molecules dictate distinct requirements for the size and shape of vesicles. Although the precise mechanisms by which these distinct morphologies are created, in many cases they require either specific COPII paralogs (noted in red) or additional cargo adaptors. In yeast, GPI-anchored proteins (GPI-APs) and the multimeric Pma1 complex depend on the Sec24 paralog, Lst1/Sfb2, for efficient capture into vesicles that are markedly larger than standard COPII vesicles. Pro-collagen assembles into long rods that are too long for a standard vesicle and are likely packaged into a more tubular structure, although these carriers have not been directly visualized. Efficient ER export of pro-collagen relies on the putative adaptor protein, TANGO-1, and is sensitive to mutations in SEC23B and knock-down of SEC13. Pre-chylomicrons are large lipid particles that accumulate in the ER when SAR1B is mutated.
Although the primary raison d’etre of the COPII coat is to initiate the intracellular itinerary of nascent secretory proteins, the cargo proteins themselves may also contribute to vesicle morphogenesis. Some have suggested that cargo proteins may be passive participants, e.g. bulk flow may be achieved by stochastic sampling of the ER membrane and lumen as vesicles form on the surface of the ER [28]. However, many cargo proteins expose sorting signals that interact directly with the COPII coat to more efficiently drive capture into vesicles [29]. In the case of soluble secretory proteins, this connection is indirect, using cargo receptors to bridge the membrane and connect cargo to coat [30]. Whether cargo proteins can initiate vesicle production remains to be seen, although recent experiments that link the GTP cycle of the COPII coat to the cargo adaptor subunit, Sec24, are suggestive of a coat system that responds to cargo occupancy [31]. Aside from potentially acting as vesicle nucleators, cargo proteins almost certainly confer distinct physical properties on the membrane, acting as barriers to membrane curvature. Asymmetrically distributed proteins seem to be particularly problematic in terms of opposing the action of the COPII coat. Yeast mutants that impede ER export of glycosylphosphatidylinositol-associated proteins (GPI-APs) also permit deletion of Sec13, suggesting that a more flexible coat is tolerated when the cargo burden of the underlying membrane is lessened [19]. This phenomenon also explains observations from human cells, where knockdown of Sec13 permitted efficient secretion of most cargo proteins but caused ER retention of pro-collagen [32], which probably also opposes curvature by virtue of its size, rigidity and elongated architecture. Indeed, pro-collagen and other large cargoes seem to have specific accessory factors that might regulate the coat directly by contributing rigidity or by inhibiting the GTP cycle of the coat and thereby preventing vesicle scission and prolonging coat assembly [27].
Regulation of the COPII coat
With outstanding molecular descriptions of the basic COPII coat in hand, the field is now turning to better appreciate how coat function might be regulated in the more complex environment of cells and tissues. In some cases, this comes in the form of direct regulation of the coat proteins themselves. Sec23 and Sec24 are phosphorylated by a Golgi resident kinase, Hrr25 [33]. In the case of Sec23, phosphorylation modulates sequential interactions with Sar1 and downstream effector proteins like TRAPP, thereby promoting the uni-directional movement of COPII vesicles towards the Golgi [33]. The function of Sec24 phosphorylation remains to be fully dissected but a role in regulated cargo binding and release seems plausible. Sec31 is also phosphorylated, which is important for its function [34], but the kinases that mediate these modifications remain undefined and the functional relevance in vivo is unclear. Human Sec31 is also ubiquitinated, a modification that is important for collagen trafficking but not bulk secretion [35]. This restricted requirement suggests a regulatory role, perhaps either in modulation of the GTPase stimulation activity or in providing additional structural rigidity to the outer coat scaffold. In this respect, it is interesting to note that the ubiquitin E3 ligase that recognizes Sec31 binds to a loop domain also bound by Sec13 [19,35]. Deletion of this loop domain is suggested to render Sec31 more rigid and able to deform the membrane surface so as to accommodate large cargo complexes [19].
