Abstract
Background
There are few clinical data to guide the use of cryoprecipitate in severely injured trauma patients. Cryoprecipitate is a rich source of fibrinogen, and has been associated with improved survival in animal as well as limited human studies. Our objective was to identify patterns and predictors of cryoprecipitate use and determine whether transfusing cryoprecipitate was associated with improved survival.
Methods
This secondary analysis of 1238 of 1245 PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study patients who had timed transfusion data included 359 (29%) who received cryoprecipitate. For this analysis, one dose of cryoprecipitate was defined as 10 units. Unadjusted predictors of cryoprecipitate use were identified using logistic regression. Multivariable time-dependent Cox models were performed to examine the association of cryoprecipitate on time to in-hospital death.
Results
Cryoprecipitate use varied significantly by center, ranging from 7–82%. Among patients who received cryoprecipitate, the median number of units infused by 24 hours was 10 (IQR: 10–20). The median time from admission to first cryoprecipitate unit was 2.7 hours (IQR: 1.7–4.4 hours). Of those who died a hemorrhagic death within six hours of admission, 72% received no cryoprecipitate. Other unadjusted predictors of cryoprecipitate use included, Injury Severity Score (ISS), initial fibrinogen levels, base deficit, INR, PT/PTT, hemoglobin, damage control surgery and surgical intervention of the chest and abdomen. Cryoprecipitate use was not associated with in-hospital mortality after adjusting for initial pH, initial hemoglobin, ED systolic blood pressure, ED GCS, blood product use, ISS and center.
Conclusions
Ten US Level 1 trauma centers vary greatly in their timing and use of cryoprecipitate in severely injured trauma patients. We could not identify any association of cryoprecipitate use with in-hospital mortality, although most patients did not receive this product. Randomized controlled studies are needed to determine if cryoprecipitate (or fibrinogen concentrates) have a beneficial effect.
Level of Evidence
II
Keywords: PROMMTT, Massive Transfusion, Bleeding, Trauma, Injury, Fibrinogen, Cryoprecipitate
INTRODUCTION
Hemorrhage remains the most common potentially preventable cause of traumatic death.1 Recent studies have refocused attention on blood component resuscitation of trauma patients suffering hemorrhagic shock and the early coagulopathy of trauma.2–4 The most severely injured patients are coagulopathic, have suffered substantial bleeding, will likely require significant transfusion,5 and the majority of these patients die from bleeding within three hours of hospital arrival.6 The high mortality risk in these transfused patients has generated studies on the early and optimal use of all blood products, including red blood cells (RBCs), fresh frozen plasma (FFP), platelets and cryoprecipitate.6–11 Early use of blood products as the primary resuscitation fluid (while minimizing crystalloid resuscitation) is one component of damage control resuscitation (DCR),3 which when implemented early is associated with improved survival, lower overall use of blood products and decreased inflammatory complications.12 While significant attention has been paid to FFP and platelets, comparatively little published data on use and outcomes after cryoprecipitate therapy exists. Interestingly, the current DCR clinical practice guideline from the Joint Theater Trauma System suggests that cryoprecipitate be transfused early, with the first units of plasma, platelets and RBCs in patients suffering substantial bleeding.13, 14
Cryoprecipitate is a pooled human blood product derived from the precipitate fraction of cold-thawed human plasma. It is manufactured by thawing a unit of FFP at temperatures just above freezing (1–6 °C), then centrifuging to remove plasma. Cryoprecipitate typically contains Factor I (fibrinogen), Factor VIII, Factor XIII, vWF, and fibronectin. Each unit should contain ≥ 80 IU of Factor VIII and ≥ 150 mg of fibrinogen in approximately 5 to 20 mL of plasma.15 Thus a “10 pack” of cryoprecipitate should contain ≥ 1.5 grams of fibrinogen. Due to variability in the manufacturing process, the actual allowed fibrinogen content varies by up to 600%. Cryoprecipitate is stored frozen at −18°C, must be thawed before infusing, and crossmatching and ABO compatibility testing are not required before infusion.15 Complications associated with cryoprecipitate use are assumed to be similar to those of FFP.
While cryoprecipitate has traditionally been transfused when plasma fibrinogen levels are < 100 mg/dL (1 g/L), it appears that this cutoff is based on six patients from a small study in 1987.16, 17 Recent reviews have raised this cutoff to 1.5–2 g/L, and document the lack of data to define critical starting and ending fibrinogen targets and thus guide rational use of this blood product.17–23 A recent survey of cryoprecipitate use failed to establish a correlation between fibrinogen level and cryoprecipitate infusion.22 In several European countries, cryoprecipitate is no longer used, instead concentrates are available that deliver consistent amounts of fibrinogen.20 In the resuscitation of bleeding trauma patients, early fibrinogen infusion appears to be associated with favorable outcomes in uncontrolled studies.24, 25 Two small randomized trials have been completed, demonstrating improved outcomes.26, 27 This experience has led to a randomized pilot study in trauma patients, exploring its use in the prehospital arena.28 (http://clinicaltrials.gov/ct2/show/NCT01475344).
Despite a dramatic increase in research efforts and numerous publications directed at optimizing resuscitation after hemorrhagic shock and reversal of acute coagulopathy, little quality data exist today to guide cryoprecipitate transfusion (or fibrinogen replacement) in rapidly bleeding trauma patients. We hypothesized that the early or increased use of cryoprecipitate in the PROMMTT patients would be associated with improved outcomes.
