Abstract
Background/Study Context
Temporal sequence learning is a critical aspect of episodic memory that may be dependent on the temporal and frontal lobes. Since amnestic mild cognitive impairment (aMCI) and normal aging may result in changes within the temporal and frontal lobes, the present study investigated temporal sequence learning in patients with aMCI, cognitively normal older adults, and young adults.
Methods
On each trial of a temporal sequence task, circles appeared one at a time at the end of each arm of a computerized radial 8-arm maze. Participants were asked to reproduce the temporal sequence by placing numbered circles (1-8) on the arms of the 8-arm maze. Participants were presented with the same fixed sequence on each trial until the sequence was replicated without any errors, or until 15 trials were presented.
Results
Individuals with aMCI required significantly more trials to learn the temporal sequence compared to older adults (p <. 05). Older adults required significantly more trials to learn the sequence than young adults (p <. 05). Older adults and individuals with aMCI committed significantly more Trial 1 errors (p <. 05) than young adults; however, there were no significant differences between the aMCI and older adult groups on Trial 1.
Conclusion
The results suggest that temporal sequence learning deficits are detectable in aMCI. These deficits may disrupt a number of cognitive processes, such as episodic memory, that are important for the execution of daily activities. The results suggest that although temporal sequence learning declines with normal aging, this decline is greater in individuals who have a diagnosis of aMCI and are at higher risk for developing AD.
Introduction
Temporal sequence learning, or the ability to learn the sequential order of items or events, is a critical aspect of episodic memory. There is converging evidence from human and animal studies supporting a role for both medial temporal lobe structures and frontal lobe cortices in the ability to learn and remember temporal sequences. Relatively selective hippocampal damage is associated with temporal order memory impairment in humans (Downes, Mayes, MacDonald, & Hunkin, 2002), and hippocampal activation is apparent on functional neuroimaging during retrieval of temporal sequences (Lehn et al., 2009). Animal studies using lesion or physiological recording techniques also show the important role of the medial temporal lobes in memory for temporal order (Fortin, Agster, & Eichenbaum, 2002; Gilbert, Kesner, & Lee, 2001; Kesner, Gilbert, & Barua, 2002). Evidence supporting the role of the frontal lobes in temporal order memory comes from studies of patients with frontal lobe lesions (Shimamura, 1995; Szatkowska, Szymanska, & Grabowska, 2004), neuroimaging studies of healthy adults (Cabeza, Anderson, Houle, Mangels, & Nyberg, 2000; Knutson, Wood, & Grafman 2004), and lesion and physiological recording studies in rats and non-human primates (Inoue & Mikami, 2006).
Since the ability to learn and recall temporal sequences likely relies on the functional integrity of the medial temporal lobes and frontal cortex, this aspect of memory may be affected early in the course of neurodegenerative disorders that involve one or both of these brain regions. One such condition is Alzheimer’s disease (AD). In its earliest clinical manifestation, AD often presents as Mild Cognitive Impairment (MCI), a term used to describe older adults with cognitive decline that is more severe than expected for healthy aging but does not meet standard criteria for dementia (Peterson et al.; 2001). Although different subtypes of MCI have been defined (e.g., amnestic versus non-amnestic; single domain versus multiple domain), the amnestic subtype has been the most widely studied and characterized form of MCI. The diagnostic criteria for amnestic MCI (aMCI) include subjective memory complaints (preferably corroborated by an informant), objective episodic memory impairment, normal performance on tests of general cognitive function, and preserved activities of daily living (Peterson et al., 2001). Amnestic MCI has been described as a transitional period between normal aging and AD (Peterson et al., 2001). Research has shown that individuals diagnosed with aMCI have a higher risk of developing AD than healthy older adults without episodic memory impairment. One study, for example, found that aMCI patients followed for 3-6 years converted to AD at a rate of 12% per year, compared with 1-2% of healthy older adults. At the end of the 6-year study, approximately 80% of the aMCI patients had developed AD (Petersen et al., 2001). There is also a higher rate of conversion to AD for individuals with aMCI compared to individuals with non-amnestic MCI, who are more likely to progress to non-AD types of dementia (Busse, Hensler, Guhne, Angermeyer, & Riedel-Heller, 2006).
