Abstract
Metabolomic approaches have begun to catalog the metabolic disturbances that accompany CKD, but whether metabolite alterations can predict future CKD is unknown. We performed liquid chromatography/mass spectrometry–based metabolite profiling on plasma from 1434 participants in the Framingham Heart Study (FHS) who did not have CKD at baseline. During the following 8 years, 123 individuals developed CKD, defined by an estimated GFR of <60 ml/min per 1.73 m2. Numerous metabolites were associated with incident CKD, including 16 that achieved the Bonferroni-adjusted significance threshold of P≤0.00023. To explore how the human kidney modulates these metabolites, we profiled arterial and renal venous plasma from nine individuals. Nine metabolites that predicted CKD in the FHS cohort decreased more than creatinine across the renal circulation, suggesting that they may reflect non–GFR-dependent functions, such as renal metabolism and secretion. Urine isotope dilution studies identified citrulline and choline as markers of renal metabolism and kynurenic acid as a marker of renal secretion. In turn, these analytes remained associated with incident CKD in the FHS cohort, even after adjustment for eGFR, age, sex, diabetes, hypertension, and proteinuria at baseline. Addition of a multimarker metabolite panel to clinical variables significantly increased the c-statistic (0.77–0.83, P<0.0001); net reclassification improvement was 0.78 (95% confidence interval, 0.60 to 0.95; P<0.0001). Thus, the addition of metabolite profiling to clinical data may significantly improve the ability to predict whether an individual will develop CKD by identifying predictors of renal risk that are independent of estimated GFR.
Given the significant morbidity and mortality attributable to established CKD, early markers of CKD risk are needed.1 The kidneys can modulate circulating small-molecule levels through a variety of mechanisms, such as filtration, reabsorption, secretion, and metabolism (including both catabolism and anabolism). Currently available metrics of kidney function (serum creatinine and urea) primarily reflect relatively advanced impairment in renal filtration. In contrast, early and specific disease markers may reflect impairments along other axes of renal small-molecule handling.
Metabolite profiling technologies enable high-throughput, high-resolution metabolic phenotyping of human plasma. Applied to well-characterized human cohorts, these techniques have the potential to identify novel disease biomarkers and to highlight their underlying metabolic pathways. For example, we have previously applied liquid chromatography-mass spectrometry (LC-MS)–based metabolite profiling to identify numerous metabolite alterations that accompany ESRD.2 Similar findings have been observed using capillary electrophoresis-MS–based metabolite profiling of plasma obtained from individuals across a spectrum of extant kidney disease.3,4 Whether any of these metabolite perturbations presage the development of clinically overt kidney disease is unknown.
Here, we report the application of metabolite profiling to plasma obtained from participants in the Framingham Heart Study (FHS). Prior work in the FHS has highlighted branched-chain and aromatic amino acids as robust predictors of future type 2 diabetes.5 Because of access to archived plasma samples, detailed phenotyping, and longitudinal follow-up on clinical outcomes, this sample provides an ideal opportunity to identify novel markers of CKD risk. To explore how select CKD predictors are modulated by the human kidney, we also performed metabolite profiling of plasma and urine samples obtained from individuals undergoing aortic and renal vein catheterization. Taken together, these studies demonstrate the broad effect kidney function has on the plasma metabolome and show how this perspective can improve CKD prediction beyond estimated GFR (eGFR) and other established CKD risk factors.
Results
Baseline Characteristics of the Epidemiologic Study Sample
We performed a prospective study of incident CKD in the FHS. Among all 1434 eligible participants with an eGFR ≥ 60 ml/min per 1.73 m2 at baseline, we identified 123 individuals who developed new-onset CKD (eGFR < 60 ml/min per 1.73 m2) during an 8-year follow-up period (see Concise Methods). Baseline characteristics of the FHS study sample are shown in Table 1. The participants who subsequently developed CKD were older (61 versus 54 years), had a higher baseline prevalence of hypertension (62% versus 33%) and diabetes (17% versus 5%), and had a trend for a higher prevalence of proteinuria (33% versus 24%). Mean baseline eGFR was 86.5±30.1 ml/min per 1.73 m2 for those who subsequently developed CKD and 94.3±25.7 ml/min per 1.73 m2 for those who remained CKD-free during the study interval (P=0.0017). At 8 years of follow-up, eGFR was 49.7±8.8 ml/min per 1.73 m2 for cases and 89.0±17.1 ml/min per 1.73 m2 for those who remained free of CKD (P<0.0001).
Table 1.
Baseline characteristics of the FHS study sample
| Clinical Characteristics | Individuals Who Developed CKD (n=123) | Individuals Who Did Not Develop CKD (n=1311) |
|---|---|---|
| Age (yr) | 61±8a | 54±9 |
| Women (%) | 59b | 50 |
| Body mass index (kg/m2) | 28.1±4.4 | 27.6±5.0 |
| Hypertension (%) | 62a | 33 |
| Diabetes (%) | 17a | 5 |
| eGFR (ml/min per 1.73 m2) | 86.5±30.1b | 94.3±25.7 |
| eGFR at follow-up (ml/min per 1.73 m2) | 49.7±8.8a | 89.0±17.1 |
| Dipstick proteinuria (%) | 33 | 24 |
Values expressed with plus/minus sign mean ± SD.
P<0.0001.
P<0.05.