METHODS
The Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study enrolled 1,245 adult trauma patients from 10 US Level 1 trauma centers during July 2009–October 2010.6 Real-time data collection on timed infusions and other treatments and their indications was initiated on consecutive patients until active resuscitation ended or patients were identified to be ineligible for the study. Information on in-hospital mortality, complications, and later treatment were recorded daily from the medical record until death or discharge. Additional details regarding the design and main results of PROMMTT have been previously published.6, 29 PROMMTT was approved by the local Institutional Review Boards at all ten clinical sites and the Data Coordinating Center as well as the US Army Human Research Protections Office.
For this secondary analysis, we examined the use of cryoprecipitate and the primary outcomes of interest were in-hospital mortality at 6 and 24 hours, and 30 days. Demographics, injury, and treatment characteristics were compared using the Mann-Whitney test for continuous variables and the Pearson Chi-square for categorical variables. Several different multivariable analyses were performed to answer different clinically-relevant questions about cryoprecipitate use and mortality. For all models, purposeful variable selection strategies were utilized, confounders were retained in the model if they changed the univariable association ≥ 10%,30, 31 and center was included as a random effect using shared frailty. In these multivariable models, units of cryoprecipitate, RBCs, plasma, and platelets were modeled as uncumulated time-dependent variables, all other covariates were modeled at baseline only, and time intervals were defined by the occurrence of death. The rationale for not cumulating blood products was the focus on the fibrinogen component of cryoprecipitate and other blood products, which is consumed in the formation of clots.
Two sets of time-dependent Cox proportional hazards models were constructed with risk of death beginning at minute 31 and in-hospital mortality evaluated at three time points (6 and 24 hours and 30 days). Model set 1 included all patients and model set 2 was only among coagulopathic patients (INR≥1.3). The proportional hazards assumption was tested for every model using the global test. Models were then stratified on time in three intervals (0–6 hours, 6–24 hours, and 24 hours–30 days) because hazards were not proportional in the 0–24 hour and 0–30 day time period.
In order to examine the association of early cryoprecipitate on mortality, another set of Cox proportional hazards models was developed defining early use as infusion of cryoprecipitate within two hours of admission (dichotomous variable). We also performed a sensitivity analysis alternately defining the exposure as receiving cryoprecipitate within one hour of admission.
Because fibrinogen is found in plasma, platelets, and cryoprecipitate, we used the following formula to calculate a fibrinogen:RBC ratio, utilizing average amounts of fibrinogen. Fibrinogen levels in plasma are 3 grams/L, thus 160–200 mL of plasma in a unit of FFP contains approximately 0.5 grams; 10 U of cryoprecipitate contains approximately 2.0 grams; and the 200–300 mL of plasma in a unit of apheresis platelets contains about 0.75 grams of fibrinogen (personal communication, John Hess). Using these estimates, we created five groups of fibrinogen:RBC ratios and analyzed how these ratios changed over 24 hours.
RESULTS
Among the 1238 patients with cryoprecipitate information recorded, 359 received cryoprecipitate within the first 24 hours after admission (29%). Among patients classified as substantially bleeding (≥4 units of RBCs transfused within 6 hours of admission or died of hemorrhage within 6 hours of admission) cryoprecipitate was infused in 37% within 24 hours. Patients receiving cryoprecipitate were more likely to be Caucasian (77% vs. 64%, p<0.001). Age, sex and Hispanic ethnicity were not different among patients receiving cryoprecipitate compared to those who did not. Cryoprecipitate use varied significantly by center, ranging from 7 to 82% in transfused patients (p<0.001) (Table 1).
Table 1.
Characteristics of PROMMTT patients by cryoprecipitate status
| No cryo (N=879) | Any cryo (N=359) | ||||||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | N non- missing | Median (IQR) | N (%) | N non- missing | Median (IQR) | N (%) | P- value |
| Age (years) | 878 | 38 (25–54) | 359 | 38 (23–54) | .579 | ||
| Male sex | 879 | 646 (73) | 359 | 273 (76) | .352 | ||
| Race (white) | 875 | 561 (64) | 357 | 275 (77) | <.001 | ||
| Hispanic ethnicity | 829 | 158 (19) | 339 | 81 (24) | .063 | ||
| Center | 879 | 359 | <.001 | ||||
| 1 | 305 | 285 (93) | 305 | 20 (7) | |||
| 2 | 136 | 94 (69) | 136 | 42 (31) | |||
| 3 | 61 | 40 (66) | 61 | 21 (34) | |||
| 4 | 128 | 23 (18) | 128 | 105 (82) | |||