Given that the earliest neuropathological changes in AD appear in the entorhinal cortex and hippocampus, the majority of neuroimaging and histological studies in aMCI have focused on the medial temporal lobe region. Magnetic resonance imaging (MRI) studies of individuals with aMCI have revealed volumetric decreases in medial temporal lobe structures (Apostolova et al., 2006; Jack et al., 1999). Histological studies have documented abnormalities characteristic of AD (i.e., neurofibrillary tangles and neuritic plaques) in the medial temporal lobes of individuals with aMCI (Bennett, Schneider, Bienias, Evans, & Wilson, 2005). Several studies have reported that hippocampal changes in aMCI are intermediate between those of normal controls and patients with early AD (Jack et al., 2000; Peterson et al., 2006), supporting the notion that aMCI is a transitional period between normal aging and early AD. Although the majority of studies have focused on medial temporal lobe structures, some studies have also found abnormalities in the frontal lobes of individuals with aMCI (van der Flier et al., 2002).
Although it is well documented that temporal order memory is impaired in AD patients compared to healthy older adults (Madsen & Kesner, 1995), it is less clear that temporal order memory is impaired in individuals with MCI. One recent study examined temporal order memory in groups of aMCI and nonamestic MCI patients (Schmitter-Edgecombe, Woo, & Greeley, 2009), and found that both groups were impaired compared to healthy controls in judging the temporal order of 8 non-related tasks that were previously completed during the testing session. In addition, temporal order memory task performance was associated with decline in instrumental activities of daily living in the MCI groups. This is an interesting finding; however, temporal sequence learning and performance may have been influenced by differential salience (i.e., memorability) among the 8 non-related tasks. In addition, the study did not examine the effect of normal aging on temporal sequence learning (e.g. Cabeza et al., 2000). Therefore, the current study aimed to examine the effects of age and aMCI on temporal sequence learning using a well-controlled visuospatial learning task. To our knowledge, no study has rigorously examined the ability of aMCI patients to learn temporal sequences of visuospatial locations, even though this may be an important aspect of episodic memory.
Methods
Individuals diagnosed with aMCI (n = 10) were recruited from the University of California, San Diego (UCSD) Shiley-Marcos Alzheimer’s Disease Research Center through which they received detailed clinical, neurological, and neuropsychological evaluations. Inclusion criteria for aMCI diagnosis were consistent with criteria outlined by Petersen et al. (2001) and included: 1) a subjective memory complaint; 2) objective memory impairment (e.g., a score of −1.5 SD relative to normative data on the Wechsler Memory Scale – Revised Logical Memory Test); 3) no impairment in other cognitive domains; and 4) preserved activities of daily living (established through interview with a knowledgeable informant). All aMCI participants had a global rating of 0.5 on the Clinical Dementia Rating Scale. Cognitively normal older adults over 65 years of age (n = 10) were recruited from the San Diego Community. Cognitively normal young adults (n = 10) were recruited from a pool of college students at San Diego State University (SDSU). All participants were provided written informed consent documents according to the Declaration of Helsinki and approved by both San Diego State University and the University of California, San Diego.
The three groups did not differ significantly in education level, F(2,27) = 1.58; p = .22. The mean education level was 15.0 years (SE = .9) for aMCI participants, 15.2 years (SE = .6) for older adults, and 13.8 years (SE = .4) for young adults. The three groups did not differ in gender distribution, χ2(2) = 4.8; p = .09. The cognitively normal older adults (M = 77.4 years, SE = 2.3) and aMCI participants (M = 76.8 years, SE = 2.3) did not differ significantly in age, F(1,18) = .035; p = .85. The younger adults had a mean age of 21 years (SE = 1.7). The aMCI participants and cognitively normal older adults were further characterized with the Dementia Rating Scale (DRS), the Geriatric Depression Scale (GDS), the Trail Making Test (TMT), the Verbal Fluency Test, and the California Verbal Learning Test (CVLT). As shown in Table 1, one-way ANOVAs revealed that the aMCI patients performed significantly worse than cognitively normal older adults on the DRS and key memory indices of the CVLT. The groups did not differ in depressive symptoms or in performance on cognitive tests of executive function (i.e., TMT Part A and B, Verbal Fluency Test).
Table 1.