Select Metabolites Are Associated with Incident CKD in FHS
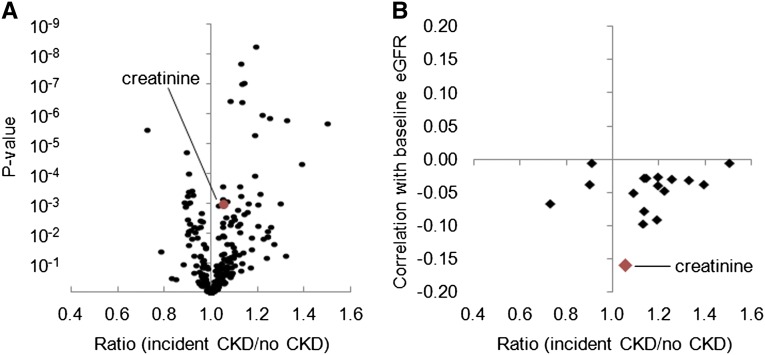
We performed metabolite profiling on fasting plasma obtained from the baseline examination. The three components of the metabolite profiling platform quantified 54 positively charged polar analytes, 59 negatively charged polar analytes, and 104 lipids. For each of these 217 metabolites, Figure 1A shows the mean ratio of each analyte in patients who went on to develop CKD (cases) versus those who did not, plotted against their corresponding P values (full results are shown in Supplementary Table 1). Sixteen metabolites achieved a conservative Bonferroni-adjusted significance threshold of P≤0.00023 (i.e., 0.05/217 metabolites), with 13 of these metabolites higher in cases relative to persons who remained CKD-free (Table 2). Several metabolites derive from the same metabolic pathway (e.g., tryptophan metabolites [kynurenic acid, kynurenine, 5-hydroxyindoleacetic acid, quinolinate], choline derivatives [choline, trimethylamine-N-oxide], citric acid cycle intermediates [aconitate, isocitrate], and purine metabolites [xanthosine, adenosine]). The only lipid analytes that achieved Bonferroni-adjusted significance, lysophosphatidylcholine 18:1 and lysophosphatidylcholine 18:2, were both lower in cases. Notably, the association between creatinine and case status did not reach Bonferroni-level significance.
Figure 1.
Metabolite profiling identifies markers of incident CKD that are not correlated with baseline eGFR. (A) The geometric mean ratio of each analyte for individuals who subsequently developed CKD (n=123) versus those who remained CKD-free (n=1311) in baseline plasma, with P values plotted on the y-axis. (B) For 16 metabolites significantly associated with case status in FHS, the geometric mean ratio for cases versus those who remained CKD-free plotted against each metabolite’s correlation with eGFR at baseline.
Table 2.
Metabolites significantly associated with incident CKD in FHS
| Metabolite | Geometric Mean Ratio in FHS (95% CI) | P Value |
|---|---|---|
| Xanthosine | 1.19 (1.13 to 1.27) | 5.2 × 10−9 |
| Citrulline | 1.13 (1.08 to 1.18) | 2.0 × 10−8 |
| Isocitrate | 1.14 (1.09 to 1.20) | 9.1 × 10−8 |
| Aconitate | 1.13 (1.08 to 1.19) | 9.4 × 10−8 |
| Choline | 1.08 (1.05 to 1.12) | 3.7 × 10−7 |
| Kynurenine | 1.13 (1.08 to 1.19) | 4.1 × 10−7 |
| β-aminoisobutyric acid | 1.22 (1.13 to 1.34) | 1.1 × 10−6 |
| Kynurenic acid | 1.25 (1.14 to 1.38) | 1.4 × 10−6 |
| Trimethylamine-N-oxide | 1.33 (1.18 to 1.49) | 1.6 × 10−6 |
| Adenosine | 1.50 (1.27 to 1.77) | 2.0 × 10−6 |
| 5-hydroxyindoleacetic acid | 0.73 (0.64 to 0.83) | 3.3 × 10−6 |
| Quinolinic acid | 1.19 (1.10 to 1.28) | 5.1 × 10−6 |
| LPC18:2 | 0.90 (0.85 to 0.94) | 1.9 × 10−5 |
| Sucrose | 1.39 (1.19 to 1.63) | 4.7 × 10−5 |
| LPC18:1 | 0.91 (0.86 to 0.95) | 1.0 × 10−4 |
| Inositol | 1.19 (1.09 to 1.30) | 1.2 × 10−4 |
LPC, lysophosphatidylcholine.
For the 16 metabolites listed in Table 2, Figure 1B plots the metabolite ratio in cases relative to participants who remained CKD-free against each metabolite’s correlation with baseline eGFR across the study sample. As expected, creatinine was negatively correlated with baseline eGFR (R=−0.16); no other metabolite had as strong a correlation with eGFR from the initial sample. Further, there was no relationship between the magnitude of metabolite associations with CKD risk and their correlation with baseline eGFR. Thus, the metabolites associated with incident CKD in FHS are not simply markers of impaired renal filtration.