| 5 | 143 | 111 (78) | 143 | 32 (22) | |||
| 6 | 110 | 93 (85) | 110 | 17 (15) | |||
| 7 | 105 | 79 (75) | 105 | 26 (25) | |||
| 8 | 121 | 64 (53) | 121 | 57 (47) | |||
| 9 | 101 | 68 (67) | 101 | 33 (33) | |||
| 10 | 28 | 22 (79) | 28 | 6 (21) | |||
| Injury characteristics | |||||||
| ISS | 877 | 24 (14–34) | 359 | 27 (18–38) | <.001 | ||
| ISS ≥ 25 | 429 (49) | 211 (59) | |||||
| AIS head | 879 | 0 (0–3) | 359 | 0 (0–4) | .013 | ||
| AIS face | 879 | 0 (0-0) | 359 | 0 (0–1) | .175 | ||
| AIS chest | 879 | 3 (0–4) | 359 | 3 (0–4) | .057 | ||
| AIS abdomen | 879 | 2 (0–3) | 359 | 2 (0–3) | <.001 | ||
| AIS extremities | 879 | 2 (0–2) | 359 | 2 (0–3) | .113 | ||
| AIS external | 879 | 0 (0–1) | 359 | 1 (0–1) | .009 | ||
| Blunt injury | 879 | 558 (63) | 359 | 233 (65) | .637 | ||
| Sites of bleeding | |||||||
| Head | 879 | 127 (14) | 359 | 51 (14) | .912 | ||
| Face | 879 | 242 (28) | 359 | 98 (27) | .934 | ||
| Neck | 879 | 39 (4) | 359 | 18 (5) | .660 | ||
| Chest | 879 | 199 (23) | 359 | 100 (28) | .052 | ||
| Abdomen | 879 | 268 (30) | 359 | 127 (35) | .094 | ||
| Pelvis | 879 | 95 (11) | 359 | 68 (19) | <.001 | ||
| Limb | 879 | 315 (36) | 359 | 125 (35) | .734 | ||
| Initial ED vital signs and laboratory data | |||||||
| Systolic blood pressure (mmHg) | 862 | 106 (86–127) | 345 | 108 (84–130) | .796 | ||
| Heart rate (bpm) | 858 | 103 (85–123) | 354 | 111 (89–128) | .007 | ||
| Temperature (°C) | 481 | 36.2 (35.6–36.6) | 149 | 36.1 (35.5–36.6) | .146 | ||
| GCS | 808 | 14 (3–15) | 323 | 10 (3–15) | .004 | ||
| Base deficit (mEq/L) | 665 | 6 (3–10) | 289 | 7 (4–11) | .012 | ||
| pH | 675 | 7.27 (7.19–7.34) | 295 | 7.26 (7.16–7.34) | .462 | ||
| INR | 751 | 1.2 (1.1–1.4) | 324 | 1.2 (1.1–1.6) | .001 | ||
| PTT (sec) | 732 | 27 (24–32) | 307 | 29 (25–37) | <.001 | ||
| PT (sec) | 692 | 14.9 (13.3–16.4) | 204 | 16.3 (14.2–19.7) | <.001 | ||
| Fibrinogen (mg/dL) | 222 | 213 (169–286) | 196 | 215 (143–307) | .449 | ||
| ≤ 100 | 12 (5) | 20 (10) | .041 | ||||
| ≤ 150 | 38 (17) | 58 (30) | .002 | ||||
| TEG MA (mm) | 221 | 64 (59–66) | 19 | 57 (52–62) | <.001 | ||
| TEG α (degrees) | 221 | 73 (68–76) | 19 | 67 (56–71) | <.001 | ||
| Platelets (109/L) | 826 | 236 (188–287) | 343 | 206 (161–254) | <.001 | ||
| Hemoglobin (g/dL) | 842 | 11.8 (10.4–13.4) | 350 | 11.5 (9.8–13.1) | .005 | ||
| Hematocrit (%) | 840 | 35.0 (31.0–39.3) | 348 | 33.8 (29.0–38.0) | .001 | ||
| Lactate (mg/dL) | 274 | 4.1 (2.7–6.3) | 129 | 4.8 (3.2–7.7) | .032 | ||
| Treatment | |||||||
| 6 hour totals (units) | |||||||
| RBC | 870 | 3 (2–6) | 354 | 6 (3–13) | <.001 | ||
| Plasma | 870 | 2 (0–4) | 354 | 4 (0–9) | <.001 | ||
| Platelet | 870 | 0 (0-0) | 354 | 0 (0–12) | <.001 | ||
| 24 hour totals | |||||||
| RBC | 879 | 4 (2–7) | 359 | 7 (4–17) | <.001 | ||
| Plasma | 879 | 2 (0–6) | 359 | 6 (2–13) | <.001 | ||
| Platelet | 879 | 0 (0-0) | 359 | 6 (0–12) | <.001 | ||
| Any LSI in ED | 879 | 419 (48) | 359 | 192 (53) | .063 | ||
| Any surgical intervention | 879 | 561 (64) | 359 | 252 (70) | .032 | ||
| Any Damage Control Surgery | 561 | 148 (26) | 252 | 91 (36) | .005 | ||
| Outcomes | |||||||
| Unadjusted in-hospital mortality | |||||||
| 6 hour | 879 | 78 (9) | 359 | 24 (7) | .204 | ||
| 24 hour | 879 | 101 (11) | 359 | 47 (13) | .431 | ||
| 30 day | 879 | 163 (19) | 359 | 95 (26) | .002 | ||
| Overall | 879 | 165 (19) | 359 | 99 (28) | .001 | ||
Cryo = cryoprecipitate; ISS = injury severity score; AIS = abbreviated injury score; LSI= life saving intervention; ED = emergency department.
Patients receiving cryoprecipitate had higher Injury Severity Score (ISS) and higher head, abdomen and external Abbreviated Injury Scale (AIS) scores compared to patients who did not receive cryoprecipitate (Table 1). Patients receiving cryoprecipitate were also more likely to have pelvic bleeding, but there were no other significant differences by bleeding site, mechanism of injury, or specific cause of injury. Patients receiving cryoprecipitate had significantly higher admission heart rates, base deficit, INR, and lactate and significantly lower admission Glasgow Coma Scale (GCS), platelet count, thrombelastography (TEG) MA and α, and hemoglobin, hematocrit. Among the 196 patients given cryoprecipitate with an admission fibrinogen level recorded, 22.9%, 7.4%, and 5.0% had a level below 150, 100, and 80 mg/dL, respectively. Patients receiving cryoprecipitate were more likely to have surgical intervention. Patients receiving cryoprecipitate received more total units of RBCs, plasma and platelets at both 6 hours and 24 hours (Table 1).
Among coagulopathic patients (INR of 1.3 or greater), the pattern of significant differences was similar to the pattern among all patients (Table 2). Among coagulopathic patients, patients receiving cryoprecipitate had significantly higher ISS, heart rate, INR, PTT/PT, and lactate and lower fibrinogen, TEG MA and α, hemoglobin, and hematocrit (Table 2).