Mean (SE) performance of older and aMCI participants on screening measures and standardized neuropsychological tests.
| Older | aMCI | F (1,18) | P | |
|---|---|---|---|---|
| DRS | 139.5 ± .92 | 133.8 ± 1.5 | 10.59 | < .01 |
| GDS | 1.1 ± .48 | .8 ± .25 | .31 | .59 |
| Verbal Fluency – | ||||
| Total Letter Fluency | 35.4 ± 3.7 | 40.7 ± 5.4 | .61 | .44 |
| Category Fluency (Animals) | 16.8 ± 1.4 | 18.3 ± 1.8 | .45 | .51 |
| Trail Making Test | ||||
| Part A | 39.6 ± 2.0 | 42.5 ± 4.0 | .43 | .52 |
| Part B | 117.6 ± 21.5 | 85.3 ± 10.9 | 1.8 | .20 |
| CVLT | ||||
| Trial 1 | 7.2 ± .63 | 4.4 ± .52 | 11.76 | < .01 |
| Trials 1-5 | 50.3 ± 3.3 | 30.4 ± 2.5 | 23.41 | < .001 |
| Short Delay Free Recall | 8.9 ± 1.0 | 3.4 ± .81 | 17.31 | < .001 |
| Short Delay Cued Recall | 11.0 ± 1.0 | 5.4 ± .79 | 19.02 | < .001 |
| Long Delay Free Recall | 10.0 ± 1.3 | 3.9 ± 1.1 | 12.94 | < .01 |
| Long Delay Cued Recall | 11.0 ± .98 | 4.7 ± .98 | 20.76 | < .001 |
| Recognition Discriminability | .90 ± .03 | .79 ± .03 | 7.44 | < .01 |
Note. Raw scores are reported. DRS = Dementia Rating Scale; GDS = Geriatric Depression Screening Scale; CVLT = California Verbal Learning Test.
The temporal sequence learning task was modeled after a previously published paradigm (Pirogovsky et al., 2009). At the beginning of each trial, the participant was presented a computerized radial eight-arm maze, 30 cm in diameter that consisted of eight arms extending from a centerpoint like spokes on a wheel. Each arm represented a different visuospatial location. The participant was told that a circle would appear at the end of each arm (i.e., visuospatial location) one at a time in a fixed sequence and that they were to remember the sequence in which the circles were presented at each location.
Each participant was randomly assigned to one of sixteen different fixed sequences. On each trial of the task, a gray circle (3 cm diameter) appeared at the end of an arm for 2 s and then a gray mask covered the entire display for 2 s to eliminate after-image effects. The maze then reappeared and the circle was shown at the end of a different, randomly-selected arm for 2 s followed by a 2 s mask. This continued until a circle had been presented at the end of each of the eight arms once. Following the sequence, the participant was presented with a printed copy of the 8-arm maze and eight gray circles numbered 1-8. The participant was asked to place the circle labeled “1” on the arm that occurred first in the sequence. The participant was then handed each of the remaining circles one at a time in numerical order and asked to place each circle on the corresponding arm in the sequence. Presentation and test of the same sequence was repeated in the same manner on subsequent trials. The participant continued to receive trials until he/she replicated the sequence without any errors. Testing was discontinued if the participant was unable to replicate the sequence without errors within 15 trials. The total number of trials required to correctly replicate the sequence (range from 1-15) was used as the dependent variable.