Arteriovenous Plasma Sampling Demonstrates Heterogeneous Renal Metabolite Handling
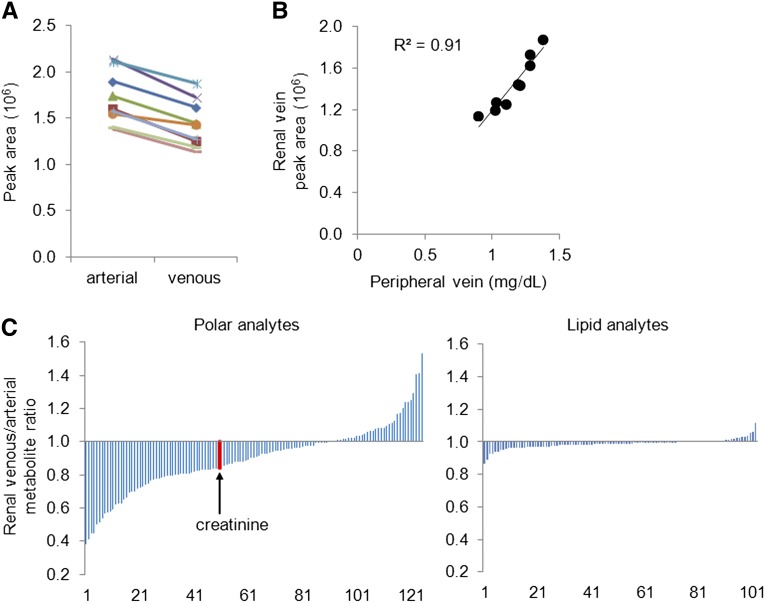
To explore the various axes of renal metabolite handling (i.e., beyond renal filtration alone), we collected arterial and renal venous plasma from nine individuals undergoing invasive catheterization (see Concise Methods). These patients had a mean eGFR of 65.4±8.7 ml/min per 1.73 m2 (Table 3). As expected, creatinine levels decreased from the arterial to renal venous plasma sample in all nine individuals (Figure 2A); the mean ratio of venous to arterial creatinine level (V/A) was 0.84 (P<0.0001). Figure 2B demonstrates the strong correlation between renal venous creatinine level as measured by the LC-MS platform and serum creatinine measured by the clinical laboratory on a peripheral venous sample drawn on the same day (R=0.95).
Table 3.
Individuals undergoing right and left heart catheterization (with renal arteriovenous plasma sampling)
| Patient | Reason for Procedure | Serum Creatinine (mg/dl) | eGFR (ml/min per 1.73 m2) | Age (yr) | Sex | Type 2 Diabetes | ACE/ARB | Coronary Disease |
|---|---|---|---|---|---|---|---|---|
| 1 | Preoperative (aneurysm repair) | 1.28 | 58.1 | 76 | M | No | No | Yes |
| 2 | Aortic stenosis | 1.1 | 67.8 | 84 | M | Yes | No | No |
| 3 | Shortness of breath | 1.19 | 65.2 | 65 | M | No | Yes | Yes |
| 4 | Preoperative (mitral valve repair) | 1.28 | 59.1 | 70 | M | No | Yes | Yes |
| 5 | Aortic stenosis | 1.38 | 51.9 | 86 | M | No | Yes | Yes |
| 6 | Aortic stenosis and mitral regurgitation | 1.21 | 61.5 | 79 | M | No | Yes | No |
| 7 | Shortness of breath | 1.03 | 78.6 | 59 | M | Yes | Yes | Yes |
| 8 | Mitral regurgitation | 0.9 | 70.7 | 49 | F | No | No | No |
| 9 | Aortic stenosis | 1.02 | 75.5 | 76 | M | Yes | Yes | Yes |
ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker; M, male; F, female.
Figure 2.
Renal arteriovenous sampling demonstrates heterogeneous metabolite handling. (A) Creatinine levels (peak areas) in aortic and renal venous plasma measured by the mass spectrometer. (B) Correlation between renal venous creatinine levels (peak areas) measured by the mass spectrometer and peripheral venous creatinine (mg/dl) measured by the clinical laboratory. (C) Mean renal venous to arterial metabolite ratios for polar (left panel) and lipid (right panel) metabolites.
Figure 2C depicts the range of arteriovenous gradients for 125 polar (left panel) and 102 lipid (right panel) analytes measured in all samples, ordered by mean V/A (full results are shown in Supplementary Table 2). Most polar analyte levels were decreased in the renal vein relative to the aorta, although several metabolite levels increased across the kidney. By contrast, lipid metabolite levels were not appreciably altered in transit from the arterial to the renal venous circulation. For any given metabolite, V/A < 1 is consistent with net uptake by the kidney, whether via filtration, secretion, and/or metabolism (catabolism), whereas V/A > 1 suggests net release by the kidney (anabolism). Thus, our physiologic data demonstrate how different metabolites could potentially serve as markers of different aspects of renal function.
Combined Epidemiologic and Physiologic Approach Nominates Markers of CKD Risk
For the 16 metabolites that achieved Bonferroni-level significance for incident CKD in FHS, the V/A values across all nine patients who underwent catheterization are shown in Table 4. Because creatinine excretion is primarily a function of glomerular filtration, we hypothesized that metabolites that decrease more than creatinine across the renal circulation are potential markers of non-GFR renal function (e.g., renal metabolism and secretion). Thus, we focused on metabolites that had a mean V/A less than that for creatinine (<0.84) and demonstrated a decrease across the kidney in all nine individuals undergoing catheterization. As a result, we highlight nine polar metabolites as potential markers of CKD risk: citrulline (urea cycle intermediate); choline; kynurenic acid, kynurenine, and 5-hydroxyindoleacetic acid (tryptophan metabolites); aconitate and isocitrate (citric acid cycle intermediates); xanthosine (purine metabolite); and β-aminoisobutyric acid (pyrimidine metabolite).
Table 4.