Table 2.
Characteristics of coagulopathic patients (INR≥1.3) by cryoprecipitate status
| No cryo (N=291) | Any cryo (N=158) | ||||
|---|---|---|---|---|---|
| Demographic characteristics | Median (IQR) | N (%) | Median (IQR) | N (%) | P- value |
| Age (years) | 35 (23–54) | 30 (21–50) | .06 | ||
| Male sex | 212 (73) | 126 (80) | <.001 | ||
| Race (white) | 191 (66) | 125 (79) | <.001 | ||
| Hispanic ethnicity | 49 (17) | 32 (20) | .37 | ||
| Center | <.001 | ||||
| Injury characteristics | |||||
| ISS | 29 (17–40) | 34 (23–43) | <.001 | ||
| Blunt injury | 201 (69) | 107 (68) | .77 | ||
| Initial ED vital signs and laboratory data | |||||
| Systolic blood pressure (mmHg) | 101 (84–120) | 102 (85–126) | .18 | ||
| Heart rate (bpm) | 110 (89–130) | 114 (98–142) | <.001 | ||
| Temperature (°C) | 36.0 (35.4–36.5) | 36.0 (35.5–36.5) | .58 | ||
| GCS | 3 (3–14) | 3 (3–14) | .08 | ||
| Base deficit (mEq/L) | 8 (4–12) | 8 (5–12) | .17 | ||
| pH | 7.23 (7.12–7.31) | 7.22 (7.12–7.32) | .70 | ||
| INR | 1.5 (1.3–1.7) | 1.6 (1.4–2.0) | <.001 | ||
| PTT (sec) | 32.3 (28.0–39.8) | 36.0 (30.6–47.1) | <.001 | ||
| PT (sec) | 17.1 (15.9–18.9) | 18.5 (16.7– 22.3) | <.001 | ||
| Fibrinogen (mg/dL) | 182 (140–219) | 143 (106– 195) | <.001 | ||
| ≤ 100 | 10 (11) | 19 (20) | .08 | ||
| ≤ 150 | 29 (32) | 54 (58) | <.001 | ||
| TEG MA (mm) | 61 (57–65) | 55 (39–57) | .005 | ||
| TEG α (degrees) | 71 (66–74) | 58 (54–69) | .003 | ||
| Platelets (109/L) | |||||
| Hemoglobin (g/dL) | 11.1 (9.6–12.4) | 10.2 (8.6– 12.0) | <.001 | ||
| Hematocrit (%) | 32.9 (28.8–37.2) | 30.0 (26.0– 35.0) | <.001 | ||
| Lactate (mg/dL) | 4.6 (2.9–7.6) | 5.6 (3.7–9.9) | .03 | ||
| Treatment | |||||
| 6 hour totals (units) | |||||
| RBC | 4 (2–7) | 9 (4–18) | <.001 | ||
| Plasma | 3 (1–6) | 7 (4–13) | <.001 | ||
| Platelet | 0 (0-0) | 6 (0–12) | <.001 | ||
| 24 hour totals | |||||
| RBC | 5 (3–9) | 11 (5–24) | <.001 | ||
| Plasma | 5 (2–9) | 8 (5–19) | <.001 | ||
| Platelet | 0 (0–6) | 6 (0–12) | <.001 | ||
| Any LSI in ED | 158 (54) | 98 (62) | .11 | ||
| Any surgical | |||||
| intervention | 164 (56) | 117 (74) | <.001 | ||
| Any Damage Control Surgery | 54 (33) | 46 (39) | .27 | ||
| Outcomes | |||||
| Unadjusted in-hospital mortality | |||||
| 6 hour | 44 (15) | 17 (11) | .20 | ||
| 24 hour | 54 (19) | 21 (13) | .15 | ||
| 30 day | 82 (28) | 60 (38) | .03 | ||
| Overall | 83 (29) | 63 (40) | .01 | ||
Cryo = cryoprecipitate; ISS = injury severity score; ED = emergency department; GCS = Glasgow coma score: INR = international normalized ratio; PTT = Partial thromboplastin time ; PT = Prothrombin time; TEG = Thrombelastography; MA = maximal amplitude; LSI= life saving intervention.
Multivariable predictors of cryoprecipitate use, adjusted for site as a random effect, included admission fibrinogen < 100 mg/dL, hemoglobin as a continuous variable, and pelvic bleeding (Table 3).
Table 3.
