Abstract
The identity of the peritubular population of cells with mesenchymal phenotype thought responsible for producing erythropoietin in humans remains unclear. Here, renal CD133+/CD73+ progenitor cells, isolated from the human renal inner medulla and described as a population of mesenchymal progenitors, released erythropoietin under hypoxic conditions. CD133− cells did not synthesize erythropoietin, and CD133+ progenitor cells stopped producing erythropoietin when they differentiated and acquired an epithelial phenotype. Inhibition of prolyl hydroxylases, using either dimethyloxalylglycine or a small hairpin RNA against prolyl hydroxylase-2, increased both hypoxia-inducible factor-2α (HIF-2α) expression and erythropoietin transcription. Moreover, under hypoxic conditions, inhibition of prolyl hydroxylase significantly increased erythropoietin release by CD133+ progenitors. Finally, blockade of HIF-2α impaired erythropoietin synthesis by CD133+ progenitors. Taken together, these results suggest that it is the renal CD133+ progenitor cells that synthesize and release erythropoietin under hypoxia, via the prolyl hydroxylase-HIF-2α axis, in the human kidney. In addition, this study provides rationale for the therapeutic use of prolyl hydroxylase inhibitors in the setting of acute or chronic renal injury.
The glycoprotein hormone erythropoietin (EPO) regulates blood red cell production, linking decreased tissue oxygenation to an adequate erythropoietic response. In adults, the kidney is responsible for >90% of EPO production. Many efforts have been made to identify renal EPO-producing cells. In the rodent anemic kidney, EPO production is restricted to interstitial peritubular fibroblast-like cells localized in the deep cortex and outer medulla and coexpressing EPO mRNA and the mesenchymal marker CD73.1–6 In the human kidney, the precise localization of EPO-producing cells is unknown. It is conceivable that, similarly to rodents, a peritubular population of mesenchymal cells/fibroblasts is responsible for EPO production. Data obtained from a human EPO-producing cell line isolated from human kidney showed that these cells possess mesenchymal characteristics and the ability to synthesize EPO in response to hypoxia-dependent hypoxia-inducible factor-2α (HIF-2α) stabilization and activation.7 However, in human renal tissue, in situ hybridization studies showed EPO production in cells of renal tubules. In particular, EPO mRNA was expressed by epithelial distal tubular cells, collecting tubules and additionally by glomerular cells.8 High EPO levels are also released by tumor cells of renal carcinomas,9 considered to derive from transformed tubular or progenitor/stem cells. In addition, murine embryonic renal stem cells organized in an organoid and implanted in vivo in rats produced murine EPO, suggesting that EPO-producing cells derive from renal stem cells.10
Using CD133 as a marker, a population of renal resident progenitors has been localized in different segments of the nephron.11–14 In particular, CD133+ progenitors are enriched in the Henle’s loop and thin segments of the papillary region of medulla, which is characterized by a very low oxygen tension.15 In vitro, hypoxia was shown to be a key factor in the maintenance of the progenitor phenotype and stem properties of these cells.13
In this study, we investigated whether CD133+ renal progenitors could be a source of EPO within the kidney.
Results
EPO Production by Renal CD133+ Cells in Normoxia and Hypoxia
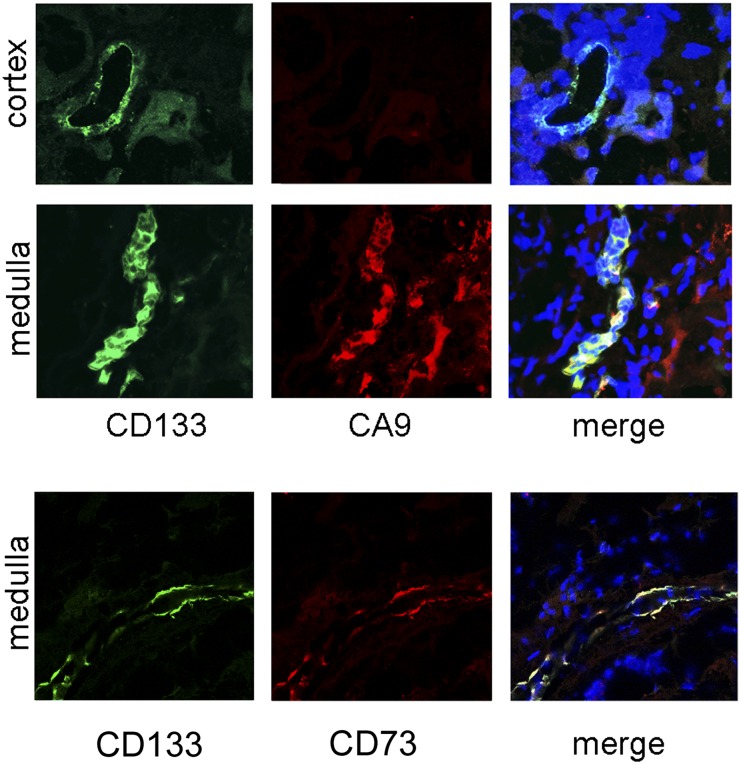
CD133+ cells located within the Henle’s loop in the human inner medulla coexpressed the hypoxic marker carbonic anhydrase IX (CAIX) (Figure 1), indicating their continuous exposure to an hypoxic environment. Isolated CD133+ progenitors from renal inner medulla displayed a mesenchymal-like phenotype,11–13 including expression of CD73, CD29, CD44, CD90, and vimentin (Figure 2, A and F). All 16 different CD133+ cell preparations used in the study, isolated by magnetic sorting from human specimens of renal medulla, showed coexpression of CD133, vimentin, and CD73 (Table 1). CD73 was also coexpressed by CD133+ cells in situ within the renal tissue (Figure 1), suggesting their mesenchymal phenotype in vivo. This is in agreement with a previous study showing vimentin expression by CD133+ cells in the cortex.16
Figure 1.