Modulation of COPII function also employs accessory proteins that act in relatively poorly defined ways. Sec16 is an essential membrane-associated protein that interacts with all of the COPII coat proteins and is thought to scaffold coat assembly [36]. In metazoans, Sec16 is a target of multiple kinases that regulate its association with the ER and thus indirectly influence the architecture of ER exit sites that give rise to COPII vesicles [37,38]. Recently, Sec16 has also been implicated in the catalytic regulation of COPII coat function by inhibiting recruitment of Sec31 to the Sar1/Sec23 complex and thereby reducing the GTPase activity of the coat [31,39]. This could serve to prolong the lifetime of the inner coat on the ER membrane, or could delay the scission event that uses GTP hydrolysis to cause vesicle release. That this effect of Sec16 is partially dependent on an interaction with Sec24 is suggestive of a somewhat coordinated GTP cycle, with cargo occupancy by Sec24 potentially influencing catalysis indirectly through Sec16 [31]. Interestingly, Sec16 shares some structural features with Sec31, interacting with Sec13 via a similar -propellor domain insertion motif [40]. This interaction is not essential for yeast viability, but may serve to regulate the timing of coat assembly or vesicle scission in ways that remain to be fully characterized.
Additional accessory proteins are only beginning to be defined in mechanistic terms. TANGO1 and its partner, cTAGE5, are integral membrane proteins that couple pro-collagen to the COPII coat (Figure 2) [41,42]. The N-terminal lumenal domain of TANGO1 binds pro-collagen and a cytoplasmic proline-rich domain interacts with Sec23/Sec24 [41]. One model posits that interaction between TANGO1 and Sec23 precludes Sec31 recruitment, thereby delaying the GTP cycle of the coat and either prolonging coat assembly or preventing vesicle release, much like the role described above for Sec16 [27]. Another function for TANGO1 may derive from its topology whereby one of its transmembrane domains seems to dip into the bilayer in a hairpin structure. Depending on which leaflet this helix inserts in, TANGO1 may either oppose the curvature induced by the COPII coat (inner leaflet) or augment its membrane bending function (outer leaflet). Both situations could be reconciled with models that would require TANGO1 to prevent premature formation of a spherical structure or to contribute extra force to bend the membrane around a rigid cargo.
Lipid modification is another avenue of potential regulation that remains to be fully explored. Phospholipase D is stimulated by Sar1 activation and in turn is required for tubulation by Sar1 [43]. Since phosphatidic acid (PA), the product of phospholipase D, is a conical lipid that can induce lipid packing defects, perhaps local generation of PA at initial sites of Sar1 insertion creates a lipid bilayer that is favorable for further membrane association by additional molecules of Sar1, thereby stimulating coat assembly. This stimulation event might be further enhanced by the action of a Sec23-interacting protein, p125, which contains homology to PA-preferring phospholipase A, and binds to both Sec23 and Sec31, although its precise function remain to be determined [44,45]. Phosphatidylinositols are also linked to COPII function and ER exit site architecture, although the mechanisms by which these less abundant lipids act remains to be determined [46,47].
COPII function in human disease and development
Most of the COPII genes in yeast are single copy and essential. SEC24 is the exception, with three genes encoding paralogs that each assembles with a single Sec23 to form heterodimers able to discriminate the full range of cargo proteins that must be sorted in the ER. Not surprisingly, the situation in mammals is more complex with two paralogs of Sar1, two of SEC23, four of SEC24 and two of SEC31 [48]. An additional SEC24 helps accommodate the much more complex proteome of secretory and membrane proteins handled by COPII in mammals. Part of this complexity is distributed among different tissues that specialize in the traffic of major secreted and membrane proteins.
As a result of tissue specific cargo protein expression, a requirement for individual paralogs of SEC24 in the transport of key proteins has emerged from genetic studies on mouse mutants. In one instance, two groups conducting forward genetic screens for mouse neural tube defects identified early chain terminating mutations in SEC24B, a brain specific paralog [49,50]. The mutations, likely null alleles, produced a severe defect in neural tube closure, chraniorachischisis, which is also seen in deletions of key neural epithelial cell surface signaling receptors such as frizzled. Further genetic, localization and biochemical studies showed that another key signaling receptor, Vangl, depends on Sec24B for its capture into COPII vesicles and thus in the absence of Sec24B, at least one of two Vangl paralogs, Vangl2, is retained in the ER and fails to appear on the proximal surface of neural epithelial cells. Sec24B almost certainly accommodates the capture of many other cargo proteins, but if so, none are required before around 11.5 days of embryonic development, which may explain why the SEC24B mutants showed a delayed developmental defect.