Multivariable predictors of cryoprecipitate use in PROMMTT using logistic regression*
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Admission fibrinogen < 100 mg/dL | 3.8 (1.6–8.9) | 0.003 |
| Pelvic bleeding | 2.0 (1.1–3.7) | 0.027 |
| Hemoglobin g/dL | 0.88 (0.79–0.98) | 0.018 |
also adjusted for center as a random effect
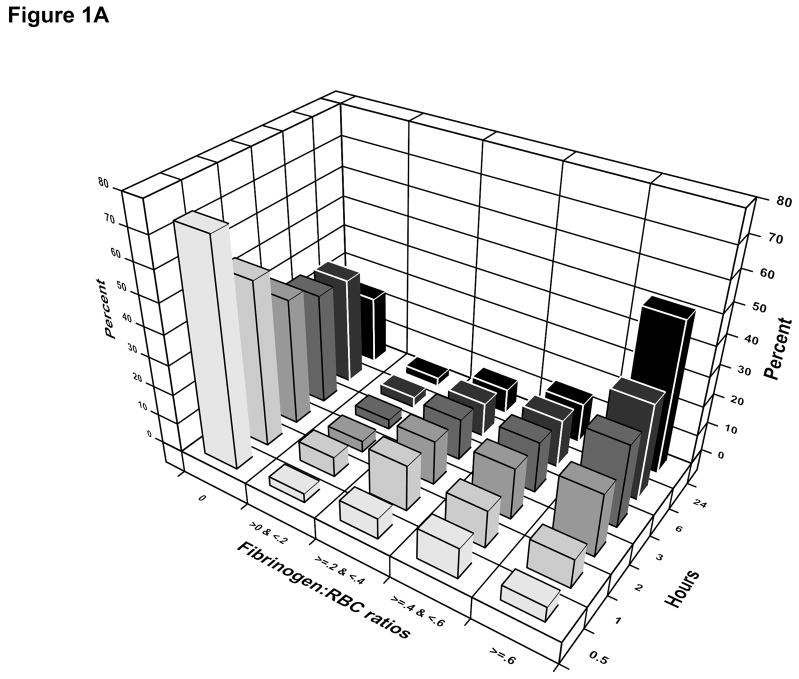
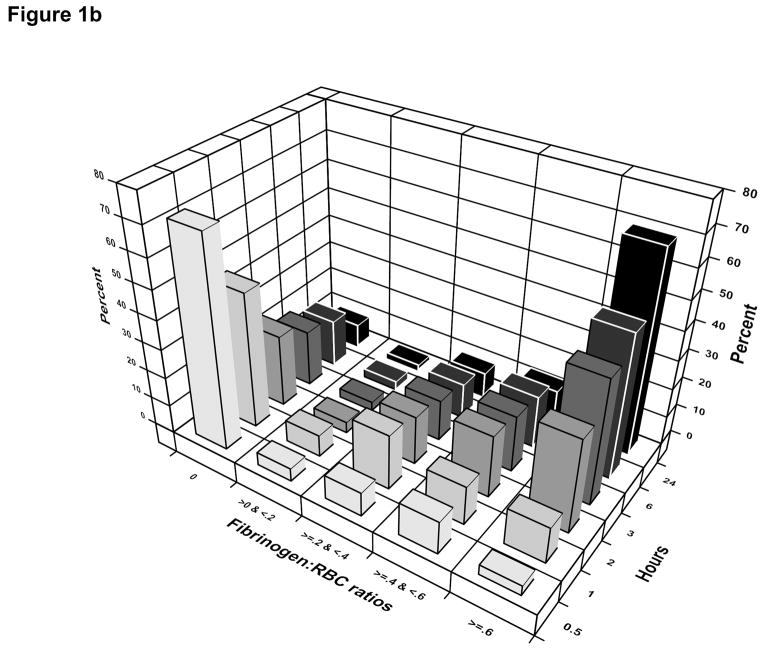
Among patients who received cryoprecipitate, the median number of units infused by 24 hours was 10 (IQR: 10–20) and the median time from admission to first cryoprecipitate unit was 2.8 hours (IQR: 1.7–4.5 hours). The median number of units of RBCs, plasma, and platelets at the time of first dose of cryoprecipitate received was 8 (IQR: 4–16), 6 (IQR: 3–12), and 6 (IQR: 0–6) respectively. The median time from admission to receipt of any fibrinogen from cryoprecipitate, plasma, or platelets was 70 minutes (IQR: 36–133 minutes). Ninety-four percent of patients receiving fibrinogen from any source received plasma first, 5% received platelets first, and 1% received cryoprecipitate first. The distribution of the ratio of grams of fibrinogen to units of RBC over time shows that 60% of patients received fibrinogen from any source before 2 hours (Figure 1a). The distribution of the ratio of grams of fibrinogen to units of RBC over time in patients with an INR > 1.3 shows that 80% of patients received some fibrinogen by 3 hours after admission (Figure 1b).
Figure 1.
Fibrinogen:RBC ratio over the first 24 hours after admission.
1a. All PROMMTT patients
1b. PROMMTT patients with an admission INR ≥ 1.3
Finally, in exploratory analyses, we examined the association of cryoprecipitate use on in-hospital mortality in several different ways (Table 4); however none showed a significant adjusted association of cryoprecipitate on survival. In general, regardless of how cryoprecipitate was modeled, associations of cryoprecipitate on 6 hour mortality were near the null or slightly protective. Associations for the 6–24 hour time interval were generally less stable because of fewer deaths during the interval.
Table 4.
Cox proportional hazards models examining the association of cryoprecipitate with in-hospital mortality during different time intervals1
| Exposure of interest | Population | 6 hour mortality | 6 – 24 hour mortality | 24 hour – 30 day mortality |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Time- dependent cryoprecipitate2 | All patients | 1.07 (0.44–2.63) | 1.75 (0.78–3.90) | 1.08 (0.61–1.89) |
| Time- dependent cryoprecipitate2 | Coagulopathic patients (INR≥1.3) | 0.84 (0.27–2.63) | 1.45 (0.47–4.43) | 1.41 (0.66–3.01) |
| Early cryoprecipitate (within 2 hours of admission)3 | Coagulopathic patients (INR≥1.3) | 0.97 (0.47–2.01) | 1.59 (0.48–5.28) | 0.77 (0.27–2.15) |
| Early cryoprecipitate (within 1 hour of admission) 3 | Coagulopathic patients (INR≥1.3) | 0.73 (0.22–2.46) | 1.70 (0.33–8.86) | 0.89 (0.11–6.85) |
| Time- dependent fibrinogen2 | All patients | 1.08 (0.67–1.73) | 0.91 (0.57–1.44) | 1.15 (0.95–1.41) |
All models are adjusted for ISS, center (as a random effect), and admission GCS, systolic blood pressure, pH, and hemoglobin
Also adjusted for time dependent RBC, plasma, and platelets
Also adjusted for receipt of plasma and platelets within 1 hour
DISCUSSION
In this study, we found wide differences in practice exist in the use of cryoprecipitate at ten level 1 trauma centers (Table 1), similar to the wide variability in blood product transfusion practices found in a separate study.4 Among these centers, use of cryoprecipitate varied between 7 to 82%. In patients who suffered substantial bleeding, cryoprecipitate was infused in only 37%. Patients who were transfused cryoprecipitate were more injured and physiologically perturbed than those who were not. In PROMMTT patients, we could not discern a survival advantage to cryoprecipitate transfusion.