Selected renal arteriovenous metabolite gradients
| Metabolite | Mean V/A | P Value | Patient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| Metabolites associated with incident CKD in FHS | |||||||||||
| Xanthosine | 0.45 | 6.7 × 10−5 | 0.37 | 0.35 | 0.27 | 0.51 | 0.56 | 0.70 | 0.31 | 0.32 | 0.62 |
| β-aminoisobutyric acid | 0.50 | 4.0 × 10−6 | 0.56 | 0.39 | 0.40 | 0.57 | 0.47 | 0.69 | 0.44 | 0.42 | 0.55 |
| Choline | 0.58 | 1.4 × 10−4 | 0.46 | 0.46 | 0.44 | 0.75 | 0.75 | 0.75 | 0.43 | 0.47 | 0.67 |
| Kynurenic acid | 0.59 | 7.0 × 10−5 | 0.61 | 0.39 | 0.82 | 0.49 | 0.69 | 0.53 | 0.55 | 0.55 | 0.69 |
| Citrulline | 0.65 | 9.0 × 10−6 | 0.55 | 0.66 | 0.63 | 0.58 | 0.74 | 0.75 | 0.52 | 0.74 | 0.68 |
| Aconitate | 0.70 | 9.9 × 10−5 | 0.67 | 0.60 | 0.60 | 0.55 | 0.78 | 0.85 | 0.69 | 0.85 | 0.71 |
| Kynurenine | 0.70 | 3.1 × 10−5 | 0.82 | 0.56 | 0.72 | 0.63 | 0.71 | 0.85 | 0.65 | 0.65 | 0.73 |
| 5-hydroxyindoleacetic acid | 0.71 | 9.3 × 10−4 | 0.98 | 0.63 | 0.82 | 0.73 | 0.62 | 0.85 | 0.48 | 0.65 | 0.67 |
| Adenosine | 0.73 | 2.8 × 10−2 | 0.77 | 0.49 | 0.43 | 0.42 | 1.00 | 0.40 | 0.70 | 1.70 | 0.67 |
| Isocitrate | 0.81 | 4.0 × 10−5 | 0.85 | 0.77 | 0.74 | 0.79 | 0.91 | 0.87 | 0.78 | 0.73 | 0.86 |
| Inositol | 0.83 | 4.5 × 10−3 | 0.76 | 0.70 | 0.75 | 0.69 | 0.94 | 0.81 | 0.81 | 0.84 | 1.13 |
| Trimethylamine-N-oxide | 0.85 | 4.9 × 10−3 | 0.87 | 0.84 | 0.92 | 0.90 | 0.95 | 0.79 | 0.63 | 0.96 | 0.82 |
| Quinolinic acid | 0.86 | 3.1 × 10−3 | 0.84 | 0.86 | 0.97 | 0.83 | 1.08 | 0.79 | 0.84 | 0.76 | 0.83 |
| LPC18:1 | 0.97 | 1.9 × 10−1 | 0.96 | 1.02 | 1.03 | 0.92 | 0.98 | 0.86 | 1.00 | 1.06 | 0.93 |
| LPC18:2 | 0.99 | 6.6 × 10−1 | 0.97 | 1.03 | 0.95 | 0.92 | 0.96 | 0.84 | 0.99 | 1.10 | 0.93 |
| Sucrose | 1.00 | 5.5 × 10−1 | 0.79 | 0.70 | 1.08 | 0.43 | 0.86 | 1.30 | 0.83 | 0.88 | 2.10 |
| Metabolites that undergo net release by the kidney | |||||||||||
| Serine | 1.53 | 4.6 × 10−4 | 1.26 | 1.49 | 1.48 | 2.04 | 1.48 | 1.07 | 1.99 | 1.26 | 1.73 |
| α-glycerophosphocholine | 1.24 | 6.5 × 10−3 | 1.05 | 1.16 | 1.57 | 1.24 | 1.64 | 1.08 | 1.10 | 1.22 | 1.07 |
| Arginine | 1.20 | 9.6 × 10−4 | 1.11 | 1.15 | 1.53 | 1.25 | 1.17 | 1.22 | 1.14 | 1.19 | 1.06 |
| Niacinamide | 1.17 | 2.9 × 10−3 | 1.07 | 1.24 | 1.16 | 1.44 | 1.27 | 1.15 | 1.00 | 1.14 | 1.07 |
| Alanine | 1.16 | 1.4 × 10−4 | 1.23 | 1.03 | 1.09 | 1.23 | 1.11 | 1.16 | 1.24 | 1.16 | 1.23 |
| Threonine | 1.11 | 3.1 × 10−4 | 1.12 | 1.07 | 1.09 | 1.25 | 1.08 | 1.06 | 1.14 | 1.07 | 1.11 |
| Serine | 1.53 | 4.6 × 10−4 | 1.26 | 1.49 | 1.48 | 2.04 | 1.48 | 1.07 | 1.99 | 1.26 | 1.73 |
LPC, lysophosphatidylcholine.
Plasma and Urinary Profiling of Select Metabolites Identifies Markers of Renal Metabolism and Secretion
In addition to identifying metabolites that decrease more than creatinine across the renal arteriovenous circulation, our physiologic data identify several metabolites that increased from artery to vein in all nine individuals undergoing catheterization, indicating net release by the kidney (Table 4). For the current study, we emphasize the increase in plasma arginine across the kidney (mean V/A, 1.20; P=0.0010), consistent with the kidney’s established role in extracting circulating citrulline and converting it to arginine.6,7 We also note the uniform increase in plasma α-glycerophosphocholine (mean V/A, 1.24; P=0.0065) across the kidney in our study sample, as net release of α-glycerophosphocholine would be contingent on net uptake of choline or choline derivatives from the circulation. Of note, α-glycerophosphocholine is an osmolyte known to be produced in the renal medulla in response to changes in extracellular tonicity.8 Betaine, another renal osmolyte derived from choline, increased from artery to renal vein in seven of the nine individuals undergoing catheterization (Supplementary Table 2).9
Using stable isotope dilution, we compared absolute levels of select metabolites in plasma with the corresponding levels in urine obtained from individuals undergoing catheterization. Using the urine-to-plasma creatinine ratio as an index of glomerular filtration, we assessed the urinary fractional excretion of citrulline, choline, and kynurenic acid (Table 5). Across these individuals, the fractional excretion for citrulline and choline were significantly <100%, consistent with their net uptake and metabolism (e.g., to arginine and α-glycerophosphocholine, respectively) by the kidney. By contrast, the fractional excretion for kynurenic acid was >100%, invoking tubular secretion in addition to glomerular filtration as a means of kynurenic acid excretion. Taken together, these data are consistent with the hypothesis that metabolites with mean V/A less than that for creatinine are markers of non-GFR renal function.