CD133+ cells in human inner medulla express CAIX and CD73. (Upper panels) Representative confocal immunofluorescence micrographs showing staining of different regions of the normal human kidney with CD133 (clone AC133–1; green) and the hypoxic marker CAIX (red). In cortical tissue, CD133+ tubules do not express CAIX. In inner medulla, CD133+ structures morphologically corresponding to the Henle’s loop express CAIX. (Lower panels) Representative confocal immunofluorescence micrographs showing costaining of CD133 and CD73. Nuclear staining is performed with Hoechst dye 33342. Original magnification, ×400.
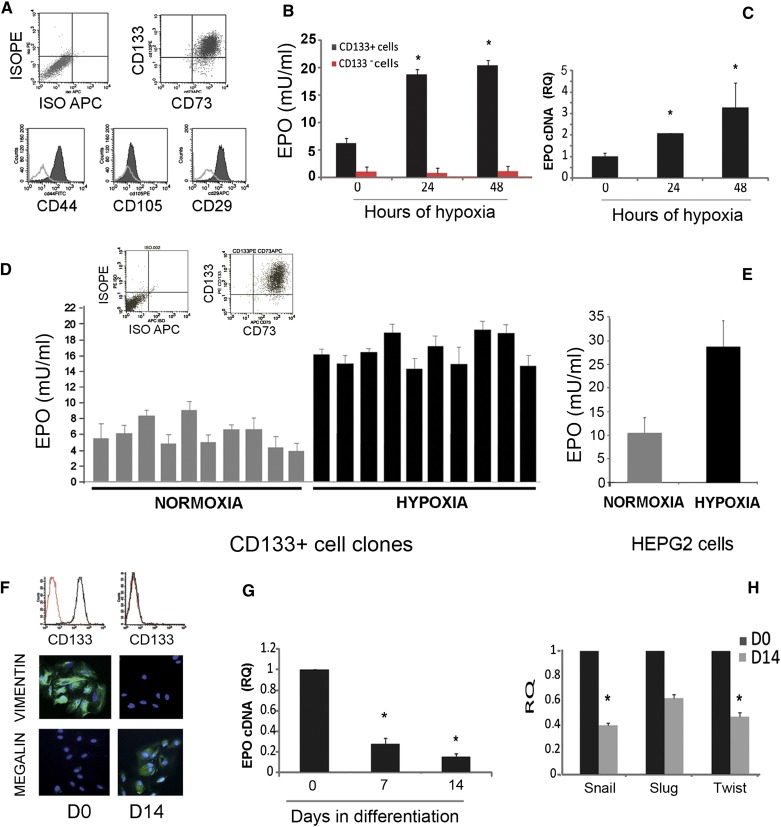
Figure 2.
EPO synthesis by renal CD133+/CD73+ cells under hypoxia and during differentiation. (A) FACS analysis showing expression of CD133 and of mesenchymal markers CD73, CD44, and CD29, but not CD105 by isolated cells. Data are representative of all cell lines in the study. (B) Increase in EPO release by CD133+ but not CD133− cells after 24 or 48 hours of hypoxia. Data are mean ± SD of different experiments performed in triplicate using six different cell isolates. (C) Quantitative RT-PCR analysis showing increase in mRNA encoding for EPO after 24 or 48 hours of hypoxia. Data are normalized to TBP mRNA and to 1 for time 0 and are the mean ± SD of three different experiments performed using three different cell isolates. ANOVA with Dunnett’s comparison test was performed for B and C. *P<0.05 versus time 0. (D and E) EPO release by 10 different CD133+/CD73+ cell clones or by HepG2 cells in normoxia and after 24 hours of hypoxia. EPO release is tested in triplicate by cells plated in 24-well plates (250,000 cells per well). A typical cytofluorimetric analysis of CD133/CD73 expression is also shown. (F) Representative cytofluorimetric histogram of CD133 expression and representative micrographs of the immunofluorescence staining of cells at day 0 and 14 after differentiation. Nuclei are stained in blue with Hoechst dye 33342. Data are representative of all cell lines in the study. (G and H) Quantitative RT-PCR analysis showing decrease in mRNAs encoding for EPO (E) and mesenchymal transcription factors (F) after 7 or 14 days of epithelial differentiation. Data are normalized to TBP mRNA and to 1 for time 0 and are the mean ± SD of three different experiments performed using three different cell isolates. ANOVA with Dunnett’s comparison test (G) or t test (H). *P<0.05. TBP, TATA binding protein. Original magnification, ×400 in F.