Reviewing massive transfusion guidelines in effect at the start of PROMMTT (July 2009) revealed a variety of potential practice standards for cryoprecipitate transfusion. Three sites made no mention of cryoprecipitate in their guideline, and two sites transfused cryoprecipitate based on the presence of ongoing bleeding and a fibrinogen <100 mg/dl. The remaining five sites recommended transfusing cryoprecipitate after 6 to 15 units of RBCs had been infused. Only one site had a stated fibrinogen goal (>100 mg/dl) after cryoprecipitate infusion. Inclusion of cryoprecipitate in the massive transfusion protocols of PROMMTT sites varied significantly and likely resulted in the wide variation in the actual use of cryoprecipitate, justifying our adjustment for center as a random effect. How sites actually complied with their existing guidelines is not known.
Supporting our impression that clinicians are unsure how to use cryoprecipitate and therefore utilize the product “late,” the median time from admission to first cryoprecipitate unit was 2.8 hours (IQR: 1.7–4.5 hours). This timing is similar to the median time to hemorrhagic death in the overall PROMMTT study of 2.6 hours.6 Nascimento, et al. found that cryoprecipitate was transfused at a median of 4.5 hours.17 These data suggest that cryoprecipitate is infused very late, essentially as a last resort while the patient is dying. Clinicians recognize this as “throwing the kitchen sink” at the patient in a last ditch effort to save their patient. Interestingly, most patients suffering hemorrhagic death did not receive cryoprecipitate. Of the 98 patients who died of exsanguination within 24 hours of admission, 67% never received any cryoprecipitate. Seventy-two percent of those who exsanguinated within 6 hours of admission never received cryoprecipitate. It appears that the faster the patients died, the less likely they were to receive cryoprecipitate.
It is also clear from our data that patients who do receive cryoprecipitate are more severely injured, have lower admission fibrinogen and hemoglobin levels, are acidotic, coagulopathic, and have a lower MA and α-angle on their admission thromboelastogram (TEG) (Table 3). A study of admission TEG showed an association between increased mortality and α-angle < 56 and MA< 55 (related to function of fibrinogen).35 In PROMMTT 34 of 243 patients (14%) who received a TEG demonstrated a low α-angle or MA and thus met criteria for rapid fibrinogen replacement within 15 minutes of admission. All efforts to stop bleeding, interrupt this vicious cycle and restore the coagulation system toward normal are important. Thus replacing lost fibrinogen stores with cryoprecipitate is plausible.
The biologic plausibility of the beneficial effect of replacing fibrinogen is compelling. Factor 1 (fibrinogen) is essential to hemostasis as it is converted by thrombin to fibrin. Fibrinogen levels in non-injured humans average 2–3 g/L. Bleeding trauma patients rapidly deplete their fibrinogen levels, reaching plasma levels of 1g/L or worse. While thawed plasma is immediately available for resuscitation, it is difficult to raise fibrinogen levels using plasma-based resuscitation alone because the fibrinogen content of FFP is 1.5 g/L. Cryoprecipitate replenishment of fibrinogen levels in bleeding trauma patients is theoretically possible; however there is huge variability in the fibrinogen content of this product (between 7–30 g/L). Cryoprecipitate must also be thawed before it can be transfused, leading to inherent delays.
Some studies have found an association between cryoprecipitate transfusion and survival. Stinger, et al. found that transfusion of an increased fibrinogen:RBC ratio was independently associated with improved survival to hospital discharge, primarily by decreasing death from hemorrhage.10 Martini showed that lactated Ringers resuscitation causes an acute decrease in fibrinogen concentration and clot strength, followed by increases in fibrinogen concentration and clot strength within 24 hours due to an increase in fibrinogen synthesis among hemorrhagic shock patients.32, 33 Fries and colleagues have championed the early use of fibrinogen concentrates in rapidly bleeding trauma patients and have shown in animal studies that infusion of fibrinogen concentrates will decrease blood loss after severe liver injury.34 Sørensen et al. also concluded that thromboelastometry is the preferred method of measuring functional fibrinogen, especially when artificial colloids have been infused. 23, 26, 36 Their group has also published a small randomized study, showing that fibrinogen supplementation significantly decreased RBC transfusion after radical cystectomy.26
Rourke and colleagues recently showed that fibrinogen depletion occurs in bleeding trauma patients with acute traumatic coagulopathy and without replacement, worsens during resuscitation.24 In this study they utilized a 1:1:1:2 ratio of FFP:platelets:cryo:RBCs, which did not normalize the fibrinogen level. In this small study only 103 patients received ≥ 4 units of RBCs and just 39 received cryoprecipitate. Because they were transfusing a standardized ratio, they infused cryoprecipitate in nearly half the time than in the current study, a median of 103 min (IQR, 78–134 min) from admission. Interestingly, only high-dose supplementation of fibrinogen, either as cryoprecipitate or concentrate, was able to correct the fibrinogen-related coagulopathy after 6–12 units of RBCs. Lower fibrinogen levels were associated with poor outcomes, which seemed to improve with increasing fibrinogen administration.