Table 5.
Fractional excretion of citrulline, choline, and kynurenic acid
| Patient | Creatinine: U/P | Citrulline | Choline | Kynurenic acid | |||
|---|---|---|---|---|---|---|---|
| U/P | FE (%) | U/P | FE (%) | U/P | FE (%) | ||
| 6 | 42.3 | 0.14 | 0.3 | 1.15 | 2.7 | 53.9 | 128 |
| 7 | 52.7 | 0.10 | 0.2 | 2.70 | 5.1 | 118.6 | 225 |
| 8 | 29.3 | 0.04 | 0.1 | 1.42 | 4.8 | 64.9 | 221 |
| 9 | 33.3 | 0.17 | 0.5 | 1.95 | 5.9 | 49.0 | 147 |
| Mean | 39.4 | 0.11 | 0.3 | 1.81 | 4.6 | 71.6 | 180 |
U/P, urine-to-plasma ratio; FE, fractional excretion.
Metabolite Predictors of Future CKD after Multivariable Adjustment
Because we found that select candidate metabolites decreased more than creatinine across the renal circulation and confirmed that three of these are markers of renal metabolism or secretion, we hypothesized that these analytes would be associated with incident CKD in FHS, even after accounting for differences in glomerular filtration. Thus, logistic regression models were fitted to assess the association between the nine candidate metabolites and future CKD, adjusting for eGFR, as well as other established CKD risk factors, including age, sex, diabetes, hypertension, and proteinuria at baseline (Table 6). Among the candidate metabolites, kynurenic acid had the largest odds ratio (OR) per SD increment in log marker for incident CKD (OR per SD, 1.53 [95% confidence interval (CI), 1.25 to 1.88]; P<0.0001), followed by kynurenine (OR per SD, 1.49 [95% CI, 1.22 to 1.83]; P=0.0001), citrulline (OR per SD, 1.48 [95% CI, 1.19 to 1.83]; P=0.0004), choline (OR per SD, 1.46 [95% CI, 1.17 to 1.82]; P=0.0007), xanthosine (OR per SD, 1.46 [95% CI, 1.21 to 1.76]; P<0.0001), β-aminoisobutyric acid (OR per SD, 1.41 [95% CI, 1.15 to 1.72]; P=0.0008), aconitate (OR per SD, 1.32 [95% CI, 1.07 to 1.62]; P=0.0092), isocitrate (OR per SD, 1.28 [95% CI, 1.05 to 1.58]; P=0.017), and 5-hydroxindoleacetic acid (OR per SD, 0.62 [95% CI, 0.51 to 0.76]; P<0.0001).
Table 6.
Relationship of baseline metabolite levels and incident CKD
| Metabolite | Odds Ratio per SD (95% CI) | P Value |
|---|---|---|
| Kynurenic acid | 1.53 (1.25 to 1.88) | <0.0001 |
| Kynurenine | 1.49 (1.22 to 1.83) | 0.0001 |
| Citrulline | 1.48 (1.19 to 1.83) | 0.0004 |
| Choline | 1.46 (1.17 to 1.82) | 0.0007 |
| Xanthosine | 1.46 (1.21 to 1.76) | <0.0001 |
| β-aminoisobutyric acid | 1.41 (1.15 to 1.72) | 0.0008 |
| Aconitate | 1.32 (1.07 to 1.62) | 0.0092 |
| Isocitrate | 1.28 (1.05 to 1.58) | 0.017 |
| 5-hydroxyindoleacetic acid | 0.62 (0.51 to 0.76) | <0.0001 |
Odds ratios for CKD obtained from logistic regressions. All models adjusted for eGFR, age, sex, diabetes, hypertension, and proteinuria at baseline.
To assess the potential value of combining markers identified by our epidemiologic and physiologic studies, we used a stepwise logistic model to construct a multimarker panel of five metabolites: kynurenic acid, xanthosine, 5-hydroxyindoleacetic acid, kynurenine, and citrulline. The incremental predictive value of the multimarker panel on top of the base model is shown in Table 7. The OR per SD increment in log multimarker panel for incident CKD was 2.41 (95% CI, 1.93 to 3.02). The addition of the multimarker panel led to an improvement in discrimination, as shown by the increase in the c-statistic (0.77–0.83; P<0.0001). Furthermore, the multimarker panel also led to a significant improvement in classification accuracy, with a “category-free” net reclassification improvement of 0.78 (95% CI, 0.60 to 0.95; P<0.0001) and integrated discrimination index of 0.074 (95% CI, 0.050 to 0.097; P<0.0001). These results were minimally attenuated with a more parsimonious multimarker panel consisting of kynurenic acid, xanthosine, and 5-hydroxyindoleacetic acid (Table 7).