Table 1.
Phenotypic and functional characteristics of the cell isolates in the study
| Isolate No. | CD133 (% Expression) | CD73 (% Expression) | Vimentin (% Positive Cells) | Epithelial Difference (% Megalin/THP Positive Cells) | EPO (mU/ml) |
|---|---|---|---|---|---|
| 1 | 97.5 | 95.5 | >95 | >95/>95 | 6.4 |
| 2 | 95.7 | 97.3 | >95 | >90/>95 | 7.0 |
| 3 | 92.7 | 96.5 | >95 | >95/>95 | 5.1 |
| 4 | 90.0 | 96.5 | >95 | >95/>95 | 6.2 |
| 5 | 94.2 | 98.9 | >95 | >95/>95 | 4.8 |
| 6 | 97.0 | 95.7 | >95 | >95/>95 | 7.4 |
| 7 | 98.5 | 91.9 | >95 | >95/>95 | 5.9 |
| 8 | 94.2 | 95.7 | >95 | N.D. | 6.2 |
| 9 | 87.7 | 98.5 | >95 | >90/>95 | 4.2 |
| 10 | 96.7 | 99.5 | >95 | >95/>95 | 5.5 |
| 11 | 94.0 | 94.4 | >95 | >95/>95 | 7.5 |
| 12 | 93.3 | 96.3 | >95 | >95/>95 | 8.3 |
| 13 | 94.6 | 98.0 | >95 | >95/>95 | 8.0 |
| 14 | 91.5 | 92.7 | >95 | >95/>95 | 5.4 |
| 15 | 95.9 | 96.8 | >95 | N.D. | 6.8 |
| 16 | 96.4 | 98.7 | >95 | N.D. | 5.1 |
Expression of CD133 and of the mesenchymal markers CD73 and vimentin by the cell isolates in the study was evaluated by cytofluorimetric analysis or immunofluorescence staining at the first culture passage. Epithelial differentiation was determined by the ability to acquire the nephron segment-specific markers megalin, expressed by proximal tubular epithelial cells, and Tamm-Horsfall protein (THP), expressed by the ascending limb of the loop of Henle and by distal convolute tubules. Cells were cultured in epithelial differentiative medium for 14 days, and differentiation evaluated by immunofluorescence staining. EPO release into the cell supernatant was assessed in normoxia.
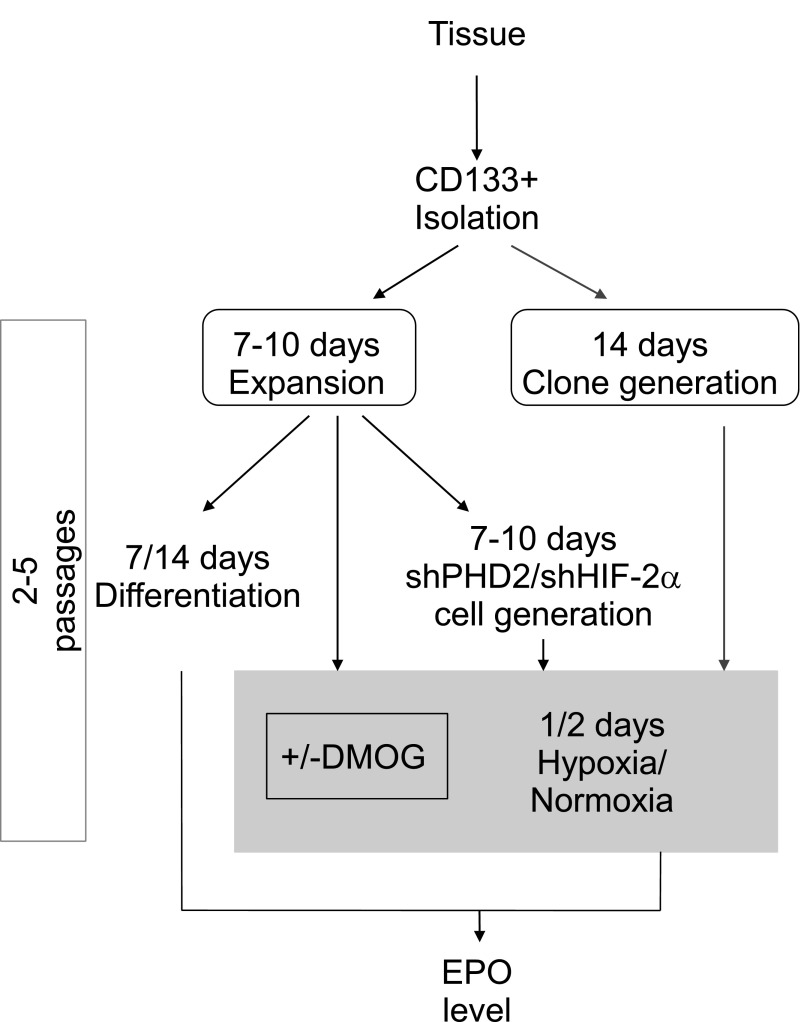
The production of EPO by CD133+/CD73+ isolates and clones was tested in normoxia and hypoxia, as schematically depicted in Figure 3. Basal EPO production was detected in all isolates (Figure 2B and Table 1). After 24–48 hours in hypoxia (1% oxygen, corresponding roughly to 7.6 mmHg), EPO release into the cell supernatant of CD133+ progenitors, but not of CD133− tubular epithelial cells, was significantly increased (Figure 2B). Concomitantly, CD133+/CD73+ progenitors undergoing hypoxia significantly upregulated EPO mRNA (Figure 2C). To verify the cell selectivity of EPO release, three different CD133+ cell isolates were subjected to clone generation by limiting dilution technique in 96-well plates. A total of 91 clones were collected (cloning efficiency