Finally, Ranucci and Solomon have published an interesting and provocative perspective on the use of fibrinogen concentrate in patients with congenital fibrinogen deficiency versus cryoprecipitate in patients with substantial bleeding and an acquired fibrinogen deficiency.20 They feel that this constitutes a double standard and exposes bleeding patients to a product with uncertain amounts of fibrinogen plus the risks of a pooled allogeneic product.
The strengths of this study are the multicenter prospectively acquired data collection, documenting the wide variability in practice that exists at trauma centers in the US. We successfully captured the timing of cryoprecipitate use in 359 bleeding trauma patients of which 196 had an admission fibrinogen level. The main weakness is that by design, this observational study did not prescribe a transfusion strategy, so the effect of either an early or consistent transfusion plan could not be determined. Additionally, serial blood samples were not collected, nor were additional fibrinogen levels recorded other than at admission. TEG on admission was regularly used at only one center and thus little data are available.
Cryoprecipitate is transfused to replenish consumed or inactivated fibrinogen under the assumption that this will strengthen clots and decrease bleeding. Unfortunately, there are scant high-quality data supporting this practice, so poorly informed and highly variable practice continues. In addition to infectious and immunologic risks similar to other blood products, cryoprecipitate is pooled, thereby increasing the number of donor exposures. Another issue regarding cryoprecipitate use is lag time between admission, recognition of need, ordering the product, thawing and actual infusion. In the substantially bleeding patient this lag time likely poses significant risk. Cryoprecipitate is still used in the US and UK, but fibrinogen concentrates are now available in many countries and randomized trials are ongoing. We await the results of these efforts. Lastly it is underappreciated how variable the actual fibrinogen content is in cryoprecipitate. Future studies should include the measurement of fibrinogen content prior to the transfusion of each product.
In conclusion, it appears that clinicians are unsure when to initiate cryoprecipitate therapy and in what amount. This is largely due to the lack of convincing data demonstrating efficacy. Cryoprecipitate continues to be used because of its biologic plausibility, rather than quality outcome data. Like many products without quality data guiding appropriate use, cryoprecipitate is used late, or not at all, with 72% receiving no cryoprecipitate despite bleeding to death. These observational data serve to describe the current state of practice and as a foundation for a pilot intervention trial. Optimal use of cryoprecipitate (or fibrinogen concentrates) is yet another area that requires prospective randomized data collection.
Acknowledgments
Funding/Support: This project was funded by the U.S. Army Medical Research and Materiel Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center was supported by CTSA funds from NIH grant UL1 RR024148.
Role of the Sponsor: The sponsors did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit this manuscript for publication.
Footnotes
Disclaimer: The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
Previous Presentation of the Information Reported in the Manuscript: These data were presented at the PROMMTT Symposium held at the 71st Annual Meeting of the American Association for the Surgery of Trauma (AAST) on September 10-15, 2012 in Kauai, Hawaii.
AUTHOR CONTRIBUTIONS
Study concept and design: Holcomb, del Junco, Rahbar, Fox
Acquisition of data: Alarcon, Brasel, Bulger, Cohen, Cotton, Holcomb, Muskat, Myers, Phelan, Schreiber
Analysis and interpretation of data: Fox, Zhang, Holcomb
Drafting of the manuscript: Holcomb, Fox
Critical revision of the manuscript for important intellectual content: Holcomb, Fox, Zhang, White, del Junco, Rahbar, Wade, Alarcon, Brasel, Bulger, Cohen, Cotton, Muskat, Myers, Phelan, Schreiber
Statistical analysis: Fox, Zhang
Obtained funding: Rahbar
Administrative, technical, or material support: Rahbar, Holcomb, Fox, del Junco, Alarcon, Brasel, Bulger, Cohen, Cotton, Muskat, Myers, Phelan, Schreiber, Wade
Study supervision: Rahbar, Holcomb
Conflict of Interest Disclosures: Dr Holcomb reported serving on the board for Tenaxis, the Regional Advisory Council for Trauma, and the National Trauma Institute; providing expert testimony for the Department of Justice; grants funded by the Haemonetics Corporation, and KCI USA, Inc. and consultant fees from the Winkenwerder Company. Dr Wade reported serving on the Science Board for Resuscitation Products, Inc. and the Advisory Board for Astrazeneca. No other disclosures were reported.
Contributor Information
Erin E Fox, Email: erin.e.fox@uth.tmc.edu.
Xuan Zhang, Email: Xuan.Zhang@uth.tmc.edu.
Nathan White, Email: whiten4@u.washington.edu.
Charles E Wade, Email: charles.e.wade@uth.tmc.edu.
Bryan A Cotton, Email: bryan.a.cotton@uth.tmc.edu.
Deborah J del Junco, Email: Deborah.j.deljunco@uth.tmc.edu.
Eileen M Bulger, Email: ebulger@u.washington.edu.
Mitchell J Cohen, Email: mcohen@sfghsurg.ucsf.edu.
Martin A Schreiber, Email: schreibm@ohsu.edu.
John G Myers, Email: myersjg@uthscsa.edu.
Karen J Brasel, Email: kbrasel@mcw.edu.
Herb A Phelan, Email: herb.phelan@utsouthwestern.edu.
Louis H Alarcon, Email: AlarconL@ccm.upmc.edu.
Peter Muskat, Email: muskatp@UCMAIL.UC.EDU.
Mohammad H Rahbar, Email: mohammad.h.rahbar@uth.tmc.edu.