Table 7.
Multimarker panels and prediction of incident CKD
| Variable | Multimarker Panel: 5 Metabolites | Multimarker Panel: 3 Metabolites |
|---|---|---|
| OR per SD | 2.41 (1.93 to 3.02) | 2.15 (1.74 to 2.65) |
| c-statistics | ||
| Base model | 0.77 | 0.77 |
| Base model + multimarker panel | 0.83 | 0.82 |
| P value | <0.0001 | 0.0002 |
| NRI (category-free) | 0.78 (0.60 to 0.95) | 0.79 (0.61 to 0.96) |
| P value | <0.0001 | <0.0001 |
| IDI | 0.074 (0.050 to 0.097) | 0.062 (0.041 to 0.083) |
| P value | <0.0001 | <0.0001 |
Five metabolites were kynurenic acid, xanthosine, 5-hydroxyindoleacetic acid, kynurenine, and citrulline. Three metabolites were kynurenic acid, xanthosine, and 5-hydroxyindoleacetic acid. Values in parentheses are 95% CIs.
Discussion
Our study has two principal findings. First, we provide a broad perspective on the metabolite perturbations that are associated with incipient kidney disease. Second, we highlight a subset of these metabolites that decrease more than creatinine across the renal arteriovenous circulation and find that these metabolites predict incident CKD even after adjustment for eGFR, age, sex, diabetes, hypertension, and proteinuria at baseline. Taken together, these findings highlight the potential value of integrating different axes of renal function in an assessment of kidney health and prognosis.
At present, clinical assessment of kidney function relies primarily on measurement of serum creatinine levels. Because creatinine is freely filtered at the glomerulus, is not reabsorbed, and undergoes only limited tubular secretion, serum creatinine is used as a noninvasive index of GFR.10 In current clinical practice, equations that incorporate demographic variables (e.g., age and sex) along with serum creatinine are used to calculate eGFR.11,12 Recent studies show that refined estimating equations and the use of alternative filtration markers (i.e., cystatin C) can further improve the precision of GFR assessment.13,14 By design, these measures do not assess fundamental renal functions beyond glomerular filtration.
Our study of individuals undergoing catheterization demonstrates the heterogeneous effect of human kidney function on the plasma metabolome. Although arterial and renal venous metabolite levels alone do not quantify the relative effects of glomerular filtration, tubular absorption/secretion, and organ metabolism, we highlight select metabolites at each extreme of V/A (Figure 2C). For example, a metabolite with a V/A > 1 is to some extent being made in the kidney. In that regard, we note that our finding of renal anabolism of arginine, serine, and alanine recapitulates prior studies that have focused on renal amino acid handling.15,16 By contrast, metabolites with V/A substantially lower than that for creatinine are to some extent being metabolized and/or secreted, as corroborated by our fractional excretion data for citrulline, choline, and kynurenic acid.
Regardless of CKD cause, tubulointerstitial injury is an invariant pathologic feature of chronic renal disease. Indeed, the degree of tubulointerstitial disease correlates better than glomerular injury with disease prognosis, even for primary glomerular diseases.17,18 In the current study, we show that markers of renal metabolism and tubular secretion predict incident CKD in the FHS, even after adjustment for eGFR and other established CKD risk factors. Because deeper, medullary components of the kidney are exposed to significantly lower oxygen tension, many energy-dependent metabolic functions reside in the renal cortex.19 For example, studies in cellular and animal systems have shown that citrulline and choline metabolism occur primarily in proximal tubular cells.20–22 Kynurenic acid, as would be expected for protein-bound solutes, has been identified as a substrate for organic anion transporters (hOAT1 and hOAT3) expressed on proximal tubular cells.23,24 Thus, elevations in plasma citrulline, choline, and kynurenic acid may signal underlying tubulointerstitial dysfunction. Further studies are required to determine whether other polar metabolites with V/A < 0.84 highlighted by our studies are also markers of renal metabolism and/or secretion (i.e., tubulointerstitial function).
Although we hypothesize that the metabolite predictors of CKD in FHS provide information about renal health orthogonal to GFR, we recognize that select risk markers could also play a functional role in CKD progression. Impaired metabolism of citrulline could limit arginine bioavailability for nitric oxide synthesis. Elevated levels of choline, as well as trimethylamine-N-oxide, have recently been implicated in atherogenesis.25 IDO1 encodes the major enzyme that catalyzes the first and rate-limiting step of tryptophan to kynurenine, which is subsequently metabolized to kynurenic acid.26 Ischemia reperfusion injury induces renal tubular Ido1 expression in mice, and Ido1−/− mice are protected from ischemia reperfusion injury compared with wild-type mice.27 Further, functional roles for both kynurenine and kynurenic acid have been described in the context of inflammation and vascular tone.28,29 Notably, 5-hydroxyindoleacetic acid production results from an alternative pathway of tryptophan catabolism, and 5-hydroxyindoleacetic acid levels were lower in incident CKD cases than in persons who remained CKD-free in our study.