equal to 35.1%). Ten clones, showing CD133/CD73 expression >95% cells, were expanded and tested for EPO release. All these clones released EPO in the normoxic or hypoxic condition (Figure 2D). The levels of EPO release were comparable with those previously reported in renal EPO releasing human cells in vitro7 and were roughly half of those obtained by HepG2 cells (Figure 2E).17 The selective EPO expression by CD133+ progenitors was further confirmed by the reduction of EPO mRNA in CD133+ progenitors undergoing differentiation into renal epithelial cells. EPO mRNA downregulation was observed after 7 days and reached significance after 14 days of differentiation (Figure 2G). In parallel, CD133+ cells underwent mesenchymal to epithelial differentiation, as shown by loss of the CD133 marker and of the mesenchymal marker vimentin, reduction of the mesenchymal transcription factors Snail, Slug, and Twist and acquirement of epithelial nephron markers (Figure 2, F and H, and Table 1), as previously reported.13
Figure 3.
Schematic representation of the experimental procedures used in the study, illustrating the temporal relationship between the CD133+ cell isolation, expansion/cloning and experimental treatment, and the final EPO analysis.
EPO Regulation by Prolyl Hydroxylase-2 Inhibition
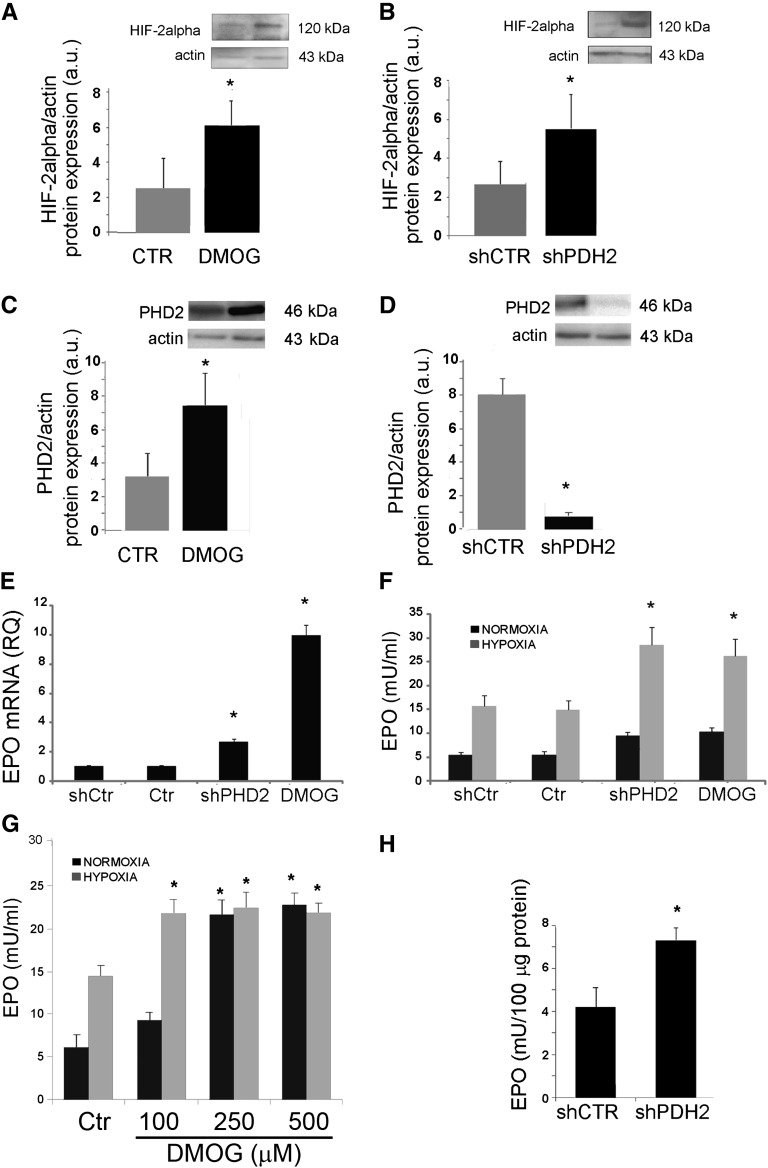
HIF stabilization required for EPO production is modulated through prolyl hydroxylases (PHDs), the enzymes that target the α subunit of HIF-1 and HIF-2 for proteasomal degradation.18 CD133+/CD73+ progenitors expressed PHD2 and upregulated its levels under hypoxic conditions (Supplemental Figure 1). We therefore evaluated the effect of a nonspecific PHD inhibitor dimethyloxalylglycine (DMOG)19 on EPO synthesis. No significant cytotoxic effect was induced by DMOG at 100–500 μM of on CD133+ progenitors after 24-hour incubation (cell vitality evaluated by Annexin V/propidium iodide staining using cytofluorimetric analysis, was >95%). In addition, we specifically inhibited PHD2, the PHD isoform known to be involved in EPO synthesis,20 using two different small hairpin RNA (shRNA). PHD inhibition with 100 μM DMOG induced HIF-2α protein increase in CD133+/CD73+ cells (Figure 4A). An increase in HIF-1α protein levels was also observed (Supplemental Figure 2A). In parallel, PHD2 was upregulated in CD133+/CD73+ cells treated with DMOG (Figure 4C), as expected in virtue