Among the human SEC24 paralogs, A and B are homologous and serve partially overlapping functions. C and D are closer to each other than to A and B. Deletion of C or D produces an early embryonic lethal phenotype distinct from that observed in the SEC24B mutants (David Ginsburg lab, unpublished). Surprisingly, deletion of SEC24A has no effect on development, but instead leads to an unusually low level of free and lipoprotein-bound cholesterol [51]. The effect on cholesterol production has been traced to a requirement for SEC24A in the packaging of a secreted serum protein, PCSK9, which controls the itinerary of the cell surface LDL receptor. In normal circumstances, PCSK9 bound to the extracellular ligand-binding domain of the LDL receptor diverts the receptor to the lysosome where it is degraded and thus unable to recycle to the cell surface. As a result, the internalization of LDL particles is reduced, leading to enhanced expression of the rate-limiting enzyme in cholesterol biosynthesis, HMG CoA-reductase. When the level of PCSK9 declines, a recycling itinerary of the LDL receptor is restored, which establishes a more balanced control of HMG CoA reductase activity. Human patients missing the gene for PCSK9 have a lower level of cholesterol and suffer fewer heart attacks [52]. Because PCSK9 is a soluble secreted protein, it would be oriented within the lumen of the ER where it could not make direct contact with the cytoplasmic COPII coat. One must therefore invoke a receptor protein in the ER membrane that bridges PCSK9 through the ER membrane to the SEC24A subunit. Another such cargo receptor, LMAN1, is required for the efficient secretion of two blood-clotting factors, V and VIII. Lesions in the LMAN1 gene result in a combined Factor V, VIII form of hemophilia [53].
Although the other COPII subunits do not appear to be directly involved in cargo sorting, their tissue specific loss in human mutants results in distinct pathologies. The two paralogs of SEC23, which encode the heterodimer partner subunit of SEC24, have largely overlapping patterns of tissue expression but the differences explain genetic lesions that lead to rather specific diseases. Mutations in SEC23B are associated with a rare form of anemia that results in deficient red cell production [54]. For reasons that are not yet clear, erythroid precursor cells express the SEC23B locus in preference to SEC23A, and any of a number of different mutations in SEC23B produce the same red cell deficit. SEC23A and B are expressed in most tissues but SEC23B appears to predominate in calvarial osteoblasts and skin fibroblasts [55]. Patients with point mutations in conserved residues of SEC23A present with a craniofacial disorder (CLSD) that affects the secretion of collagen and possibly other connective tissue proteins [56]. These mutations define a surface feature of Sec23 facing the cytoplasm and involved in forming a productive contact with Sec31 [18]. Skin fibroblasts cultured from homozygous CLSD patients display grossly distorted ER cisternae and smooth tubular projections that emanate from the ER exit face and appear to represent aborted efforts to form COPI vesicles. From this we conclude that Sec23 engagement with the Sec31 complex is crucial to compete the vesicle fission event that results in a COPII vesicle.
The SAR1B paralog appears to be most highly expressed in the intestinal epithelium and its loss in enterocytes results in a disease of lipid malabsorption, Anderson’s or Chylomicron Retention Disease [57]. Enterocytes may depend on Sar1A for the transport of most of the secretory proteome, but for some reason possibly related to the enormous size of chylomicrons – from 150–500nm in diameter – Sar1B may have a specialized role in adapting the COPII coat to a larger circumference. This same consideration may apply to the packaging of procollagen rigid rods into COPII vesicles, although no obvious connective tissue problem is associated with Anderson’s Disease.