We note several limitations of this study. First, serum creatinine measurements provide an imperfect assessment of GFR, and we acknowledge that more precise measures, such as inulin clearance, would improve renal phenotyping in the FHS. Such measures, however, are impractical in the context of a community-based study. Of note, our study of renal arteriovenous plasma sampling shows that select CKD predictors are markers of renal metabolism and secretion, not simply markers of baseline differences in glomerular filtration. Second, although we set a conservative significance threshold for metabolite discovery in FHS, we acknowledge the possibility of false discovery. Furthermore, because the FHS included middle-aged to older individuals of predominantly European ancestry, additional cohorts will be required to both validate our findings and extend their generalizability to younger individuals or other racial/ethnic groups. Third, given the clinical indications for right and left heart catheterization, our physiologic study included several individuals with abnormal kidney function. Ideally, further study of healthy individuals, along with more systematic surveys of urinary fractional excretion, will provide a more thorough picture of normal renal metabolite handling. Nevertheless, we believe that the complete epidemiologic and physiologic data sets we present in this study (Supplementary Tables 1 and 2) will serve as a valuable resource for understanding the interaction between kidney function and the metabolome in humans.
Increasing interest has been directed toward the application of metabolite profiling to plasma from individuals with existing CKD or ESRD.2–4,30–32 Here, we profile plasma obtained from individuals up to 8 years before disease onset, on the principle that non-GFR markers of renal metabolite handling may be sensitive indicators of incipient kidney dysfunction. Although metabolite profiling alone may not be sufficiently specific to identify individuals who go on to develop CKD, it may contribute to clinical models of CKD prediction that contain other variables. Thus, future efforts will be directed at measuring specific metabolites in more diverse cohorts, across distinct CKD causes, and adjudicating to what extent these biomarkers add to established clinical predictors. In parallel, efforts to understand how nonrenal determinants (e.g., diet and de novo synthesis) modulate select metabolite levels will be required to further clarify their relation to CKD risk.
Concise Methods
FHS
The Framingham Offspring Study was initiated in 1971, when 5124 individuals were enrolled into a longitudinal cohort study; 3799 of these individuals attended the fifth examination of this cohort, which took place between 1991 and 1995.33 The fifth examination is designated as the baseline examination for the current study. At this and subsequent quadrennial visits, participants underwent a physician-administered physical examination, medical history, and routine laboratory tests. The presence of CKD was ascertained at the fifth and seventh examinations. Of the 3799 attendees at the baseline examination, we have thus far applied all three components of our metabolite profiling platform to baseline plasma from 2069 individuals; 378 of these participants were chosen in the context of a nested case-control study of incident type 2 diabetes,5 and the subsequent 1691 were chosen randomly. Individuals were excluded from the current study if they had CKD at the baseline exam (n=89), had unknown CKD status at baseline (n=240), or had unknown CKD status at the seventh examination (n=306). Therefore, of the 2069 attendees of the baseline examination with available metabolite data, 1434 individuals were eligible for the current study. By the seventh examination (up to 8 years after the baseline examination), 123 individuals had developed new-onset CKD and were designated as cases, and 1311 individuals remained without CKD.
CKD Assessment
We used eGFR to estimate kidney function, using the abbreviated Modification of Diet in Renal Disease study equation. CKD was defined as an eGFR < 60 ml/min per 1.73 m2 (i.e., CKD stage 3 or worse).11,12 The modified Jaffe method was used to measure serum creatinine, and creatinine levels were calibrated using a two-step process as described previously.34 Proteinuria was defined as a value of trace or higher on a urine dipstick assay (Ames Labstix, Elkhart, IN).
Arterial and Renal Venous Plasma Sampling
We recruited patients referred to the Massachusetts General Hospital Cardiac Catheterization Laboratory for right and left heart catheterization (n=9). In addition to the steps required in each individual’s routine clinical care, the protocol included introduction of a Judkins catheter into the ostium of a renal vein, with plasma sampling from this renal venous catheter and from a catheter positioned in the abdominal aorta at the level of the renal arteries before coronary artery catheterization (and administration of iodinated contrast medium). All participants were fasting at the time of their procedure. First morning voided urine was obtained from the final four study participants.
The study protocols were approved by the institutional review boards of Boston University Medical Center and Massachusetts General Hospital. All participants provided written informed consent.
Metabolite Profiling
We applied three distinct LC-MS–based methods to distinct plasma aliquots for each experimental sample. Amino acids, amino acid derivatives, urea cycle intermediates, nucleotides, and other positively charged polar metabolites were profiled as previously described using 10 μl of plasma.5 Lipids, including lysophosphatidylcholines, lysophosphatidylethanolamines, phosphatidylcholines, sphingomyelins, cholesterol esters, diacylglycerols, and triacylglyerols, were profiled as previously described using 10 μl of plasma.35 For organic acids, sugars, bile acids, and other negatively charged polar metabolites, 30 μl of plasma were used and MS data were acquired using a 5500 QTRAP triple quadrupole mass spectrometer (AB SCIEX, Foster City, CA) using electrospray ionization and multiple reaction monitoring in the negative ion mode. Detailed methods are provided in the Supplemental Methods.
Statistical Analyses
Metabolite levels were log transformed because the raw data did not exhibit normal distributions. For the study of individuals undergoing invasive catheterization, we compared arterial and renal venous metabolite levels before iodinated contrast using two-tailed paired t tests. In FHS, we examined the association between the plasma level of each metabolite and incident CKD in FHS. Baseline metabolite levels were compared in persons who developed CKD versus those who did not using two-tailed t tests for the 217 measured metabolites. To account for multiple testing, we used a Bonferroni-corrected P value threshold of 0.00023 (0.05/217).