of the rapid induction of its synthesis by HIFs.18 Indeed, DMOG does not affect the PHD protein levels because it acts as a competitive inhibitor.19 The specific PHD2 inhibition by lentiviral shRNA for PHD2 (shPHD2 cells) enhanced HIF-2α protein levels in CD133+ cells and downregulated PHD2 expression (Figure 4, B and D). HIF-1α protein levels were also increased (Supplemental Figure 2B). In CD133+/CD73+ cells, PHD2 inhibition, using either shPHD2 delivery with two different lentiviral vectors or DMOG administration, induced an upregulation of EPO mRNA (Figure 4E and Supplemental Figure 3A). In particular, DMOG treatment upregulated the expression of EPO mRNA >9-fold. The evaluation of EPO release into the cell supernatant, however, did not show a significant increase in normoxia (Figure 4F and Supplemental Figure 3B). At variance, PHD2 inhibition significantly enhanced the EPO release at 24 hours when cells were submitted to hypoxia (1% oxygen) (Figure 4F and Supplemental Figure 3B). In normoxia, increasing concentrations of the nonspecific PHD inhibitor DMOG were required to induce EPO release (Figure 4G), whereas its release in hypoxia was already at plateau at the lower DMOG concentration. Indeed, in shPHD2 cells, EPO increased in normoxia at an intracellular level (Figure 4H), indicating a possible hypoxia-based PHD2 independent control of EPO release.
Figure 4.
Increase in EPO released by CD133+/CD73+ cells after PHD2 inhibition. CD133+/CD73+ cells are infected with shRNA for PHD2 (shPHD2) or with scrambled vector (shCtr) or treated with DMOG (100 μM for 24 hours). (A–D) Western Blot micrographs and densitometric analysis of HIF-2α (A and B) and PHD2 (B and D) expression. Data, shown as arbitrary units, are representative of three different experiments performed using three different cell isolates and are normalized to actin expression. (E) Quantitative RT-PCR analysis showing increase in mRNA encoding for EPO in shPHD2 and DMOG treated CD133+/CD73+ cells. (F and G) EPO release by shPHD2 and DMOG treated CD133+ cells in normoxia or hypoxia. Data are the mean ± SD of experiments performed in triplicate using three (shPHD2) or six (DMOG) different cell isolates. (H) Detection of EPO in the cell lysates of shCtr and shPHD2 cells in normoxia or hypoxia. Data are the mean ± SD of three different experiments. The t test is performed. *P<0.05 DMOG versus Ctr or shPHD2 versus shCtr.