Results
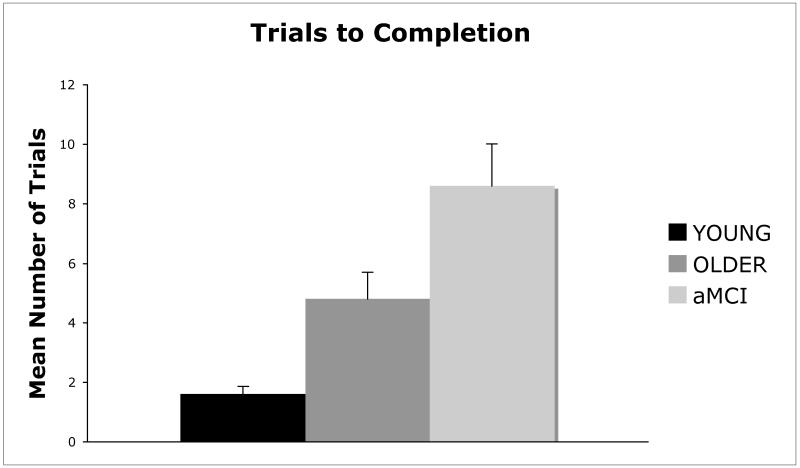
A one-way ANOVA with group (young, healthy older, aMCI) as the between-group factor and trials to completion as the dependent variable revealed a significant main effect of group, F(2,27) = 12.83; p < .001; η2 = .487. Newman-Keuls post hoc comparison tests revealed that the number of trials to complete the sequence was significantly higher (p <. 05) for aMCI participants than for cognitively normal older adults, and significantly higher (p <. 05) for cognitively normal older adults than for young adults (Figure 1). All of the young adults and cognitively normal older adults learned the task in 15 trials or less. Two of the 10 aMCI patients did not learn the sequence within 15 trials (i.e., the discontinue criterion).
Figure 1.
Mean (+SE) number of trials to replicate the sequence without any errors in young adults, healthy older adults, and aMCI patients.
The first trial of the temporal sequence task could be considered to be analogous to a visuospatial span working memory task that may be dependent on prefrontal cortex. Examination of trial one performance with a one-way ANOVA with group as the independent variable and number of errors on the first trial as the dependent variable revealed a significant main effect of group, F(2,27) = 13.12, p < .001; η2 = .493. Newman-Keuls post-hoc tests revealed that cognitively normal older adults (M = 4.5, SE = .75) and aMCI participants (M = 5.2, SE = .55) committed significantly more trial one errors than young adults (M = 1.1, SE = .48). There was no significant difference in the number of trial one errors committed by aMCI participants and cognitively normal older adults.
Discussion
As predicted, aMCI patients required more trials than cognitively normal older adults to learn a visuospatial temporal sequence. In addition, healthy older adults required more trials to learn the sequence than younger adults. These results suggest that although temporal sequence learning declines with normal aging, this decline is greater in individuals who have a diagnosis of aMCI and are at higher risk for developing AD. The particularly prominent decline in temporal sequence learning in aMCI could be attributed to medial temporal and/or frontal lobe dysfunction since this aspect of memory may be dependent on both brain regions (Cabeza et al., 2000; Gilbert et al., 2001; Knutson et al., 2004; Shimamura, 1995). As discussed previously, most studies examining neuropathological changes in aMCI have demonstrated abnormalities in the medial temporal lobes, and some provide evidence of frontal lobe dysfunction as well.
The differential role of medial temporal lobe and frontal lobe dysfunction in producing the current pattern of results is clarified to some degree by examining performance on the first trial of the temporal sequence learning task. As discussed previously, the first trial of the task is similar to a visuospatial working memory span task, a measure that is thought to be dependent on the functional integrity of the prefrontal cortex. Impaired performance of healthy older adults relative to young adults on the first trial is consistent with evidence that normal aging is associated prefrontal cortex dysfunction (Salat et al., 2005; Raz et al., 1997). Furthermore, the comparable performance of healthy older adults and aMCI patients on the first trial raises the possibility that prefrontal cortex dysfunction contributes to the impaired performance of aMCI patients on the temporal sequence learning task. However, the performance of aMCI patients did not differ significantly from older adults on frontal lobe dependent standardized neuropsychological measures of executive function such as the trail making test or verbal fluency.
It is clear, however, that aMCI patients were impaired relative to healthy older adults beyond the first trial of the temporal sequence learning task, suggesting that their deficit is not primarily related to the prefrontal cortex dysfunction that they may share with normal aging. Rather, their temporal sequence learning deficit is likely to reflect neuropathological changes in the medial temporal lobes that they share with patients with AD. In support of this hypothesis, the performance of aMCI patients differed significantly from older adults on the CVLT1, a test shown to be related to temporal lobe function. Future studies using neuroimaging techniques that can directly examine the medial temporal lobe versus frontal lobe contribution to temporal sequencing impairments in aMCI will be useful in addressing these important issues.