Other proteins that interact with COPII subunits are known to influence collagen secretion. Monoubiquitination of Sec31 mediated by the klhl12 adaptor subunit of the Cullin E3 ligase complex is required for collagen secretion [35]. Klhl12 is poised at the ER exit site in punctae that align with Sec31. Overexpression of klhl12 creates an exaggerated COPII structure and greatly speeds the exit of procollagen from the ER. Klhl12 may modulate the polymerization of the COPII coat, which could then influence the packaging of other large particles such as lipoproteins and chylomicrons.
Among the diseases of collagen biogenesis, spondyloepiphysial dysplasia tarda (SEDT) appears to affect collagen packaging at the level of exit from the ER [58]. The protein product of the SEDT gene, Sedlin, interacts with TANGO1, the putative procollagen sorting receptor in the ER. Sedlin deficiency results in the accumulation of Sar1-GTP, thus the protein may have a direct or indirect role in the normal cycle of GTP hydrolysis and nucleotide exchange that accompanies the assembly of the COPII coat. By influencing the rate of GTP hydrolysis by Sar1, Sedlin could control the stability of the coat and promote the formation of membrane buds able to accommodate the long rigid rod of procollagen. Further progress in this area may require the reconstitution of procollagen or large lipoprotein packaging into COPII vesicles in the cell-free transport vesicle budding reaction.
Conclusions
Building on the solid foundation of genetics and biochemistry that established the COPII coat as the minimal machinery that drives ER export, the field currently seems to be moving in two different directions. The first is to take an ever more detailed view of the coat proteins themselves, building into existing structural models an understanding of the underlying physics that drives vesiculation. By fully dissecting the energetics of coat assembly and the contributions of each coat component to events like membrane deformation, we can gain a detailed molecular blueprint of the general requirements for intracellular traffic. The second approach zooms out to take a wide view of how cellular, environmental and physiological regulation impact coat function. Our growing appreciation that diverse human diseases are associated with distinct mutations in individual COPII proteins serves to highlight this complexity but also provides new tools for the more reductionist approach. The union of the two viewpoints of COPII function promises an exciting future to continue the characterization of this remarkably accommodating coat.
Acknowledgments
Research in the Miller lab is supported by the National Institute of General Medical Science of the National Institutes of Health under award numbers R01GM085089 and R01GM078186. R.S. is funded as an Investigator of the Howard Hughes Medical Institute and as a Senior Fellow of the UC Berkeley Miller Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- 2.Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- 3.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 5.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]
- 6.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 7.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/j.cell.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 8.McMahon C, Studer SM, Clendinen C, Dann GP, Jeffrey PD, Hughson FM. The structure of Sec12 implicates potassium ion coordination in Sar1 activation. J Biol Chem. 2012;287:43599–43606. doi: 10.1074/jbc.M112.420141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stagg SM, Gürkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 10.Stagg SM, LaPointe P, Razvi A, Gürkan C, Potter CS, Carragher B, Balch WE. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••11.Noble AJ, Zhang Q, O’Donnell J, Hariri H, Bhattacharya N, Marshall AG, Stagg SM. A Pseudo-atomic model of the COPII cage obtaned from CryoEM and mass spectrometry analysis. Nat Struct Biol. 2013 doi: 10.1038/nsmb.2467. Using a new gradient-fixation protocol to enrich for specific size classes of COPII cages, Noble and colleagues achieved a much higher resolution cryo-EM structure of assembled Sec13/Sec31 than was previously possible. This permitted fitting of a homology model based on the yeast crystal structure to give a pseudo-atomic view of the assembled cage. Further insight was derived from hydrogen-deuterium exchange experiments that measured coat flexibility in the unassembled and assembled forms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 13.Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 2002;21:6105–6113. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, Schekman R. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- 15.Mossessova E, Bickford LC, Goldberg J. SNARE selectivity of the COPII coat. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 16.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonny B, Schekman R. ER export: public transportation by the COPII coach. Curr Opin Cell Biol. 2001;13:438–443. doi: 10.1016/s0955-0674(00)00234-9. [DOI] [PubMed] [Google Scholar]