Role of HIF-2α in EPO Production
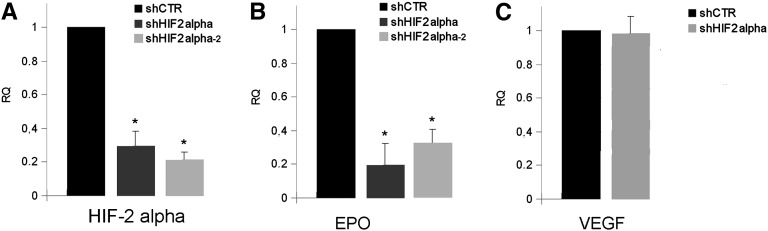
EPO production is known to be modulated by hypoxia through HIF-2α activation.7,21,22 The involvement of HIF-2α in EPO production by CD133+/CD73+ progenitors was investigated by generation of negative progenitors for HIF-2α (shHIF-2α) by infection with two different specific shRNA lentiviruses (Figure 5A). shHIF-2α cells almost completely lost the ability to synthesize EPO (Figure 5B and Supplemental Figure 3, A and B) but not vascular endothelial growth factor, used as control for maintenance of cellular activity (Figure 5C).
Figure 5.
HIF-2α is required for EPO but not VEGF synthesis by CD133+/CD73+ cells. Quantitative RT-PCR analysis showing reduction in mRNA encoding for HIF-2α (A) and EPO (B), but not for VEGF (C) in CD133+ cells infected with two different shRNA for HIF-2α (shHIF2 alpha) in respect to cells infected with a scrambled vector (shCtr). Data are normalized to TBP mRNA and to 1 for shCtr and are the mean ± SD of three experiments performed using three different cell isolates. The t test is performed. *P<0.05 shHIF2 alpha versus shCtr. VEGF, vascular endothelial growth factor.
Discussion
Taken together, the results of this study identify CD133+/CD73+ renal progenitors as a possible source of EPO and show that EPO release in hypoxic conditions can be enhanced by PHD2 inhibition. CD133+/CD73+ progenitors therefore appear as a new source of EPO production within the human kidney. Similarly to EPO-producing renal cells described in murine studies or isolated from human kidney, CD133+/CD73+ cells present a mesenchymal phenotype. The ability to synthesize EPO was restricted to CD133+ progenitors because it was lost after epithelial differentiation and it was absent in CD133− cells. Indeed, in epithelial cells, the mechanisms underlying the repression of EPO gene expression were recently shown to depend on the GATA promoter motif,23 underlying a cell type– dependent inhibitory mechanism that could be relevant in the epithelial differentiation of renal cells. In contrast with the described localization of EPO-producing cells in the interstitium of rat and mouse kidney,1–6,21 CD133+ progenitors have been identified along the renal nephron.11–14 This observation overlaps the described localization of EPO-producing cells in different segments of the human nephron by in situ hybridization studies.8,9 These dissimilar results may depend on differences in species. Indeed, a population of cells corresponding to the human CD133+ cells has not been identified in mice and rats because the AC133 antibodies recognize a glycosylation-dependent stem cell–specific isoform of CD133 only in human cells.24 Alternatively, it can be speculated that CD133+/CD73+ cells within the nephron in the inner medulla represent an additional source of EPO within the kidney, together with a fibroblast-like interstitial population. Indeed, nonclassic sites of EPO production have also been described in different organs such as brain, lung, heart, and bone marrow.25 In this context, EPO may play tissue-specific physiologic roles possibly unrelated to erythropoiesis such as modulation of angiogenesis and cell survival.26,27
The molecular pathways involved in the control of oxygen sensing and leading to EPO synthesis have been fully elucidated in recent years.27 In vivo studies in rats as well as in vitro studies on EPO-producing cell lines clearly showed that EPO production depends on HIF-2α activation,7,21,22 which, in turn, is modulated by the hydroxylation of its proline residues by PHD2.27 This was supported both in genetic murine studies as well as in human clinical settings. In fact, the loss of PHD2 function, either by inherited mutation or by genetic deletions of Egln1 (the gene codifying for PHD2), or the increase in HIF-2α function in patients with Hif2a inherited mutation is associated with excessive EPO and polycythemia.28–30 We here confirmed the involvement of PHD2–HIF-2α axis in hypoxia-induced EPO synthesis by renal CD133+/CD73+ progenitor cells. However, as PHD2 inhibition in normoxia increased intracellular EPO production, but not its release, at variance with the nonspecific PHD inhibitor DMOG, the presence of additional PHD2-independent hypoxia-related mechanisms controlling EPO release can be envisaged. Indeed, hypoxia is known to control exocytosis trafficking31 and to modulate specific receptor turnover independently by PHD2.32 Moreover, a previous work in a renal carcinoma cell line showed the presence of an intracellular pool of preformed EPO that could be rapidly released in response to an increase of cAMP.33 Although these mechanisms still require elucidation, the hypoxic environment within the renal medulla appears instrumental for EPO release by CD133+/CD73+ progenitors.