For nine metabolites meeting this P value threshold (kynurenic acid, kynurenine, citrulline, choline, xanthosine, β-aminoisobutyric acid, aconitate, isocitrate, and 5-hydroxyindoleacetic acid), we performed logistic regression analyses to estimate the OR of CKD at different metabolite values. Metabolites were analyzed as continuous variables (log transformed and scaled to SD of 1), and regressions were adjusted for eGFR, age, sex, hypertension, diabetes, and proteinuria at baseline. To assess the joint predictive ability of these metabolites, we first ran a stepwise logistic model including all nine metabolites. Five metabolites—kynurenic acid, xanthosine, 5-hydroxyindoleacetic acid, kynurenine, and citrulline—remained significant in the multivariable model (P threshold=0.05). We constructed a multimarker score based on the regression coefficients of these five metabolites, and then assessed whether a model including all clinical risk factors plus the multimarker panel improves CKD prediction compared with the model including clinical risk factors only. We used c-statistics to compare model discrimination, “category-free” net reclassification improvement to assess the ability of the model to correctly reclassify risk groups, and integrated discrimination improvement to examine the ability of the model to increase average sensitivity without reducing average specificity. We repeated these analyses for a more parsimonious multimarker panel composed of kynurenic acid, xanthosine, and 5-hydroxyindoleacetic acid because this subset of metabolites had the strongest P values in the stepwise logistic model.
All analyses were performed using SAS software, version 9.1.3 (SAS Institute, Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) contract N01-HC-25195 to the Framingham Heart Study, R01-DK-HL081572 (R.E.G. and T.J.W.), the Leducq Foundation (R.E.G.), and an Established Investigator Award from the American Heart Association (R.E.G.). E.P.R. received support from NIH award K08-DK-090142.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012101006/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE: Metabolite profiling identifies markers of uremia. J Am Soc Nephrol 21: 1041–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, Momose A, Toki N, Sato H, Nakayama M, Hozawa A, Tsuji I, Ito S, Soga T, Abe T: Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 33: 944–952, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Toyohara T, Suzuki T, Morimoto R, Akiyama Y, Souma T, Shiwaku HO, Takeuchi Y, Mishima E, Abe M, Tanemoto M, Masuda S, Kawano H, Maemura K, Nakayama M, Sato H, Mikkaichi T, Yamaguchi H, Fukui S, Fukumoto Y, Shimokawa H, Inui K, Terasaki T, Goto J, Ito S, Hishinuma T, Rubera I, Tauc M, Fujii-Kuriyama Y, Yabuuchi H, Moriyama Y, Soga T, Abe T: SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J Am Soc Nephrol 20: 2546–2555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE: Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME: Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol 259: E437–E442, 1990 [DOI] [PubMed] [Google Scholar]
- 7.van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH: Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am J Clin Nutr 79: 185–197, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Zablocki K, Miller SP, Garcia-Perez A, Burg MB: Accumulation of glycerophosphocholine (GPC) by renal cells: Osmotic regulation of GPC:choline phosphodiesterase. Proc Natl Acad Sci U S A 88: 7820–7824, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Vance DE: Phosphatidylcholine and choline homeostasis. J Lipid Res 49: 1187–1194, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Perrone RD, Madias NE: Serum creatinine and renal function. Annu Rev Med 39: 465–490, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen EE, Robinson RR: Amino acid extraction and ammonia metabolism by the human kidney during the prolonged administration of ammonium chloride. J Clin Invest 42: 263–276, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C: Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Invest 65: 1162–1173, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Wirthensohn G, Guder WG: Renal substrate metabolism. Physiol Rev 66: 469–497, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Miller B, Schmid H, Chen TJ, Schmolke M, Guder WG: Determination of choline dehydrogenase activity along the rat nephron. Biol Chem Hoppe Seyler 377: 129–137, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Levillain O, Hus-Citharel A, Morel F, Bankir L: Localization of arginine synthesis along rat nephron. Am J Physiol 259: F916–923, 1990 [DOI] [PubMed] [Google Scholar]
- 22.O'Donoghue N, Sweeney T, Donagh R, Clarke KJ, Porter RK: Control of choline oxidation in rat kidney mitochondria. Biochim Biophys Acta 1787: 1135–1139, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Koepsell H, Endou H: The SLC22 drug transporter family. Pflugers Arch 447: 666–676, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Uwai Y, Honjo H, Iwamoto K: Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res 65: 254–260, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL: Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray MF: The human indoleamine 2,3-dioxygenase gene and related human genes. Curr Drug Metab 8: 197–200, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Mohib K, Wang S, Guan Q, Mellor AL, Sun H, Du C, Jevnikar AM: Indoleamine 2,3-dioxygenase expression promotes renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 295: F226–F234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barth MC, Ahluwalia N, Anderson TJ, Hardy GJ, Sinha S, Alvarez-Cardona JA, Pruitt IE, Rhee EP, Colvin RA, Gerszten RE: Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem 284: 19189–19195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Jr, Hunt NH, Stocker R: Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 16: 279–285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato E, Kohno M, Yamamoto M, Fujisawa T, Fujiwara K, Tanaka N: Metabolomic analysis of human plasma from haemodialysis patients. Eur J Clin Invest 41: 241–255, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Goek ON, Döring A, Gieger C, Heier M, Koenig W, Prehn C, Römisch-Margl W, Wang-Sattler R, Illig T, Suhre K, Sekula P, Zhai G, Adamski J, Köttgen A, Meisinger C: Serum metabolite concentrations and decreased GFR in the general population. Am J Kidney Dis 60: 197–206, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Shah VO, Townsend RR, Feldman HI, Pappan KL, Kensicki E, Vander Jagt DL: Plasma metabolomic profiles in different stages of CKD. Clin J Am Soc Nephrol 8: 363–370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP: An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110: 281–290, 1979 [DOI] [PubMed] [Google Scholar]
- 34.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE: Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121: 1402–1411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.