References
- 1.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34:158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 2.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study: Comparative Effectiveness of a Time-Varying Treatment With Competing Risks. Arch Surg. 2012:1–10. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, Ling Q, Harris RS, Hillyer CD. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50:493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 9.Duchesne JC, Islam TM, Stuke L, Timmer JR, Barbeau JM, Marr AB, Hunt JP, Dellavolpe JD, Wahl G, Greiffenstein P, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67:33–37. doi: 10.1097/TA.0b013e31819adb8e. [DOI] [PubMed] [Google Scholar]
- 10.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, Hess JR, Dubick MA, Simon CD, Beekley AC, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–S85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 11.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 12.Cotton BA, Reddy N, Hatch QM, Lefebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Army Institute of Surgical Research. [Accessed October 22,2012.];Joint theater trauma system clinical practice guideline: damage control resuscitation at level IIb/III treatment facilities. Available at: http://www.usaisr.amedd.army.mil/assets/cpgs/Damage_Control_Resuscitation_11_Oct%202012.pdf.
- 14.Duke MD, Guidry C, Guice J, Stuke L, Marr AB, Hunt JP, Meade P, McSwain NE, Jr, Duchesne JC. Restrictive fluid resuscitation in combination with damage control resuscitation: Time for adaptation. J Trauma Acute Care Surg. 2012;73:674–678. doi: 10.1097/TA.0b013e318265ce1f. [DOI] [PubMed] [Google Scholar]
- 15.AABB. [Accessed October 22,2012.];Circular of information for the use of human blood and blood components. Available at: http://www.aabb.org/resources/bct/Documents/coi0809r.pdf.
- 16.Ciavarella D, Reed RL, Counts RB, Baron L, Pavlin E, Heimbach DM, Carrico CJ. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol. 1987;67:365–368. doi: 10.1111/j.1365-2141.1987.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 17.Nascimento B, Rizoli S, Rubenfeld G, Fukushima R, Ahmed N, Nathens A, Lin Y, Callum J. Cryoprecipitate transfusion: assessing appropriateness and dosing in trauma. Transfus Med. 2011;21:394–401. doi: 10.1111/j.1365-3148.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- 18.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon C, Pichlmaier U, Schoechl H, Hagl C, Raymondos K, Scheinichen D, Koppert W, Rahe-Meyer N. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth. 2010;104:555–562. doi: 10.1093/bja/aeq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranucci M, Solomon C. Supplementation of fibrinogen in acquired bleeding disorders: experience, evidence, guidelines, and licences. Br J Anaesth. 2012;109:135–137. doi: 10.1093/bja/aes227. [DOI] [PubMed] [Google Scholar]
- 21.Fenger-Eriksen C, Moore GW, Rangarajan S, Ingerslev J, Sorensen B. Fibrinogen estimates are influenced by methods of measurement and hemodilution with colloid plasma expanders. Transfusion. 2010;50:2571–2576. doi: 10.1111/j.1537-2995.2010.02752.x. [DOI] [PubMed] [Google Scholar]
- 22.Tinegate H, Allard S, Grant-Casey J, Hennem S, Kilner M, Rowley M, Seeney F, Stanworth S. Cryoprecipitate for transfusion: which patients receive it and why? A study of patterns of use across three regions in England. Transfus Med. 2012;22:356–361. doi: 10.1111/j.1365-3148.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen B, Tang M, Larsen OH, Laursen PN, Fenger-Eriksen C, Rea CJ. The role of fibrinogen: a new paradigm in the treatment of coagulopathic bleeding. Thromb Res. 2011;128 (Suppl 1):S13–S16. doi: 10.1016/S0049-3848(12)70004-X. [DOI] [PubMed] [Google Scholar]
- 24.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10:1342–1351. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 25.Schochl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenger-Eriksen C, Jensen TM, Kristensen BS, Jensen KM, Tonnesen E, Ingerslev J, Sorensen B. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Flinck A, Skrtic S, Jeppsson A. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102:137–144. doi: 10.1160/TH08-09-0587. [DOI] [PubMed] [Google Scholar]
- 28.Fries D. [Accessed October 22,2012.];Fibrinogen concentrate (FGTW) in trauma patients, presumed to bleed. Available at: http://clinicaltrials.gov/ct2/show/NCT01475344.
- 29.Rahbar MH, Fox EE, del Junco DJ, Cotton BA, Podbielski JM, Matijevic N, Cohen MJ, Schreiber MA, Zhang J, Mirhaji P, et al. Coordination and management of multicenter clinical studies in trauma: Experience from the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study. Resuscitation. 2012;83:459–464. doi: 10.1016/j.resuscitation.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: John Wiley & Sons; 1999. pp. 159–187. [Google Scholar]
- 32.Martini WZ, Holcomb JB. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann Surg. 2007;246:831–835. doi: 10.1097/SLA.0b013e3180cc2e94. [DOI] [PubMed] [Google Scholar]
- 33.Martini WZ, Chung KK, Dubick MA, Blackbourne LH. Daily profiles of fibrinogen metabolism for 5 days following hemorrhage and lactated ringer’s resuscitation in pigs. Shock. 2012;37:605–610. doi: 10.1097/SHK.0b013e3182522e2c. [DOI] [PubMed] [Google Scholar]
- 34.Fries D, Haas T, Klingler A, Streif W, Klima G, Martini J, Wagner-Berger H, Innerhofer P. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy--a porcine model. Br J Anaesth. 2006;97:460–467. doi: 10.1093/bja/ael191. [DOI] [PubMed] [Google Scholar]
- 35.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen B, Fries D. Emerging treatment strategies for trauma-induced coagulopathy. Br J Surg. 2012;99 (Suppl 1):40–50. doi: 10.1002/bjs.7770. [DOI] [PubMed] [Google Scholar]