Pharmacologic modulation of the local renal EPO production is a major goal in nephrology and several pharmaceutical companies are believed to have an interest in PHD-based drug discovery.34 Indeed, PHD inhibitors have been shown to induce EPO production in mouse and rhesus macaque models.35,36 Besides EPO production, PHD inhibitors were also shown to display renoprotective activity in AKI,37 possibly due to the effect of EPO on survival of endothelial and renal tubular cells. In this context, the finding that pharmacologic inhibition of PHD, and particularly of PHD2, enhances EPO release by CD133+/CD73+ progenitors under hypoxia may support and provide a new rationale for the use of PHD inhibitors in clinical settings of acute or chronic renal injury. Moreover, isolated CD133+/CD73+ cells could represent a useful model to dissect the mechanisms of EPO synthesis and release from human renal cells.
Concise Methods
Isolation and Culture Conditions
Renal progenitor cells were obtained from the normal portion of the inner medulla obtained from surgically removed kidneys, after approval by the Ethical Committee for the Use of Human Tissue of the University of Torino, as described,13 and cultured in endothelial basal medium medium plus supplement kit (Cambrex BioScience, East Rutherford, NJ) without serum addition. Cell isolates, obtained from different renal specimens (n=16), were used between passages 2 and 5 (Figure 3 and Table 1). The CD133− cell population obtained after magnetic sorting was plated in RPMI plus 10% FCS and used after 1–2 culture passages. To generate clones, CD133+ cells were seeded using a limiting dilution technique in 96-well plates. After 12 hours, wells not containing single cells were discarded by microscopical visualization and clones derived from a single cell were expanded in expansion medium. CD133 expression was evaluated by FACS analysis.
Epithelial differentiation was obtained by culturing cells for 14 days in expansion medium with 10 ng/ml human hepatocyte growth factor (Sigma Aldrich, St. Louis, MO) and 10 ng/ml human fibroblast growth factor-4 (Sigma Aldrich). When cultured in hypoxic conditions, cells were placed in hypoxic chambers with 1% O2. DMOG (100 μM; Frontier Scientific, Logan, UT) dissolved in PBS was added to the cells for the indicated amount of time.
Cell Infection and Transfection
The Block-it Pol II miR RNAi Expression Vector Kit (Life Technologies, Grand Island, NY) was used to construct pcDNA6.2-GW/EmGFP-miR vectors (Life Technologies) expressing the target miRNA (miR-PHD2) according to the manufacturer’s instructions. The Rapid BP/LR Recombination Reaction (Block-it Lentiviral Pol II miR RNAi Expression System; Life Technologies) between pDONR 221, pcDNA6.2-GW/EmGFP-miR, and pLenti6/V5-DEST was performed to generate the pLenti6/V5-GW/EmGFP-miR expression construct. For knockdown of HIF-2α, a pGIPZ lentiviral vector (Open Biosystems, Lafayette, CO) carrying shHIF-2a was used. The constructs were then transfected with the 293T cell line using the ViraPower Packaging Mix (Life Technologies) for lentivirus production. To confirm the results, the following lentiviral particles carrying other hairpin RNA were obtained from Sigma Aldrich: scrambled PLKO.1 and shRNA for HIF-2α (HIF-2α 2, ΤPΧΝ0000082303) and scrambled PLVX.1 and shRNA for PHD2 (PHD2 2, TRCN0000001042). After titering the lentiviral stock, CD133+ progenitors were transduced with lentiviral particles following the manufacturer’s instructions.
Immunofluorescence and Western Blot Analyses
Immunofluorescence and immunohistochemistry were performed using the following antibodies: anti-CD133/1 (clone AC133, 293C3; Miltenyi Biotec, Auburn, CA), anti-CD73 (Becton Dickinson, San Jose, CA), anti-megalin and anti-Tamm-Horsfall protein (Santa Cruz Biotechnology, Santa Cruz, CA), anti-vimentin (Sigma), anti-PHD2 (Abcam, Cambridge, UK), and anti-HIF-1α, anti-HIF-2α, and CA IX (Novus Biologicals, Littleton, CO). Details are reported in the Supplemental Materials and Methods.
RNA Preparation and RT-PCR
Total RNA was isolated from different cell preparations using the RNAqueos-Micro isolation kit (Ambion, Life Technologies) according to the manufacturer’s protocol. RNA was then quantified spectrophotometrically (NanoDrop ND-1000; NanoDrop Products, Wilmington, DE). For gene expression analysis, quantitative real-time PCR was performed in 20 μl of reaction mixture containing 5 ng of cDNA template, sequence-specific oligonucleotide primers (purchased from MWG-Biotech AG, Ebersberg, Germany), and Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). TATA binding protein mRNA is used to normalize RNA inputs. Fold-change expression with respect to control was calculated for all samples. Sequence-specific oligonucleotide primers are reported in the Supplemental Materials and Methods.
ELISA for EPO
EPO protein in cell culture supernatants and in cell lysates was measured by Platinum ELISA (eBioscience, San Diego, CA) according to the manufacturer’s recommendations.