- 18.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23. Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••19.Copic A, Latham CF, Horlbeck MA, D’Arcangelo JG, Miller EA. ER Cargo Properties Specify a Requirement for COPII Coat Rigidity Mediated by Sec13p. Science. 2012 doi: 10.1126/science.1215909. This study probed the mechanistic function of Sec13 using a combination of genetics and biochemistry to demonstrate that the essential function of Sec13 is in partner with Sec31, likely contributing rigidity to the rod-like edge element of the COPII cage. This rigidity is particularly important when cells export asymmetrically distributed cargo proteins that likely confer a barrier to the curvature generated by the coat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka K, Morimitsu Y, Uchida K, Schekman R. Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol Cell. 1998;2:703–708. doi: 10.1016/s1097-2765(00)80168-9. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Nakano A. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol. 2005;12:167–174. doi: 10.1038/nsmb893. [DOI] [PubMed] [Google Scholar]
- 24.Sheetz M, Singer S. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •25.Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane bending by protein-protein crowding. Nature Cell Biology. 2012;14:944–949. doi: 10.1038/ncb2561. Wading into the complex issue of how membrane curvature is generated, this paper describes a relatively simple model whereby molecular crowding of surface-attached proteins can drive membrane bending on synthetic liposomes. This new paradigm relies simply on entropic effects of the attached proteins and may apply to many different vesicle generation systems. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerberg J, Kozlov M. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra V, Erlmann P. Protein export at the ER: loading big collagens into COPII carriers. EMBO J. 2011;30:3475–3480. doi: 10.1038/emboj.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thor F, Gautschi M, Geiger R, Helenius A. Bulk flow revisited: transport of a soluble protein in the secretory pathway. Traffic. 2009;10:1819–1830. doi: 10.1111/j.1600-0854.2009.00989.x. [DOI] [PubMed] [Google Scholar]
- 29.Barlowe C. Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol. 2003;13:295–300. doi: 10.1016/s0962-8924(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 30.Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79:777–802. doi: 10.1146/annurev-biochem-061608-091319. [DOI] [PubMed] [Google Scholar]
- •31.Kung LF, Pagant S, Futai E, D’Arcangelo JG, Buchanan R, Dittmar JC, Reid RJ, Rothstein R, Hamamoto S, Snapp EL, et al. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 2012;31:1014–1027. doi: 10.1038/emboj.2011.444. Starting with a novel mutation in yeast Sec24 that broadly impacts vesicle production, this study links the cargo-binding coat component to the GTPase cycle of the coat. The authors discover a mechanistic function for Sec16 in modulating the Sec31-stimulated GTPase activity of the coat by competing with Sec31 for binding to Sec23/Sar1. The Sec24 mutation reduces the impact of Sec16, leading to heightened GTPase activity of the assembled coat and the generation of smaller vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, Stephens DJ. Efficient coupling of Sec23–Sec24 to Sec13–Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci. 2008;121:3025–3034. doi: 10.1242/jcs.031070. [DOI] [PubMed] [Google Scholar]
- ••33.Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature. 2011;473:181–186. doi: 10.1038/nature09969. This is one of the first papers to delve into the question of how post-translational modification of the coat may participate in coat function. The authors define several phosphorylation sites on Sec23 that govern sequential interaction with Sar1, TRAPP and the Golgi-localized kinase that modifies Sec23. This cascade of interactions ensures that COPII vesicles move forward to the Golgi, where uncoating and fusion can occur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salama NR, Chuang JS, Schekman RW. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–217. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••35.Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. A second type of post-translational modification of the coat is characterized in this study, which identified an E3 ligase that ubiquitinates Sec31. This event is important for the efficient export of pro-collagen, linking specific coat modifications to important aspects of coat assembly (polymerization and/or GTPase activity) that influence generation of non-canonical carriers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller EA, Barlowe C. Regulation of coat assembly--sorting things out at the ER. Curr Opin Cell Biol. 2010;22:447–453. doi: 10.1016/j.ceb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farhan H, Wendeler MW, Mitrovic S, Fava E, Silberberg Y, Sharan R, Zerial M, Hauri H-P. MAPK signaling to the early secretory pathway revealed by kinase/phosphatase functional screening. J Cell Biol. 2010;189:997–1011. doi: 10.1083/jcb.200912082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zacharogianni M, Kondylis V, Tang Y, Farhan H, Xanthakis D, Fuchs F, Boutros M, Rabouille C. ERK7 is a negative regulator of protein secretion in response to amino-acid starvation by modulating Sec16 membrane association. EMBO J. 2011;30:3684–3700. doi: 10.1038/emboj.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Yorimitsu T, Sato K. Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites. Mol Biol Cell. 2012;23:2930–2942. doi: 10.1091/mbc.E12-05-0356. This study dissects the function of yeast Sec16 in vivo and in vitro, showing that Sec16 modulates the GTPase cycle of the COPII coat by interfering with normal hierarchical coat assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittle JRR, Schwartz TU. Structure of the Sec13–Sec16 edge element, a template for assembly of the COPII vesicle coat. J Cell Biol. 2010;190:347–361. doi: 10.1083/jcb.201003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, Katada T. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22:2301–2308. doi: 10.1091/mbc.E11-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pathre P, Shome K, Blumental-Perry A, Bielli A, Haney CJ, Alber S, Watkins SC, Romero G, Aridor M. Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. EMBO J. 2003;22:4059–4069. doi: 10.1093/emboj/cdg390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimoi W, Ezawa I, Nakamoto K, Uesaki S, Gabreski G, Aridor M, Yamamoto A, Nagahama M, Tagaya M, Tani K. p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J Biol Chem. 2005;280:10141–10148. doi: 10.1074/jbc.M409673200. [DOI] [PubMed] [Google Scholar]
- 45.Ong YS, Tang BL, Loo LS, Hong W. p125A exists as part of the mammalian Sec13/Sec31 COPII subcomplex to facilitate ER-Golgi transport. J Cell Biol. 2010 doi: 10.1083/jcb.201003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell. 2006;11:671–682. doi: 10.1016/j.devcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Shindiapina P, Barlowe C. Requirements for transitional endoplasmic reticulum site structure and function in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:1530–1545. doi: 10.1091/mbc.E09-07-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 49.Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2009 doi: 10.1038/ncb2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 2010;137:1067–1073. doi: 10.1242/dev.041434. [DOI] [PubMed] [Google Scholar]
- ••51.Chen X-W, Wang H, Bajaj K, Zhang P, Meng Z-X, Ma D, Bai Y, Liu H-H, Adams E, Baines A, Yu G, Sartor MA, Zhang B, Yi Z, Lin J, Young SG, Schekman R, Ginsburg D. SEC24A deficiency lowers plasma cholesterol through reduced PCSK9 secretion. eLife. 2013 doi: 10.7554/eLife.00444. in press Faced with the surprising viability of a SEC24A knockout mouse, the authors identify a very specific defect: reduced circulating cholesterol. This phenotype is traced to a decrease in secretion of the regulatory protein PCSK9, which modulates that fate of the LDL receptor and thereby influences cholesterol uptake from the blood and intracellular cholesterol homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B, Cunningham MA, Nichols WC, Bernat JA, Seligsohn U, Pipe SW, McVey JH, Schulte-Overberg U, de Bosch NB, Ruiz-Saez A, et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet. 2003;34:220–225. doi: 10.1038/ng1153. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, Chen W, Paw BH, Hopfner K-P, Holzmann K, Russo R, et al. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet. 2009;41:936–940. doi: 10.1038/ng.405. [DOI] [PubMed] [Google Scholar]
- 55.Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–634. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, Zhang G, Hamamoto S, Schekman R, Ravazzola M, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- 57.Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- 58.Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Mironov A, Jr, Zelante L, et al. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 2012;337:1668–1672. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang M, Weissman JT, Beraud-Dufour S, Luan P, Wang C, Chen W, Aridor M, Wilson IA, Balch WE. Crystal structure of Sar1-GDP at 1. 7 A resolution and the role of the NH2 terminus in ER export. J Cell Biol. 2001;155:937–948. doi: 10.1083/jcb.200106039. [DOI] [PMC free article] [PubMed] [Google Scholar]