In conclusion, individuals with aMCI show impaired temporal sequence learning relative to healthy older adults, who in turn show poorer temporal sequence learning than young adults. Although future studies with larger sample sizes and longitudinal designs are needed, these results suggest that temporal sequence learning deficits are detectable in aMCI and add to our understanding of cognitive deficits associated with this condition. The present temporal sequence task involves a visuospatial component and impaired visuospatial function in the MCI patients could have contributed to poor performance on this task. Performance on the temporal sequence task did improve in aMCI patients across trials and some patients did learn the task, suggesting that the deficits in this group were not solely due to visuospatial dysfunction. Although this was not a focus of the current study, future studies should examine the impact of visuospatial impairment on performance in this task and/or examine temporal sequence learning using tasks that do not depend on visuospatial function. In addition, slowed processing speed and/or basic difficulty following a sequence may contribute to poor performance on the temporal sequence task. However, given the lack of differences between healthy older adults and aMCI patients on the Trails A test of the Trail Making Test, it is unlikely that slowed processing speed or inability to follow a sequence was contributing to impairment on the temporal sequence task in aMCI. Impaired temporal order memory may disrupt a number of cognitive processes important for the execution of daily activities. For example, episodic memory involves the ability to encode and subsequently retrieve an event in a spatial and temporal context. Thus, deficits in temporal order processing in aMCI patients may contribute to their overall episodic memory deficit. Future investigations examining the relationship between temporal order memory and performance on episodic memory tasks may further our understanding of the component processes of episodic memory impairment in aMCI.
Acknowledgements
This research was supported by a National Institutes of Health Grant (#AG034202) from the National Institute on Aging awarded to Paul E. Gilbert. We thank Genevive Brusati for her assistance with data collection. We also thank all of the participants in this study for their contributions.
Footnotes
A potentially interesting analysis would involve a comparison between learning trials on the CVLT and the temporal sequence task. However, the comparison cannot be accomplished due to the discontinuation rule implemented on the temporal sequence task. As a result of the discontinuation rule, some participants completed the temporal sequence task in the first few trials, while others required more trials to complete the task. This is in contrast to the CVLT, in which participants complete five learning trials regardless of the number of words recalled on each trial.
The authors have no conflict of interest.
References
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. Brain: A Journal of Neurology. 2006;129:2867–2873. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;6:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Lee AC, Parr A, Owen AM. Frontal lobe involvement in spatial span: Converging studies of normal and impaired function. Neuropsychologia. 2006;44:229–237. doi: 10.1016/j.neuropsychologia.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. Journal of Cognitive Neuroscience. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Daum I, Gräber S, Schugens MM, Mayes AR. Memory dysfunction of the frontal type in normal ageing. Neuroreport. 1996;7:2625–2628. doi: 10.1097/00001756-199611040-00043. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R,, Stern Y, Tabert MH, De Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: Prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Mayes AR, MacDonald C, Hunkin NM. Temporal order memory in patients with Korsakoff’s syndrome and medial temporal amnesia. Neuropsychologia. 2002;40:853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster K, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: Double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order for a sequence of odors. Behavioral Neuroscience. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Lehn H, Steffenach A, van Strien NM, Veltman DJ, Witter MP, Håberg AK. A specific role of the human hippocampus in recall of temporal sequences. The Journal of Neuroscience. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E, Goldstein J, Peavy G, Jacobson MW, Corey-Bloom J, Gilbert PE. Temporal order memory deficits prior to clinical diagnosis in Huntington’s disease. Journal of the International Neuropsychological Society. 2009;15:662–670. doi: 10.1017/S1355617709990427. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23:168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Memory and the prefrontal cortex. Annals of the New York Academy of Sciences. 1995;769:151–159. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- Szatkowska I, Szymanska O, Grabowska A. The role of the human ventromedial prefrontal cortex in memory for contextual information. Neuroscience Letters. 2004;364:71–75. doi: 10.1016/j.neulet.2004.03.084. [DOI] [PubMed] [Google Scholar]
- Van Der Flier WM, Van Den Heuvel DM, Weverling-Rijnsburger AW, Split A, Bollen EL, Westendrop RG, Middelkoop HA, Van Buchem MA. Cognitive decline in AD and mild cognitive impairment is associated with global brain damage. Neurology. 2002;24:874–879. doi: 10.1212/wnl.59.6.874. [DOI] [PubMed] [Google Scholar]