Statistical Analyses
Statistical analysis was performed using the t test, or ANOVA with Dunnett’s multiple comparison tests, as appropriate. A P value of <0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the Regione Piemonte, PISTEM project.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080772/-/DCSupplemental.
References
- 1.Rosenberger C, Rosen S, Paliege A, Heyman SN: Pimonidazole adduct immunohistochemistry in the rat kidney: Detection of tissue hypoxia. Methods Mol Biol 466: 161–174, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Koury ST, Bondurant MC, Koury MJ: Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 71: 524–527, 1988 [PubMed] [Google Scholar]
- 3.Lacombe C, Da Silva JL, Bruneval P, Fournier JG, Wendling F, Casadevall N, Camilleri JP, Bariety J, Varet B, Tambourin P: Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J Clin Invest 81: 620–623, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL, Koury ST, Nejfelt MK, Gearhart JD, Antonarakis SE: Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci U S A 88: 8725–8729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann S, Le Hir M, Eckardt KU: Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJ, Johnson MH, Ratcliffe PJ: Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Frede S, Freitag P, Geuting L, Konietzny R, Fandrey J: Oxygen-regulated expression of the erythropoietin gene in the human renal cell line REPC. Blood 117: 4905–4914, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Beirão I, Moreira L, Barandela T, Lobato L, Silva P, Gouveia CM, Carneiro F, Fonseca I, Porto G, Pinho E Costa P: Erythropoietin production by distal nephron in normal and familial amyloidotic adult human kidneys. Clin Nephrol 74: 327–335, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Da Silva JL, Lacombe C, Bruneval P, Casadevall N, Leporrier M, Camilleri JP, Bariety J, Tambourin P, Varet B: Tumor cells are the site of erythropoietin synthesis in human renal cancers associated with polycythemia. Blood 75: 577–582, 1990 [PubMed] [Google Scholar]
- 10.Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, Benigni A, Remuzzi G: In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23: 1857–1868, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G: Isolation of renal progenitor cells from adult human kidney. Am J Pathol 166: 545–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP: TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J 24: 514–525, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bussolati B, Moggio A, Collino F, Aghemo G, D’Armento G, Grange C, Camussi G: Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am J Physiol Renal Physiol 302: F116–F128, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Ward HH, Romero E, Welford A, Pickett G, Bacallao R, Gattone VH, 2nd, Ness SA, Wandinger-Ness A, Roitbak T: Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules. Biochim Biophys Acta 1812: 1344–1357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyman SN, Khamaisi M, Rosen S, Rosenberger C: Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol 28: 998–1006, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ: Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg MA, Glass GA, Cunningham JM, Bunn HF: The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci U S A 84: 7972–7976, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Cunliffe CJ, Franklin TJ, Hales NJ, Hill GB: Novel inhibitors of prolyl 4-hydroxylase. 3. Inhibition by the substrate analogue N-oxaloglycine and its derivatives. J Med Chem 35: 2652–2658, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J: HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082–4090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M: Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS ONE 6: e25839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH: Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117: 1068–1077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obara N, Suzuki N, Kim K, Nagasawa T, Imagawa S, Yamamoto M: Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood 111: 5223–5232, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW: AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90: 5002–5012, 1997 [PubMed] [Google Scholar]
- 25.Haase VH: Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol 299: F1–F13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahlmann FH, Fliser D: Erythropoietin and renoprotection. Curr Opin Nephrol Hypertens 18: 15–20, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Wenger RH, Hoogewijs D: Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am J Physiol Renal Physiol 298: F1287–F1296, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Minamishima YA, Kaelin WG, Jr: Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 329: 407, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS: A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A 103: 654–659, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrotta S, Della Ragione F: The HIF2A gene in familial erythrocytosis. N Engl J Med 358: 1966–, author reply 1966–1967., 2008 [PubMed] [Google Scholar]
- 31.Wang Y, Roche O, Xu C, Moriyama EH, Heir P, Chung J, Roos FC, Chen Y, Finak G, Milosevic M, Wilson BC, Teh BT, Park M, Irwin MS, Ohh M: Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc Natl Acad Sci U S A 109: 4892–4897, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragonés J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P: Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136: 839–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherwood JB, Burns ER, Shouval D: Stimulation by cAMP of erythropoietin secretion by an established human renal carcinoma cell line. Blood 69: 1053–1057, 1987 [PubMed] [Google Scholar]
- 34.Muchnik E, Kaplan J: HIF prolyl hydroxylase inhibitors for anemia. Expert Opin Investig Drugs 20: 645–656, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, Lin A, Smith R, Rodgers GP, Donahue RE, Klaus SJ, Tisdale JF: HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood 110: 2140–2147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safran M, Kim WY, O’Connell F, Flippin L, Günzler V, Horner JW, Depinho RA, Kaelin WG, Jr: Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: Assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A 103: 105–110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernhardt WM, Câmpean V, Kany S, Jürgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckardt KU: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.